Original research
Peer reviewed
Association between clinical signs and high serum titers of porcine reproductive and respiratory syndrome virus (PRRSV) in nursery pigs under field conditions
L. Cuartero, MS, DVM; S. Dee, DVM, PhD, Diplomate ACVM; J. Deen, DVM, PhD, Diplomate ABVP; A. Ruiz, DVM; C. Pijoan, DVM, PhD
Swine Disease Eradication Center, University of Minnesota College of Veterinary Medicine, St Paul, MN 55108.
Cuartero L, Dee S, Deen J, et al. Association between clinical signs and high serum titers of porcine reproductive and respiratory syndrome virus (PRRSV) in nursery pigs under field conditions. J Swine Health Prod. 2002;10(3):119-122. Also available as a PDF.
Summary
Objective: To establish clinical categories to identify nursery pigs infected with porcine reproductive and respiratory syndrome virus (PRRSV) and to quantify their PRRSV serum titers, in order to select the proper pigs for gilt acclimatization.
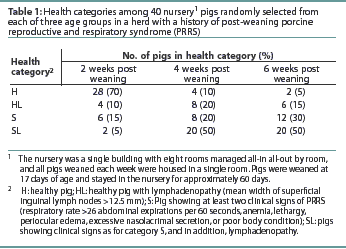
Methods: Nursery pigs were studied during an acute outbreak of PRRS in a single herd. Four clinical categories were defined: healthy pigs (H), healthy pigs with superficial inguinal lymphadenopathy (HL), sick pigs (S), and sick pigs with superficial inguinal lymphadenopathy (SL). For each category, 40 pigs were selected from each of three different age groups (2, 4, and 6 weeks after weaning) and organized by clinical category. Blood was collectedfrom each pig and tested by quantitative polymerase chain reaction assay for PRRSV and by ELISA for PRRSV antibodies.
Results: Rectal temperatures and mean superficial lymph node widths were greatest at 4 weeks post weaning (PW), and ELISA titers were greater at 4 and 6 weeks PW than at 2 weeks PW. At 2 weeks PW, only one pig, classified SL, was viremic. At 4 and 6 weeks PW, 30 to 100% of pigs in each clinical category were viremic, but there was no significant association between occurrence of viremia and clinical category. The association between lymphadenopathy and PRRS viremia was not significant in pigs 4 weeks or 6 weeks PW.
Implication: Nursery pigs with clinical signs of PRRS (fever, respiratory signs, lymphadenopathy) are potential sources of PRRSV for use in gilt acclimatization protocols; however, viremia cannot be predicted solely on the basis of these signs.
Key words : swine, post
weaning, porcine reproductive and respiratory syndrome virus,viremia,
superficial inguinal lymph node
: swine, post
weaning, porcine reproductive and respiratory syndrome virus,viremia,
superficial inguinal lymph node
Received: April 25, 2001
Accepted: November 14, 2001
Nursery pigs are commonly in-fected with multiple patho-gens.1-4 Besides porcine reproductive and respiratory syndrome virus (PRRSV), other agents that have been recoveredinclude Streptococcus suis, Hae-mophilus parasuis, Mycoplasma hyopneu-moniae, and swine influenza virus.1-4 Clinical signs associated with post-weaning PRRSV infection consist of periocular edema, nasolacrimal secretion, hyperpnea, dyspnea, fever, reduced growth rate, lymphadenopathy, and increased mortality.3-5 Frequently, pigs are reported to be healthy at the time of weaning, but become clinically affected 3 to 4 weeks later. Inability to control the circulation of PRRSV throughout the weaned pig population is associated with improper introduction of actively infectedreplacement breeding stock.2 Therefore, gilt acclimatization constitutes one of the main control strategies to reduce the risk of PRRSV outbreaks in PRRS-positive herds.
A widely practiced acclimatization strategy in the industry today is exposure and infection of gilts with farm-specific strains of PRRSV through direct contact with infected animals.6 Because young pigs have a prolonged viremia, they are often commingled with incoming replacement gilts during acclimatization.6 However, due to the wide range of clinical signs during outbreaksof post-weaning PRRSV infection, it is very difficult to visually recognize the pigs that are infected with PRRSV. Therefore, the goal of this project was to establish clinical categories to identify PRRSV-infected nursery pigs and to quantifythe magnitude of their PRRSV titers, in order to select the proper pigs to provide a source of PRRSV during the acclimatizationprocess.
Materials and methods
Selection of study herd
Prior to initiation of the study, specific criteriawere established for herd selection, including a breeding herd inventory capable of providing approximately 2000 pigs weaned per week at approximately 16 to 20 days of age, use of segregated production, a nursery facility located on a separate site consisting of exactly eight rooms, use of all in-all out management by room for nursery pigs, use of open gating between nursery pens to allow nose-to-nose contact, a previous clinical and diagnostic history of post-weaning PRRSV infection, and no history of PRRSV vaccine use.
Description of facility and animal flow
A facility in the midwest United States that fulfilled all criteria was selected. The nursery consisted of one building with eight rooms, managed all in-all out by room. Pigs were weaned at 17 days of age and remained in the nursery until 60 days of age. All of the pigs weaned each week were housed in the same nursery room, with 25 pigs per pen. Nursery rooms each contained four rows of eight pens with plastic slatted flooring.
Selection of clinical signs
A set of measurable parameters based on previously published data for nursery pigs infected with PRRSV was proposed, including rectal temperature, respiratory rate, and the width of the superficial inguinal lymph nodes.7,8 Observational parameters included level of physical activity, body condition, skin color, and the occurrence of periocular edema and excessive nasolacrimal secretion.1-4
During a preliminary visit to the farm, clinical signs characteristic of post-weaning PRRSV infection were observed. Pigs appeared to be in excellent health at the time of weaning; however, 4 weeks later, fever, anorexia, lethargy, dyspnea ("thumping"), and increasing mortality were observed.1-4
Dyspnea was defined as greater than 26 inspirations per 60 seconds;7 a stopwatch was used to record an exact 60-second observational period.
In order to determine the presence or absence of lymphadenopathy, the right and left superficial lymph nodes were measured. Pigs were manually restrained in an inverted position by grasping both rear limbs. The medial-lateral size of each lymph node was determined using Vernier calipers, and measurements were quantified using a millimeter ruler. The two measurements were added together and the mean width calculated. Lymphadenopathy was determined to be absent when the mean width of the superficial inguinal lymph nodes was <=12.5 mm. Lymphadenopathy was considered to be present when the mean width of the superficial inguinal lymph nodes was >12.5 mm (Figure 1).
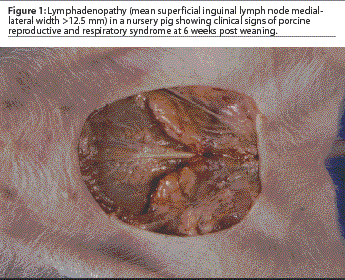
A febrile pig was defined as having a rectal temperature of greater than 39°C.7
Other clinical signs of PRRS included anemia (pigs having visible skin pallor); lethargy (pigs displaying a reduced level of activityand frequently found in ventral or lateral recumbency); poor body condition (pigs displaying extensive weight loss with visible vertebra, ribs, or both); periocular edema (swelling or hyperemia around the circumference of the eye, or both); and nasolacrimal discharge (visible discharge from the nasolacrimal (ocular) duct).
Definition of clinical categories
Clinical categories were defined on the basisof the clinical signs described above.
Category H: healthy pigs. A pig was classified as healthy if it had respiratory rate <=26 abdominal inspirations per 60 seconds, mean width of superficial inguinal lymph nodes <=12.5 mm, and no visual evidenceof anemia (skin pallor), lethargy, poor body condition, periocular edema, or excessive nasolacrimal secretion.
Category HL: healthy pigs with lymphadenopathy. Clinical parameters were the same as for category H, except for the presence of lymphadenopathy as defined by a mean superficial inguinal lymph node width of >12.5 mm.
Category S: sick pigs with no detectable lymphadenopathy. These pigs showed a minimum of two of the following clinical signs: respiratory rate >26 abdominal expirations per 60 seconds, anemia, lethargy, periocular edema, excessive nasolacrimal secretion, or poor body condition, with mean superficial inguinal lymph node width of <=12.5 mm.
Category SL: sick pigs with lymphadenopathy. These pigs showed a minimum of two of the clinical signs listed for category S pigs, and in addition, a mean superficial inguinal lymph node width of >12.5 mm.
Experimental design
A cross-sectional study was conducted with nursery pigs in three age groups tested on a single day. Groups were tested 2 weeks after entering the nursery, at the approximate onset of clinical signs (4 weeks after entering the nursery), and prior to leaving the facility (6 weeks after entering the nursery). A total of 120 pigs (40 pigs per age group) were randomly selected from three different nursery rooms according to the desired ages. Within each age group, pigs were classified into the categories H, HL, S, or SL. This sample size allowed detection of at least one pig with PRRS at the 95% confidence interval if the prevalence was higher than 10.5% for the age group and higher than 30% for the category group. The statistical program Statcalc (Version 6; EpiInfo, CDC Division of Surveillance and Epidemiology, Paris) was used for these calculations.
Clinical examination and sample collection
Data were collected in the following order: respiratory rate was counted, lymph node width was measured, rectal temperature was recorded, and blood was collected. Blood samples were kept on ice during the collection process, then centrifuged at 1500g for 15 minutes and immediately submitted to the University of Minnesota Diagnostic Laboratory, 1333 Gortner Avenue,St Paul, MN 55108. Sera were tested for PRRSV RNA using a semi-quantitative Taqman polymerase chain reaction (PCR) (Perkin-Elmer Applied Biosystems, Foster City, California) and for PRRSV antibodies by ELISA (HerdChek PRRS ELISA, IDEXX Laboratories, Westbrook, Maine).9,10 The Taqman PCR provided information on PRRSV titers expressed as median tissue culture infective doses (TCID50) per mL. The ELISA expressed PRRSV antibodies as sample-to-positive (S:P) ratios. An S:P ratio of >=0.4 was consideredpositive.
Statistical analysis
Data were analyzed using Statistica (StatSoft Inc, Tulsa, Oklahoma). A multivariate linear regression model was used with PRRSV titer as the dependent variable. The independent variables included the means (+/- SD) for each age group and for each clinical category for the following parameters: rectal temperature, respiratory rate, individual superficial inguinal lymph node widths, and ELISA S:P ratio. Data were analyzed by age group and clinical category. A level of significance of P<=.05 was used throughout the study.
Results
The numbers of pigs in each health category at each time post weaning are shown in Table 1.
Two weeks post weaning. Means for rectal temperature, width of the superficial inguinal lymph nodes, and ELISA S:P ratio are shown in Table 2. The mean respiratory rate was 18+/-1.7 expirations per 60 seconds. Only one of the 40 animals tested (Taqman PCR) was viremic (2.5%), and this pig was classified as category SL, with a PRRSV titer of 1.24 x 106 TCID50 per mL.
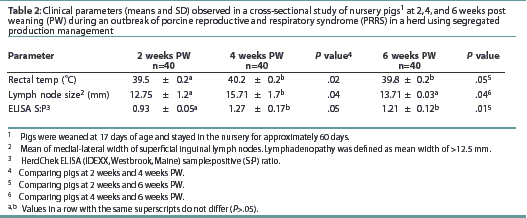
Four weeks post weaning. Means for rectal temperature,
width of the superficial inguinal lymph nodes, and ELISA S:P ratio
are shown in Table 2. The mean rectal temperaturewas higher than
that of pigs 2 weeks post weaning. The mean width of the superficial
inguinal lymph nodes and the mean ELISA S:P ratio were greater
than for pigs 2 weeks after weaning. The mean respiratory rate,
17.85+/-1.5 expirations per 60 seconds, did not differ (P
= .36) from that of pigs 2 weeks post weaning. The mean PRRSV
titer was 1.25 x
106 TCID50 per mL. Viremia was detected
in 33 of the 40 selected pigs (prevalence 82.5%). Viremia occurred
in all four pigs (100%) in category H, five of eight pigs (62.5%)
in category HL, six of eight pigs (75%) in category S, and 18
of 20 pigs (90%) in category SL. The association between viremia
and lymphadenopathy was not significant (P=.22). The 28
pigs with lymphadenopathy (categories HL and SL) had a mean PRRSV
titer of 1.98 x 106 TCID50 per mL, while
the 12 pigs without lymphadenopathy (categories H and S) had a
mean PRRSV titer of 1.30 x 105 TCID50 per
mL.
Six weeks post weaning. Means for rectal temperature, width of the superficial inguinal lymph nodes, and ELISA S:P ratio are shown in Table 2. The mean rectal temperature was higher than that of pigs at 2 weeks post weaning, but did not differ from that of pigs at 4 weeks post weaning. Mean superficial inguinal lymph node width was smaller than that of pigs at 4 weeks post weaning, but did not differ (P=.22) from that of pigs 2 weeks post weaning. The ELISA S:P ratios were higher than those of pigs 2 weeks post weaning, but did not differ from those of pigs 4 weeks post weaning (P=.31). Mean respiratory rate (expirations per 60 seconds) did not differ (P=.40) from that of pigs 2 weeks or 4 weeks post weaning. The overall prevalence of viremia was 72.5% (29 of 40 pigs), and the mean PRRSV titer was 1.38 x 106 TCID50 per mL. Viremia occurred in one of two pigs (50%) in category H, two of six pigs (30%) in Category HL, six of 12 pigs (50%) in category S, and all 20 pigs (100%) in category SL. The association between PRRS viremia and lymphadenopathy was not significant (P =.69). The 26 pigs with lymphadenopathy had a mean PRRSV titer of 1.76 x 106 TCID50 per mL, while the 14 pigs without lymphadenopathy had a mean PRRSV titer of 9.63 x 106 TCID50 per mL.
Discussion
The impact of post-weaning PRRS is frequently compounded by infection with multiple pathogens, both of a viral and a bacterial nature. The clinical effects of post-weaning PRRS may persist in endemically infected farms, resulting in increased mortality (1.9 to 10.2%), reduced average daily gain (0.38 to 0.26 kg), increased treatment costs per pig (US$1.77 to $1.91), and reduced feed efficiency (1.77 to 1.91 kg feed per kg gain).4 Inability to control PRRSV transmission in the nursery pig population has been associated with improper gilt management.5,6 Therefore, the goal of this observational study was to evaluate a clinical method to detect PRRSV-infected pigs in endemically infected nursery pig populations and to quantify their PRRSV titers, in hopes of being able to identify viremic nursery pigs as sources of PRRSV for use in acclimatization protocols.
In this study, sick pigs with lymphadenopathy had a high prevalence
of viremia and high PRRSV titers at 4 or 6 weeks after weaning,
although the association between lymphadenopathy and viral titers
was not statistically significant. It was interesting to note
that category H pigs, classified as "clinically healthy",
proved to have a prevalence of infection and viral titers similar
to those of "clinically ill" animals. This may have
been because the clinically healthy animals had not been exposed
to the virus for a sufficient period of time, or that these pigs
had not yet experienced the effects of opportunistic pathogens,
eg, S suis,
H parasuis. It was also noteworthy that no association between
ELISA S:P ratio and PRRSV titer could be demonstrated, although
high S:P ratio is frequently used as an indicator of viremia in
the field.
The primary limitation of this study was the sample size. Deficiencies in sample size may have reduced the ability to estimate the true prevalence of infected pigs in all categories after weaning, or to detect statistically significant differences between clinicalsigns described in a PRRSV-infected nursery. Also, this field study necessarily lacked controls, was carried out in only one herd, and was not repeated within the herd. The results generated for this herd might not be representative of those for other herds in the global swine industry. However, the pattern of PRRSV infection in the nursery of the study herd was representative of that observed in similar progressive, large-scale commercial production systems in North America.2-4 These results thus provide a foundation for further studies. Finally, it is not known if the clinical parameters used in the study are applicable across other farms. Therefore, practitioners and producers might collaborate to establish a set of standards for each individual herd participating in a similar program.
Implications
- Although nursery pigs showing clinical signs of PRRS (fever, respiratory signs, lymphadenopathy) are potential sources of PRRSV for use in gilt acclimatization protocols, viremia cannot be predicted solely on the basis of these clinical signs.
References - refereed
1.Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, Lum MS, Andrews JJ, Rathje JA. Comparison of the pathogenicity of two US porcine and reproductive and respiratory syndrome virus isolates to that of the Lelystad virus. Vet Pathol. 1995;32:648-660.
2. Dee SA, Joo HS. Prevention of the spread of PRRS virus in endemically infected pig populations by nursery depopulation. Vet Rec. 1994;135:6-9.
3. Yoon IJ, Joo HS, Christianson WT, Morrison RB, Dial GD. Persistent and contact infection in nursery pigs experimentally infected with PRRSV. Swine Health Prod. 1993;1(4):5-9.
4. Dee SA, Joo HS, Polson DD, Park BK, Pijoan C, Molitor TW, Collins JE, King V. Evaluation of the effects of nursery depopulation on the persistence of PRRS virus and the productivity of 34 farms. Vet Rec. 1997;140:247-248.
5. Dee SA, Joo HS. Clinical investigation of recurrent reproductive failure with PRRS virus in a swine herd. JAVMA. 1994;204:1017-1018.
6. Dee SA. An overview of production systems designed to prepare naïve replacement gilts for impending PRRSV challenge: A global perspective. Swine Health Prod. 1997;5:231-239.
7. Blood DC, Radostits OM, Henderson JA, Arundel JH, Gay CC. Clinical examination. In: Blood DC, Radostits OM, Henderson JA, eds. Veterinary Medicine. 6th ed. London: Bailliere Tindall. 1983:12-14.
8. Montane L, Bourdelle E, Bressou C. In: Bailliere JB, ed. Anatomie regionale des animaux domestiques III: Le Porc. 2nd ed. Paris: Balliere. 1964:286-289.
9. Shin J, Bautista E, Kang YB, Molitor TW. Quantitation of PRRS virus RNA in semen using single-tube reverse transcription-nested polymerase chain reaction. J Virol Methods. 1998;72:67-79.
10. Albina E, Leforban Y, Baron T, Plana Duran J, Vannier P. An enzyme linked immunosorbent assay for the detection of antibodies to porcine reproductive and respiratory syndrome (PRRS) virus. Annales de Recherches Veterinaires. 1992;23:167-173.
