Original research |
Peer reviewed |
Meloxicam as adjunctive therapy in treatment and control of porcine respiratory disease complex in growing pigs
El meloxicam como una terapia adjunta en el tratamiento y control del complejo respiratorio porcino en cerdos en crecimiento
Utilisation du meloxicam comme thérapie supplémentaire pour le traitement et le controle du complexe respiratoire porcin chez les porcs en engraissement
Ioannis E. Georgoulakis, DVM, PhD; Evanthia Petridou, DVM, Dr Med Vet; Georgios Filiousis, DVM, Dr Med Vet; Constantinos Alexopoulos, DVM, Dr Med Vet, Diplomate ECAR; Spiros C. Kyriakis, DVM, Dr Med Vet, Diplomate ECAR; Ioannis Papatsas, DVM, Dr Med Vet
IEG: School of Agriculture, Animal Production and Hydatic Environment, University of Thessaly, 382 21, Volos, Greece. EP: Laboratory of Microbiology and Infectious Diseases, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki, 541 24, Macedonia, Greece. GF: 3rd Greek Army Veterinary Hospital, Thessaloniki, Macedonia, Greece. CA: Clinic of Obstetrics and Artificial Insemination, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki, 541 24, Macedonia, Greece. SCK: Clinic of Productive Animal Medicine, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki, 541 24, Macedonia, Greece. IP: Boehringer Ingelheim Ellas AE, 551 33, Thessaloniki, Macedonia, Greece. Corresponding author: Dr Ioannis Papatsas, POB 46, GR-57010, Asvestochori, Thessaloniki, Macedonia, Greece; Tel: +30 2310 424 618; Fax: +30 2310 424 663; E-mail: papatsas@ath.boehringer-ingelheim.com
Cite as: Georgoulakis IE, Petridou E, Filiousis G, et al. Meloxicam as adjunctive therapy in treatment and control of porcine respiratory disease complex in growing pigs. J Swine Health Prod. 2006;14(5):253-257.
Also available as a PDF.
SummaryObjective: To determine the efficacy of injectable meloxicam as adjunctive therapy to antimicrobial medication in treatment and control of porcine respiratory disease complex (PRDC) in growing pigs. Methods: A total of 162 ninety-day-old pigs showing early clinical signs of PRDC were weighed (Day 0) and allocated into two treatment groups, Controls (n = 82) and Meloxicam (n = 80), in a randomized block design (approximately 20 pigs per pen, four pens per treatment per replicate). In-feed chlortetracycline (800 g per tonne; 20 mg per kg body weight per day) was administered to both groups for 8 consecutive days (Days 0 to 7). On Day 0, Controls received a single injection of a placebo and the Meloxicam group received a single injection of meloxicam (0.4 mg per kg body weight). Respiratory signs were assessed per individual animal and per treatment group Days 0 to 7. Frequency of additional injectable medications, mortality rate, and growth rate were assessed until the end of the growing phase (117 days of age; Day 27). The lungs of all dead animals were submitted for bacteriological culture and pathological examination. Results: Clinical scores, frequency of treatment with additional injectable medications, and mortality rate were lower and growth performance was better in meloxicam-treated animals than in Controls. Implications: Meloxicam as an adjunct to oral antibiotic therapy may contribute to treatment and control of PRDC by accelerating recovery from respiratory inflammation, enhancing restoration of normal growth rate, and reducing mortality rate. | ResumenObjetivos: Determinar la eficacia del meloxicam inyectable como terapia adjunta a la medicación antimicrobiana en el tratamiento y control del complejo respiratorio porcino (PRDC por sus siglas en inglés) en cerdos de crecimiento. Métodos: Se pesaron un total de 162 cerdos de noventa días de edad que mostraban los primeros signos clínicos de PRDC (Día 0) y se colocaron en dos grupos de tratamiento, Control (n = 82) y Meloxicam (n = 80), en un diseno de bloques al azar (aproximadamente 20 cerdos por corral, cuatro corrales por tratamiento por réplica). Se administró clorotetraciclina (800 g por tonelada; 20 mg por kg de peso corporal por día) a ambos grupos por 8 días consecutivos (Días 0 a 7). En el Día 0, los Controles recibieron una inyección única de placebo y el grupo de Meloxicam recibió una inyección única de meloxicam (0.4 mg por kg de peso corporal). Se valoraron los signos respiratorios por animal individual y por grupo de tratamiento los Días 0 a 7. Se valoró la frecuencia de medicamentos inyectables adicionales, índice de mortalidad, e índice de crecimiento hasta el final de la etapa de crecimiento (117 días de edad; Día 27). Los pulmones de todos los animales muertos se enviaron para cultivo bacteriológico y examen patológico. Resultados: Los puntajes clínicos, la frecuencia del tratamiento con medicamentos inyectables adicionales, y el índice de mortalidad fueron más bajos y el desempeno del crecimiento fue mejor en animales tratados con meloxicam que en los Controles. Implicaciones: El meloxicam como un adjunto a la terapia antibiótica oral puede contribuir al tratamiento y control del PRDC acelerando la recuperación de la inflamación respiratoria, al restaurar el índice de crecimiento normal, y reducir el índice de mortalidad. | ResuméObjectifs: Déterminer l‘efficacité du meloxicam injectable comme thérapie supplémentaire à l‘administration d‘antimicrobiens pour le traitement et le contrôle du complexe respiratoire porcin (PRDC) chez les porcs en engraissement. Méthodes: Un total de 162 porcs âgés de 90 jours et montrant des signes cliniques hâtifs de PRDC ont été pesés (Jour 0) et répartis en deux groupes de traitement, Témoin (n = 82) et Meloxicam (n = 80), selon un dispositif en blocs aléatoires (environ 20 porcs par parc, quatre parcs par traitement par réplication). De la chlortétracycline ajoutée à la nourriture (800 g par tonne; 20 mg par kg de poids corporel par jour) a été administrée au deux groupes pour 8 jours consécutifs (Jour 0 à 7). Au Jour 0, les animaux du groupe Témoin ont reçu une injection unique d‘un placebo et le groupe traité a reçu une dose unique de meloxicam (0.4 mg par kg de poids corporel). Les signes respiratoires ont été évalués pour chaque animal et pour chaque groupe de traitement du Jour 0 au Jour 7. La fréquence d‘injections additionnelles de médicaments, le taux de mortalité, et le taux de croissance ont été évalués jusqu‘à la fin de la période de croissance (117 jours d‘âge; Jour 27). Les poumons de tous les animaux morts ont été soumis pour culture bactérienne et examen pathologique. Résultats: Le pointage clinique, la fréquence de traitement avec des médicaments injectables additionnels, et les taux de mortalité étaient inférieure et les performances de croissance étaient meilleures chez les animaux traités avec le meloxicam que pour les animaux témoins. Implications: Le meloxicam utilisé comme thérapie supplémentaire à une thérapie antibiotique orale peut contribuer au traitement et au controle de PRDC en accélérant la guérison d‘une inflammation respiratoire, en améliorant le rétablissement d‘un taux de croissance normal, et en réduisant le taux de mortalité. |
Keywords: swine, meloxicam,
porcine respiratory disease complex
Search the AASV web site
for pages with similar keywords.
Received: April
11, 2005
Accepted: October
20, 2005
Porcine respiratory disease complex (PRDC) of growing-finishing pigs is a major economic threat to the modern pig industry worldwide. Numerous viral and bacterial agents may contribute to pathogenesis of PRDC either as primary or secondary etiologic agents, and etiology varies from farm to farm.1-4 Pathogens implicated include porcine reproductive and respiratory syndrome virus (PRRSV), pseudorabies virus (PRV), swine influenza virus (SIV), Mycoplasma hyopneumoniae, Pasteurella multocida, and Actinobacillus pleuropneumoniae (APP).1-4 Use of vaccination and medication strategies targeted against specific agents is a widely accepted method to minimize losses.4,5 Use of nonsteroidal anti-inflammatory drugs (NSAIDs) as adjunctive therapy in treatment of bovine respiratory disease has been reported in several studies in which administration of NSAIDs concurrently with appropriate antimicrobial therapy accelerated clinical improvement and moderated lung inflammation.6-8
Meloxicam, an NSAID of the oxicam class, exerts potent anti-inflammatory, analgesic, antitoxic, antipyretic, and anti-exudative activity by inhibiting modulators and mediators of the inflammatory process.9 Efficacy of meloxicam as adjunctive therapy in treatment of respiratory infections in cattle has been widely demonstrated.10-12 Meloxicam has recently been approved in many countries for treatment of the mastitis-metritis-agalactia (MMA) syndrome9 and locomotor disorders13 in pigs.
The objective of this study was to investigate the effect of meloxicam (Metacam 2%; Boehringer Ingelheim, Ingelheim, Germany) as adjunctive therapy to antimicrobials in treatment and control of PRDC in growing-finishing pigs.
Materials and methods
Study herd
The study was carried out in a 560-sow commercial farrow-to-finish swine farm located in the region of Central Macedonia (Topigs, commercial hybrid; Vught, The Netherlands). The quality of management was poor, especially in the grower and finisher, eg, animal density was high (2.5 pigs per m2 in the grower and 2.0 pigs per m2 in the finisher), ventilation was poor (approximately 1.1 m3 per kg per hour), and biosecurity measures were inconsistent (eg, use of disinfectants and sanitizers after barns were washed between groups of pigs).
Recurring PRDC episodes in this herd were associated with high mortality both in the growing phase (71 to 117 days of age; average mortality 6.2%) and finishing phase (118 to 170 days of age; average mortality 4.6%). Nursery pigs (28 to 70 days of age) were vaccinated at 30 days of age with a single-dose M hyopneumoniae vaccine (Ingelvac M hyo; Boehringer Ingelheim). Sows, boars, and gilts were routinely vaccinated with an inactivated PRRSV vaccine (Progressis; Merial, Lyon, France) and also with vaccines against PRV (Porcilis-Begonia; Intervet, Boxmeer, The Netherlands), porcine parvovirus (Nobi-Porvac-Parvo; Intervet), swine erysipelas (Nobi-Porvac-Ery; Intervet), and atrophic rhinitis (Nobi-vac AR-T; Intervet).
According to the recorded medical history, the herd was endemically infected with PRRSV, while P multocida, Streptococcus suis, and APP had been frequently isolated from cases of PRDC during the previous 2 years. Serological testing was performed 2 months before the trial began. A total of 40 blood samples were collected (10 samples per age group) from unvaccinated gilts, growing pigs 60 and 110 days of age, and finishing pigs 170 days of age. Unvaccinated gilts were housed separately from the finishing pigs, and 60-day-old and 110-day-old pigs and finishers were housed in separate rooms in the same building. For each of the tested age groups, random samples were collected by selecting pigs from different pens in various parts of the room.
All samples were tested for antibodies against PRV (gE blocking ELISA; Svanova, Uppsala, Sweden; OD < 45 considered positive); SIV (H1N1 ELISA; Idexx, Schipol-Rijk, The Netherlands; sample:positive ratio [S:P] > 0.4 considered positive); PRRSV (PRRS ELISA; Idexx; S:P > 0.4 considered positive); and porcine circovirus type-2 (PCV-2) (immunofluorescence assay; Bioscreen GmbH, Muenster, Germany; titration in sample dilutions from 1:20 to 1:1280, reciprocal titers positive). All samples were seronegative for PRV and SIV. Some animals in all tested groups were seropositive for PRRSV, including 100% of unvaccinated gilts, 30% of 60-day-old pigs, 90% of 110-day-old pigs, and 100% of 170-day-old pigs. Although no clinical signs indicative of PCV-2 infection had been observed, 90% of tested samples were seropositive.
Experimental design
In a double-blinded, randomized study, 162 growing pigs were selected (Day 0), with equal numbers of males and females, at the age of 90 ± 2 days, ie, the age of onset of clinical signs of PRDC in this herd. Selected animals were housed in eight pens (8.2 m2) in a room of the grower barn reserved for the study, and were ear-tagged and randomly allocated to two treatment groups, with 20 ± 2 pigs per pen and four pens per treatment per replicate. Average body weight in the treatment groups was similar (33.9 and 34.0 kg; P > .05) at the start of the trial. During the trial period, a standard grower ration (mash) based on corn and soybeans was fed ad libitum to all animals up to 117 days of age. Two nipple drinkers and one fully automated circular feeder were provided in each pen, with one feeder place per four animals. Both treatment groups received in-feed chlortetracycline (CTC; 800 g per tonne, 20 mg per kg body weight per day) for 8 consecutive days beginning Day 0. Selection of CTC for treatment was based on antimicrobial sensitivity testing of lung samples from recent animal groups. In addition, on Day 0, Controls (n = 82) received a single intramuscular (IM) injection of a placebo (isotonic saline) and the Meloxicam group (n = 80) received a single IM injection of meloxicam (Metacam; Boehringer Ingelheim; 0.4 mg per kg body weight).
This protocol adhered to the Greek National Presidential Decree 160/91 requirements for animal welfare treatment and was supervised by the Faculty of Veterinary Medicine of Aristotle University of Thessaloniki.
Clinical parameters
Clinical parameters associated with signs of acute respiratory infection were assessed per animal and per trial group. Average daily respiratory score (ADRS) for Days 0 to 7 (90 to 97 days of age) was scored on a scale of 0 (absence of clinical signs) to 3 (abdominal breathing and generally poor condition characterized by depression, reluctance to rise and move, inappetence, and apparent weight loss). Mean respiratory score (RS) was calculated for the 8-day period between Days 0 and 7. General health status (GHS) for Days 0 to 7 was scored on a four-point scale (0 = normal general condition and absence of clinical signs; 1 = apparent clinical signs, less active than normal pigs, no obvious growth variation; 2 = apparent clinical signs, obvious depression, and moderate growth variation; and 3 = apparent clinical signs, poor general condition, long haircoat, and obviously retarded growth). The frequency of additional injectable medications required was expressed as the percentage of animals per treatment group that received additional injectable antimicrobial medication between 91 days of age (Day 1) and 117 days of age (Day 27), ie, the end of the growing phase. Clinical index score (CIS) was the total of RS and GHS scores. Mortality rate per treatment group was calculated for Days 0 to 27.
Scoring for ADRS and GHS per individual animal was performed twice daily at a 4-hour interval, always by the same investigator, with each observation period lasting a maximum of 2 hours. Two daily values per parameter and per animal were recorded. The highest of the two recorded daily values for ADRS and GHS per animal were used in data analysis.
Growth performance data
All animals were individually weighed on Days 0 and 27. For each animal and treatment group, average daily gain (ADG) was calculated for the overall trial period (Day 0 to Day 27).
Bacteriology and pathology
Lungs of all dead animals were subjected to pathological and bacteriological examinations. Gross pathological examination was performed at the farm site. Whole lung samples were submitted 2 to 3 hours later to the Laboratory of Microbiology and Infectious Diseases of the Faculty of Veterinary Medicine (Thessaloniki) for histological examination and bacteriological testing.
Swabs from the cut surface of each lung sample were inoculated on 5% sheep blood agar (incubated at 37°C for 48 hours) for detection of Pasteurella spp and Streptococcus spp and on 5% sheep blood agar with a cross-streak of Staphylococcus epidermidis (incubated at 37°C for 24 hours) for detection of APP. Biochemical tests were used to identify P multocida and S suis, and P multocida isolates were typed using polymerase chain reaction.
Lung tissue sections (approximately 4 cm × 4 cm samples containing both healthy and affected tissue) were placed in formalin for histological examination.
Statistical analysis
Treatment-group means for average bodyweight at the start of the trial, ADRS, RS, GHS, CIS, and ADG were compared using a Student t test. Means for parameters expressed as frequencies, ie, additional injectable medications and mortality rate, were compared using Pearson‘s chi-square test. Significance levels were investigated for α = 0.05 and α = 0.01.
Results
Clinical parameters
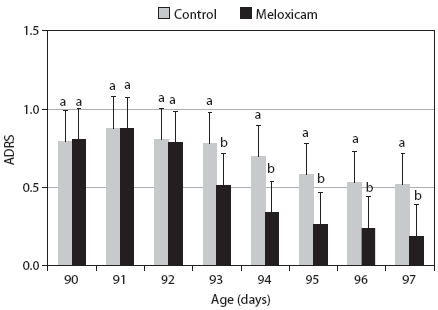
Average daily respiratory score in the Meloxicam group was significantly lower than in the Control group when pigs were 93, 94, 95, 96, and 97 days of age (Figure 1). Mean RS and CIS were significantly lower in the Meloxicam group than in the Control group (Table 1). Mean GHS was similar for both groups (Table 1). A higher proportion of animals in the Control group received additional injectable medications (Table 2). Mortality rate was lower in the Meloxicam group (Table 2).
Figure 1: Average daily respiratory score (ADRS) for two groups of grower pigs from 90 to 97 days of age (Days 0 to 7) was graded on a scale of 0 (absence of clinical signs) to 3 (abdominal breathing and generally poor condition characterized by depression, reluctance to rise and move, inappetence, and apparent weight loss). At 90 days of age, the Meloxicam group received a single injection of meloxicam (Metacam; Boehringer Ingelheim, Ingelheim, Germany; 0.4 mg/kg body weight) and Controls received a placebo injection. Both groups were treated in-feed with chlortetracycline (800 g/tonne of feed; 20 mg/kg body weight/day) on Days 0 through 7. Within a day, means with different superscripts differ significantly (Student t test; P < .01).
|
Table 1: Means (± SD) for general health status (GHS), respiratory score (RS), and clinical index score (CIS) for groups of growing pigs showing signs of porcine respiratory disease complex and treated with Meloxicam (n = 80) or untreated (n = 82)*
* Pigs 90 days of age (Day 0) were treated either with meloxicam (Metacam; Boehringer Ingelheim, Ingelheim, Germany; 0.4 mg/kg body weight) or with a placebo injection (Control). Both groups received in-feed chlortetracycline (800 g/tonne of feed; 20 mg/kg body weight/day) Days 0 through 7. † GHS scale: 0 = normal general condition and absence of clinical signs; 1 = apparent clinical signs, less active than normal pigs, no obvious growth variation; 2 = apparent clinical signs, obvious depression, and moderate growth variation; and 3 = apparent clinical signs, poor general condition, long haircoat, and obviously retarded growth. ‡ Mean RS = mean average daily respiratory score (ADRS) for Days 0 through 7, with ADRS graded on a scale of 0 (absence of clinical signs) to 3 (abdominal breathing and generally poor condition characterized by depression, reluctance to rise and move, inappetence, and apparent weight loss). § CIS = GHS + RS. ab Values within a row with different superscripts are different (Student t test; P < .05). |
|||||||||||||||||||
Table 2: Frequency of additional injectable medications (AIM) and mortality rate per treatment group for growing pigs showing signs of porcine respiratory disease complex and either treated with meloxicam or a placebo (Control) at 90 days of age*
* Pigs 90 days of age were treated either with meloxicam (Metacam; Boehringer Ingelheim, Ingelheim, Germany; 0.4 mg/kg body weight), or with a placebo injection (Control). Both groups received in-feed chlortetracycline (800 g/tonne of feed; 20 mg/kg body weight/day) from 90 to 97 days of age. † Number of treated animals/total number of animals in the group (%). ‡ Number of dead animals/total number of animals in the group (%). ab Values within a row with different superscripts differ significantly (Pearson‘s chi-square test; P < .05). |
|||||||||||||||
Growth performance
Average daily gain was higher in the Meloxicam group (0.667 kg) than in the Control group (0.635 kg) (P < .05).
Bacteriological results
The interval between death and necropsy was approximately 10 to 12 hours for most animals. Pasteurella multocida type A was isolated from four of the seven Control group lung samples and S suis was isolated from two of the seven Control group samples. Although pathological findings suggested APP infection in some animals, this organism was not isolated.
Pathological results
Most dead animals were discovered in the early morning hours, and time between death and culture of samples was at least 12 hours. In all animals that died, both macroscopic lesions (multifocal interstitial pneumonia, tracheobronchial lymphadenopathy, pleurisy with fibrinous adhesions, bronchopneumonia) and microscopic lesions (type-2 pneumonocyte and alveolar hyperplasia, exudative bronchopneumonia) were typical of complicated viral respiratory infection.
Discussion
The results of this study in growing pigs showing clinical signs of PRDC show that treatment with injectable meloxicam, in combination with appropriate antimicrobial medication, was associated with a lower prevalence of respiratory signs and consequent mortality, as well as better growth rate. The requirement for less additional injectable medication also suggests that meloxicam contributes to more rapid recovery from the respiratory inflammation triggered by viral and bacterial pathogens in PRDC infection.
The only known mechanism of meloxicam activity is inhibition of inflammatory enzymes produced by tissue inflammation.9 Recent research14 showed that treatment with meloxicam in pigs challenged with Escherichia coli endotoxins was associated with less severe clinical signs and lower levels of the inflammatory enzyme thromboxane-B2, which is greatly increased by respiratory-tract inflammation. Moreover, the role of other inflammatory enzymes, principally cyclooxygenase-2, that are expressed through the lung inflammation process and triggered by respiratory pathogens in pigs, has also been demonstrated.15-17 Further investigation of the possible inhibitory effect of meloxicam on cyclooxygenase-2 activity would enhance our knowledge concerning use of this agent as routine adjunctive therapy in respiratory infections in growing and finishing pigs.
Poor management on the study farm was an important issue. Implementation of measures to improve management and hygienic conditions on a long-term basis should be the primary goal, before antimicrobial and adjunctive therapy are prescribed for treatment and control of PRDC.
The long interval between death and dispatching of lung samples to the laboratory (ie, ≥ 12 hours) likely contributed to failure to recover APP from lung tissue in this study.
Although measurement of average daily feed intake and calculation of feed conversion ratio might have been valuable parameters in this study, the automatic feeding system, type of feeder, and available equipment in this facility did not allow collection of these data.
Variable results have been reported in a series of studies18-21 concerning efficacy of NSAIDs in treatment of respiratory infections. The present study focused on just one production phase. Further research evaluating additional growth parameters and use of meloxicam in recurring PRDC episodes, especially during the finishing phase, is needed. Different and possibly more convenient routes of administration of NSAIDs for treatment and control of PRDC, either in-feed or via drinking water, should be investigated.
Implications
- Prevalence of clinical signs of PRDC and requirement for additional injectable medications may be lower in growing pigs treated with injectable meloxicam and appropriate antimicrobial medication than in pigs treated with the same antimicrobial alone.
- Meloxicam used as adjunctive therapy in treatment and control of PRDC may minimize growth retardation and mortality in affected animals.
Acknowledgements
The authors wish to thank the owner and the technical staff of the trial farm who contributed in the process of this study, regardless of the high workload required. The assistance of Dr Gabriele Friton in the presentation of this article is also gratefully acknowledged. Dr Papatsas was employed by Boehringer Ingelheim Vetmedica GmbH while the study and analysis were being conducted.
References
1. Dee SA. The porcine respiratory disease complex: Are subpopulations important? Swine Health Prod. 1996;4:147-149.
*2. Halbur PG, Paul PS, Andrews JJ. Viral contributors to the porcine respiratory disease complex. Proc AASP. Kansas City, Missouri. 1993;343-350.
*3. Stevenson GW. Bacterial contributors to the porcine respiratory disease complex. Proc AASP. Kansas City, Missouri. 1993;351-365.
4. Christensen G, Sorensen V, Mousing J. Diseases of the respiratory system. In: Straw BE, D‘Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:913-940.
*5. Desrosiers R. Diagnosis and control of swine respiratory diseases. Proc AASP. Quebec, Canada. 1997;333-344.
6. Deleforge J, Thomas E, Davot JL, Boisrame B. A field evaluation of the efficacy of tolfenamic acid and oxytetracycline in the treatment of bovine respiratory disease. J Vet Pharmacol Therap. 1994;17:43-47.
*7. Clarke CR, Burrows GE, Ames TR. Therapy of bovine bacterial pneumonia. Vet Clin North Am: Food Anim Pract. 1991;7:669-679.
8. US Department of Agriculture. Animal and Plant Health Inspection Service. Treatment of respiratory disease in U.S. feedlots. Info Sheet. October 2001. Available at: http://www.aphis.usda.gov/vs/ceah/ncahs/nahms/feedlot/feedlot99/FD99treatresp.pdf. Accessed March 12, 2006.
9. Hirsch AC, Philipp H, Kleemann R. Investigation on the efficacy of meloxicam in sows with mastitis-metritis-agalactia syndrome. J Vet Pharmacol Therap. 2003;26:355-360.
10. Schmidt H, Philipp H, Salamon E, Okkinga K. The effects of additional treatment with Metacam® (meloxicam) on the course of acute respiratory disease in bovines. Der praktische Tierarzt. 2000;81:240-244.
11. Friton G, Cajal C, Romero Ramirez R, Kleeman R. Clinical efficacy of meloxicam (Metacam®) and flunixin (Finadyne®) as adjuncts to antibacterial treatment of respiratory disease in fattening cattle. Berliner und Munchener Tierartztliche Wochenschrift. 2004;117:304-309.
*12. Salamon E, Schmidt A, Henderson A, Okkinga K. Effects of meloxicam on thromboxane in calves with experimentally induced endotoxaemia. Proc World Buyatric Congress. 2000;8:37-38.
13. Friton G, Hagen P, Schneider T, Kleeman R. Investigation on the clinical efficacy and safety of meloxicam (Metacam®) in the treatment of non-infectious locomotor disorders in pigs. Berliner und Munchener Tierartztliche Wochenschrift. 2003;116:421-426.
*14. Schroedl W, Schmidt H, Duering F, Friton G. Effect of meloxicam (Metacam) in endotoxin challenged pigs. Proc IPVS Cong. Hamburg, Germany. 2004;606.
15. Cho WS, Chae C. In vitro effects of Actinobacillus pleuropneumoniae on inducible nitric oxide synthase and cyclooxygenase-2 in porcine alveolar macrophages. Am J Vet Res. 2003;64:1514-1518.
16. Cho WS, Chae C. Expression of cyclooxygenase-2 in swine naturally infected with Actinobacillus pleuropneumoniae. Vet Pathol. 2003;40:25-31.
17. Cho WS, Chae C. Immunohistochemical detection of cyclooxygenase-2 in lungs of pigs naturally infected with Actinobacillus pleuropneumoniae. J Comp Pathol. 2002;127:274-279.
18. Swinkels JM, Pijpers A, Vernooy JC, Van Nes A, Verheijden JH. Effects of ketoprofen and flunixin in pigs experimentally infected with Actinobacillus pleuropneumoniae. J Vet Pharmacol Therap. 1994;17:299-303.
19. Brown NC, Anderson TT, Anderson DL. Dexamethasone and indomethacin modify endotoxin-induced respiratory failure in pigs. J Appl Physiol. 1985;58:274-284.
*20. De Jong MF, Sampimon O, Arnaud JP, Theunissen G, Groenland G, Werf PJ. A clinical study with a non steroid anti inflammatory drug. Proc IPVS Cong. Bologna, Italy. 1996;659.
21. Laval A. Utilisation des anti-inflammatoires chez le porc. Recueil de Médecine Véterinaire. 1992;168:733-744.
*Non-refereed references.