Original research |
Peer reviewed |
The effect of dietary chicken egg-yolk antibodies on the clinical response in weaned pigs challenged with a K88+ Escherichia coli isolate
Larisa V. Chernysheva, DVM, MSc; Robert M. Friendship, DVM, MSc, Diplomate ABVP; Catherine E. Dewey, DVM, MSc, PhD; Carlton L. Gyles, DVM, PhD
LVC, RMF, CED: Department of Population Medicine, Ontario Veterinary College, University of Guelph, Guelph, Ontario. CLG: Department of Pathobiology, Ontario Veterinary College, University of Guelph, Guelph, Ontario. Corresponding author: Dr Robert M. Friendship, Department of Population Medicine, Ontario Veterinary College, University of Guelph, Guelph, Ontario, Canada N1G 2W1; Tel: 519-824-4120, ext 54022; Fax: 519-763-3117; E-mail: rfriends@ovc.uoguelph.ca
Cite as: Chernysheva LV, Friendship RM, Dewey CE, et al. The effect of dietary chicken egg-yolk antibodies on the clinical response in weaned pigs challenged with a K88+ Escherichia coli isolate. J Swine Health Prod. 2004;12(3):119-122..
Also available as a PDF.
Summary
Objectives: To determine if supplementing the diet of weaned pigs with a specific chicken egg-yolk-antibody product would protect against experimentally induced colibacillosis.
Methods: Four treatment groups of 12 newly weaned pigs (approximately 22 days old) were fed either a control diet (Groups 1 and 2) or the same diet containing chicken egg-yolk antibodies (IgY) at inclusion rates of 0.32% (Group 3) and 3.2% (Group 4). Pigs in Groups 2, 3, and 4 were challenged with 5 mL of a suspension containing 1011 colony forming units per mL of a K88+ isolate of Escherichia coli.
Results: Pigs in Group 1 (unchallenged controls) did not develop diarrhea. In the three groups challenged with E coli, pigs developed watery diarrhea and dehydration. Prevalence and severity of clinical signs were similar in all three groups. No appreciable levels of IgY were detected either in treated or control pigs by ELISA testing of small intestine content.
Discussion: The E coli challenge was successful in creating a clinical response similar to field cases. The presence of chicken egg-yolk antibodies in the ration did not appear to be effective in preventing the disease. It is possible that IgY activity is greatly reduced by gastric acid and pepsin, and therefore, even at high inclusion rates, egg-yolk antibodies may not be efficacious in pigs as old as 3 to 4 weeks of age.
Implications: Supplementation of chicken egg-yolk-antibody products may not be effective in controlling postweaning E coli diarrhea.
Keywords: swine, postweaning mortality, diarrhea, passive immunity
Search the AASV web site
for pages with similar keywords.
Received: July
31, 2003
Accepted: September
15, 2003
Postweaning Escherichia coli diarrhea is an important cause of economic loss for pig producers.1 Often, as a response to postweaning diarrhea, farmers incorporate antibiotics and high concentrations of zinc oxide in starter rations. However, concerns have arisen with regard to the emergence of antimicrobial resistance and to the environmental impact of heavy metals in manure. A variety of alternative approaches have been tried with disappointing results.2 Treatments that have been investigated include acidification of feed or water, probiotics, prebiotics, and vaccination.
One alternative technique that has shown potential as an inexpensive and effective control measure for postweaning E coli diarrhea is the use of chicken egg-yolk antibodies (IgY). Chickens that have been vaccinated against specific enterotoxigenic E coli (ETEC) fimbrial antigens produce high levels of IgY in their eggs. Products made from the yolks of eggs from immunized chickens have been administered orally to piglets and offer prophylactic and therapeutic value in controlling colibacillosis.3 However, in field studies, morbidity and mortality appeared to be unaffected, possibly because of the interaction of environmental and management factors and the presence of concurrent diseases.4 Possibly a better method to determine the value of chicken egg-yolk antibodies in the control of postweaning diarrhea is by using a controlled experimental E coli challenge study. However, some researchers have reported that experimental infection to create clinical disease is difficult to achieve.5
The objectives of this study were to determine whether an oral challenge using a K88+ ETEC isolate could induce diarrhea in postweaned pigs as old as 3 to 4 weeks of age, and to determine if egg-yolk antibodies were protective against an experimentally induced E coli infection.
Material and methods
All challenge studies were approved by the University of Guelph Animal Care Committee and were in accordance with the Canadian Council on Animal Care guidelines for the care and use of experimental animals.
Animals
Forty-eight purebred Yorkshire pigs from the University of Guelph's swine research farm were weaned at approximately 3 weeks of age (mean = 22.0 days, SD = 3.4 days) and assigned randomly to one of four treatment groups. All pigs appeared healthy, with no history of diarrhea, but their carrier status for ETEC was not evaluated prior to the study.
Experimental design
A total of 48 pigs were used in the three replicates of the trial, with 12 pigs allotted to each of four treatment groups in the study. In each trial, 16 pigs were assigned to four pens, with each pen representing a treatment group. At the time of arrival at the isolation facility, pigs were given an ear tag for identification purposes and weighed.
Pigs in Groups 1 and 2 were fed a control diet (a standard starter ration containing no antibiotics). Pigs in Groups 3 and 4 received a similar diet except for addition of egg-yolk-antibody product. The feed for Group 3 was prepared by including the egg-yolk-antibody product at the level recommended by the manufacturer (3.2 g per kg or 0.32% of the total diet) and for Group 4 the egg-yolk-antibody product was added at 10 times the recommended level (32 g per kg or 3.2% of the total diet). Pigs were allowed to acclimatize to the facility for 3 days. During this time, experimental diets were offered as mash ad libitum, and all pigs were observed to be eating the feed.
On the third day, pigs were challenged with a preparation of E coli isolated from a field case of postweaning colibacillosis. Five mL of a suspension containing 1011 colony forming units (CFU) per mL was given by gavage to each pig in Groups 2, 3, and 4. Group 1 was not challenged with E coli (negative control).
The experimental diets were offered ad libitum until the end of the experiment. Pigs were observed hourly postchallenge for development of clinical signs, including lethargy, inappetence, dehydration, gauntness, and diarrhea. Pigs with severe watery diarrhea were euthanized. Thirty-six hours after challenge, all remaining pigs were euthanized and necropsy examinations were performed.
Egg-yolk-antibody product
The egg-yolk-antibody product, obtained from Dr Ron Marquardt (Department of Animal Science, University of Manitoba, Winnipeg, Manitoba, Canada), contained antibodies specific to E coli fimbrial antigens K88, K99, and 987P. The method used for preparation and purification of the egg-yolk antibodies was similar to that described by Kim et al (1999).6 The powdered egg-yolk-antibody product was mixed with mash feed by hand because of the small quantities of feed used in these trials.
Challenge culture
A strain of O149 K88ac+ E coli isolated from a field case of postweaning diarrhea was used to challenge the pigs. In order to prepare sufficient inoculum, the E coli was streaked onto a blood agar plate. One colony was transferred to 15 mL of brain-heart infusion broth and incubated for 18 to 24 hours. Six 4-L baffle flasks were prepared with 800 mL of brain-heart infusion broth, and to each flask, 2.5 mL of the inoculated broth was added. The flasks were placed on a platform shaker (INNOVA; Brunswick Scientific, Edison, New Jersey) and rotated at 150 rpm overnight at 37°C. A 1-mL sample of broth was diluted logarithmically and plated on blood agar to determine the number of CFU per mL. The bacteria were pelleted by centrifugation and resuspended in phosphate buffered saline (PBS) to a concentration estimated to be 5 x 1011 CFU per 5-mL dose.
Collection of intestinal samples
Euthanasia was performed by intravenous injection of pentobarbital at a dosage of 70 mg per kg of body weight. Fecal swabs collected from all pigs at euthanasia were submitted to the Animal Health Laboratory, University of Guelph (Guelph, Ontario, Canada), for culture and typing of E coli isolates. Contents of the stomach, small intestine, and large intestine were diluted 1:6 in PBS and homogenized. Final pH of stomach content was 7.2 to 7.4. Each sample was centrifuged at 1000g for 5 minutes, and the supernatants were filtered through 0.45 mm membrane filters6 and stored at -20°C until the assay for IgY was performed.
ELISA for IgY
A direct ELISA was used to detect anti-K88+ IgY immunoglobulins in feed and gastrointestinal samples. Microtiter plates were coated with K88 antigen. The procedure for the ELISA is described by Jin et al (1998).7 A sample size of at least 2 g was used for the initial (ie, highest) dilution to obtain the best results. Antibodies were diluted to such a degree that the plate reader could analyze the wells without exceeding its absorption spectrum. For example, the recommended dilution is 1:2000 for egg-yolk antibodies and 1:10 for feed samples.
Statistical analysis
A general linear model analysis of variance procedure (GLM) (SAS Institute Inc, Cary, North Carolina) was used to determine the effect of different dosages of antibodies at initial and final time of sampling. Least squares means were computed for each dosage time and their interaction. Descriptive statistics for numerical data and test for normality were conducted with PROC UNIVARIATE in SAS program (PC-SAS 6.12; SAS Institute).
A mixed linear model (SAS PROC MIXED; SAS Institute) was used to model treatment group effects on the titre of IgY K88 antibody in the gastrointestinal tracts of pigs, while controlling for the random effects of pig nested within the fixed effects of the treatment nested within the random effects of trial. Tukey's HSD test (PROC GLM, SAS/STAT) was applied in all pair-wise comparisons of means following detection of significant interaction effects on intestinal samples. Descriptive statistics for numerical data and tests for normality were conducted with PROC UNIVARIATE in SAS. All multivariate models were run in SAS PROC MIXED. Interaction effects were included in all models and all variables were tested. A chi-square test was used to determine if the number of pigs with diarrhea was different among groups. All tests were assessed at a confidence level of 95%; the results were considered significant if P < .05.
Results
Pigs in Group 1 (negative controls) did not develop diarrhea (Table 1). In the three groups receiving the E coli challenge, diarrhea was noted in some pigs as early as 6 hours postchallenge. In all three challenged groups, some pigs showed no clinical signs of gastrointestinal disease (4, 3, and 5 of 12 pigs in each of Groups 2, 3, and 4, respectively). These pigs appeared bright and alert throughout the 36-hour postchallenge period. Some pigs in all three challenged groups were euthanized before the end of the trial because of severe watery diarrhea and dehydration (2, 3, and 2 of 12 pigs in Groups 2, 3, and 4, respectively). Necropsy examination of pigs with diarrhea revealed fluid-filled intestines and enteritis consistent with colibacillosis. There were similar levels of diarrhea in each of the challenged groups (chi-square test = 0.75, P < .69).
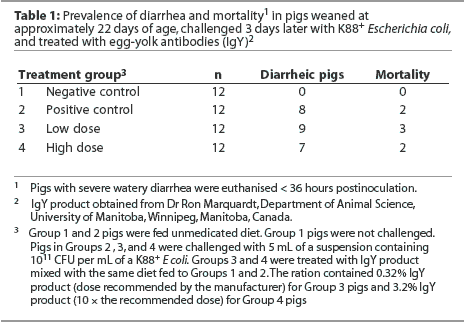
Mean titer of egg-yolk-antibody activity in the feed with 10 times the recommended level of product (Group 4) was 0.03, and for the lower level of inclusion (Group 3) the titres averaged 0.003.
Titers of IgY in stomach and intestinal content from both treated pigs (Groups 3 and 4) and pigs that had received no egg yolk (Groups 1 and 2) were extremely low and did not differ (P > .05).
The culture of feces from pigs with diarrhea revealed a pure growth of K88+ E coli.
Discussion
Results of this study indicate that the egg-yolk-antibody product did not protect susceptible pigs from developing diarrhea as a result of oral challenge with approximately 5 x 1011 CFU of K88+ E coli. Pigs that did not develop diarrhea possibly did not have intestinal receptor sites for K88+ E coli and therefore were safe from infection.8 In future studies, it would be useful to establish the presence or absence of K88 receptors in the study pigs. Because the pigs were randomly assigned to treatment, it is likely that similar numbers of pigs in each group had K88 receptors.
It is possible that the challenge dose was too great, and that the egg-yolk product might have provided protection if a lower dose of E coli had been used. However, some pigs that developed diarrhea were not severely sick, suggesting that the challenge was reasonable.
In other research studies, levels of challenge similar to the one used in this study have been used. Marquardt et al (1999)9 challenged 3-week-old pigs with 5 mL of E coli at a dose of 1012 CFU, provided them with 0.5 g of egg-yolk-antibody product three times daily for 2 days after the experimental infection, and demonstrated a positive effect. Pigs in Group 3 of our study consumed similar levels of egg-yolk product, and it is unclear why the results differed. The effect of egg-yolk antibodies appears to be dose dependent. Yokoyama et al (1997)10 found that 5.5 g of egg-yolk powder per pig per day was protective, whereas 3.5 g was not. Imberechts et al (1997)11 used 30 g of egg-yolk powder per day to prevent postweaning diarrhea. The pigs in our study consumed less than 400 g of feed per day, ie, less than 13 g of egg-yolk product per day for those in the high-dose category.
The levels of egg-yolk antibody product commonly used in the feed industry in Ontario are 0.1 to 0.2% (1 to 2 kg per tonne). At 1 kg of product per tonne of starter ration, the extra cost per tonne is about $80 (all costs reported in $US). According to Marquardt and Li (2001),3 a pig on average will consume a total of approximately 4 kg of feed during the normal feeding period (ie, 18 to 28 days of age), or about 4 g of egg yolk product at the 0.1% inclusion rate ($0.32 per pig). The higher dosage of 3.2% used in this study is probably close to the limit of what is economically feasible.
In this study, no appreciable levels of anti-K88 E coli IgY were detected in the gastrointestinal tract, even in pigs on the higher dosage level of the egg-yolk-antibody product. It is possible that the reason for failure of the product to control E coli diarrhea was that the level of egg-yolk antibody in the gastrointestinal tract was too low to be effective.
In studies involving younger weaned pigs (weaned at 10 days of age), egg-yolk antibodies appeared to provide protection against K88+ E coli.12 It is possible that in such young piglets the gastric pH is relatively high, and digestive enzymes are not as fully developed as in the 3- to 4-week-old pigs used in this trial. Some researchers have suspected that avian immunoglobulin may be destroyed by the pH and enzymes of the pig's stomach and small intestine and have advocated coating the egg-yolk product to improve its survival.13,14 However, coating the product or increasing the levels of product incorporated into the feed in order to avoid destruction in the gastrointestinal system is likely to make the use of egg-yolk antibodies prohibitively expensive.
Implications
- Under the conditions of this study, a chicken egg-yolk-antibody product was not effective in controlling postweaning E coli diarrhea.
- Clinical disease can be produced in 3- to 4-week-old pigs by challenging them with K88+ ETEC at a dose of 5 x 1011 CFU.
References
1. Amezcua R, Friendship RM, Dewey CE, Gyles CL, Fairbrother JM. Presentation of postweaning E. coli diarrhea in southern Ontario, prevalence of hemolytic E. coli serogroups involved and their antimicrobial resistance patterns. Can J Vet Res. 2002;66:73-78.
*2. Friendship RM, Dewey CE, Amezcua R, Chernysheva L, Gyles C. Failure of non-antibiotic treatments for the control of postweaning diarrhea. Proc IPVS Cong. Ames, Iowa. 2002:234.
*3. Marquardt RR, Li S. Control of diarrhea in young pigs using therapeutic antibodies. Proc Leman Swine Conf. St Paul, Minnesota. 2001:227-239.
4. Chernysheva LV, Friendship RM, Gyles CL, Dewey CE. Field trials to assess the efficacy of specific egg yolk antibody product for the control of postweaning E. coli diarrhea in pigs. Vet Ther. 2003;4:279-284.
5. Madec F, Bridoux N, Bounaix S, Cariolet R, Duval-iflah Y, Hampson DJ, Jestin A. Experimental models of porcine postweaning colibacillosis and their relationship to postweaning diarrhea and digestive disorders as encountered in the field. Vet Microbiol. 2000;72:295-310.
6. Kim JW, Jin LZ, Cho S-H, Marquardt RR, Frohlich AA, Baidoo SK. Use of chicken egg-yolk antibodies against K88+ fimbrial antigen for quantitative analysis of enterotoxigenic Escherichia coli (ETEC) K88+ by a sandwich ELISA. J Sci Food Agric. 1999;79:1513-1518.
7. Jin LZ, Baidoo SK, Marquardt RR, Frohlich AA. In vitro inhibition of adhesion of enterotoxigenic Escherichia coli K88 to piglet intestinal mucus by egg-yolk antibodies. FEMS Immunol Med Microbiol. 1998;21:313-321.
8. Bertschinger HU, Fairbrother JM. Escherichia coli infections. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:431-468.
9. Marquardt RR, Jin LZ, Kim JW, Fang L, Frohlich AA, Baidoo SK. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early weaned piglets. FEMS Immunol Med Microbiol. 1999;23:283-288.
10. Yokoyama H, Hasi K, Umeda K, Icetlo FC, Kuroki M, Ikemori Y, Kodama Y. Effect of oral egg yolk antibody infection in weaned pigs. J Vet Med Sci. 1997;59:917-921.
11. Imberechts H, Deprez P, Van Driessche E, Pohl P. Chicken egg-yolk antibodies against F18ab fimbriae of Escherichia coli inhibit shedding of F18 positive E. coli by experimentally infected pigs. Vet Microbiol. 1997;54:329-341.
12. Owusu-Asiedu A, Baidoo SK, Nyachoti CM, Marquardt RR. Response of early-weaned pigs to spray-dried porcine or animal plasma-based diets supplemented with egg-yolk antibodies against enterotoxigenic Escherichia coli. J Anim Sci. 2002; 80:2895-2903.
13. Akita EM, Nakai S. Preparation of enteric-coated gelatin capsules of IgY with cellulose acetate phthalate. In: Sim JS, Nakai S, Guenter W, eds. Egg Nutrition and Biotechnology. Wallingford, United Kingdom: CAB International; 2000:301-310.
14. Shimizu M, Miwa Y, Hashimoto K, Goto A. Encapsulation of chicken egg yolk immunoglobulin G (IgY) by liposomes. Biosci Biothec Biochem. 1993;57:1445-1449.
* Non-refereed references.
