Practice tip |
New blood collection technique for porcine reproductive and respiratory syndrome virus monitoring in boars
André Broes, DVM, PhD; Isabelle Caya, BSc; Maryse Bélanger, BSc, MSc
Biovet Inc, St-Hyacinthe, Québec, Canada. Corresponding author: Dr André Broes, Biovet Inc, 4375, avenue Beaudry, St-Hyacinthe, Quebec, Canada J2S 8W2; Tel: 450-771-7291; Fax: 450-771-7291; E-mail: andre.broes@biovet-inc.com.
Cite as: Broes A, Caya I, Bélanger M. New blood collection technique for porcine reproductive and respiratory syndrome virus monitoring in boars. J Swine Health Prod. 2007;15(1):42–44.
Also available as a PDF.
Porcine reproductive and respiratory syndrome virus (PRRSV) can be transmitted through infected semen. Consequently, early detection of PRRSV infection in boar studs is critical to prevent downstream contamination of sow herds.1,2 Boar-stud monitoring has been based on polymerase chain reaction (PCR) testing of semen for many years. However, it has been recently demonstrated that PCR testing of serum or blood samples is more sensitive in detecting early PRRSV infections than is testing of semen samples.3-7
Effective PRRSV monitoring of boar studs by PCR testing serum or blood requires repetitive blood sampling of animals.2,8 Jugular bleeding is the method of choice when large numbers of animals must be sampled.9 Sampling from the coccygeal vessels is safe and relatively easy to perform without restraint during semen collection. This method may be difficult in boars with docked tails, but may be mastered (A. Broes, personal observations, 2006). Lateral saphenous veins may be used in animals with a thin hairless skin and prominent veins. However these veins are not always accessible during semen collection (A. Broes, personal observations, 2006).
Recently, Reicks has proposed using swabs to collect blood from the ear vein for PCR testing.10 This method can easily be performed in unrestrained boars during semen collection. Blood swabs have been successfully used for PCR testing,4 but PCR using blood swabs is less sensitive than PCR using serum. Approximately 10 times fewer PRRSV genome copies are contained in a blood swab diluted in 0.75 mL of saline than are in 180 μL of serum (M. Bélanger, unpublished data). According to Reicks, using 0.5 mL of diluent would cause approximately a seven- to 11-fold dilution effect,8,10 which might restrict the possibility of pooling samples to reduce the cost of testing.10,11
Also of importance is the fact that the PRRSV genome may not be detected if significant genetic differences exist between the PRRSV isolate and the primers used in the PCR assay.12 In such cases, serological testing represents an interesting complement to detect infection. Samples other than serum have been successfully used for PRRSV serological testing. As an example, filter paper blotted with blood has been used to detect PRRSV antibodies by ELISA.13,14 However, the feasibility of using blood swabs for serological testing has not been evaluated so far.
A blood collection method applicable to unrestrained boars and able to obtain a significant volume of serum would be valuable. Recently, Yoon et al15 have suggested use of the Safe-T-Fill capillary blood collection system (RAM Scientific, Yonkers, New York). However, a maximum volume of only 300 μL of blood is collected by this system, and animals must be immobile in order to prevent air-bubble formation in the capillary tube. Dewey and Carman16 have proposed use of microtubes (Microvettes; Sarstedt Inc, Montreal, Quebec, Canada) by letting the blood flow into the capillary tube. The maximum blood volume per tube is 500 μL, and several tubes may be collected per animal.
Another capillary blood collection system offers several advantages (Bio-Tubes; Biovet Inc, St-Hyacinthe, Quebec, Canada). Again, blood is allowed to flow into the capillary tube. Up to 600 μL of blood per tube may be collected with this method. Several tubes may be collected if blood flow is sufficient. Puncturing the vein with a needle usually results in insufficient blood flow. A small scalpel blade may be used. On the other hand, the capillary tube may be inserted into an 18-gauge needle that is gently introduced into the vein. In this case, 1.0 to 1.5 mL of blood may be obtained per tube, and several tubes may be collected.
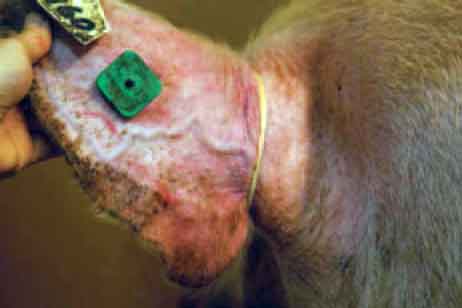
All of these collection methods may be conducted during semen collection without restraint of the boar. In all cases, it is suggested that a rubber tourniquet be placed at the base of the ear and that the ear skin be scrubbed with alcohol (ethanol or xylol) to cause congestion of the ear vein (Figure 1). Note that some boars have such small ear veins that it is virtually unfeasible to collect blood from them.
| Figure 1: A rubber band has been placed at the
base of the boar’s ear and the skin has been scrubbed with alcohol
in preparation for collection of blood from the ear vein.
|
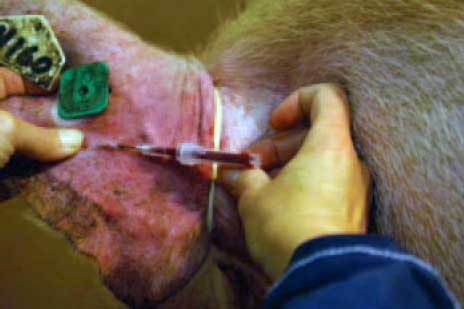
Once blood collection is completed (Figure 2), the capillary tube is removed and the tube is closed using a screw cap (Figure 3). At the laboratory, the tubes are centrifuged at 6000g for 2 minutes. After centrifugation, each tube may provide 250 to 450 μL of serum, depending on the way the blood was collected.
| Figure 2: An 18-gauge, 1” needle has been
fixed onto the capillary tube and then inserted into the ear vein of a
boar mounted on a dummy for semen collection. The capillary tube may be
filled within a few seconds.
|
| Figure 3: After collecting blood from the ear vein
of a boar using the Bio-Tube system (Biovet Inc, St-Hyacinthe, Quebec,
Canada), the capillary tube is removed and the tube is closed with a screw
cap.
|
Idexx ELISA testing (Indexx 2X-R PRRS ELISA: Idexx Laboratories, Westbrook, Maine) requires a minimum of 25 μL of serum (including the quantity necessary for one retest). The quantity of serum per PCR test may vary by laboratory. Biovet’s laboratory requires 360 μL of serum per PCR test (including the quantity necessary for one retest). Thus, one tube filled using a needle may provide enough serum for both ELISA and PCR testing.
Bio-Tubes represent a convenient tool for collection of blood from unrestrained boars during semen collection to provide enough serum for both PRRSV serological and PCR testing.
Acknowledgments
The authors would like to thank Guy-Pierre Martineau (École Nationale Vétérinaire, Toulouse, France), Robert Desrosiers (Boehringer Ingelheim Vetmedica, Burlington, Ontario, Canada) and Julie Ménard (F. Ménard, L’Ange Gardién, Quebec, Canada) for their comments and suggestions on the paper, for providing pictures, or both.
References
1. Christopher-Hennings J. Monitoring for porcine reproductive and respiratory syndrome virus (PRRSV) in the boar stud. J Swine Health Prod. 2001;9:186–188.
*2. Torremorell M, Thompson B. Monitoring boar studs. Proc AASV. Orlando, Florida. 2003;259–260.
3. Reicks DL, Muñoz-Zanzi C, Mengeling W, Christopher-Hennings J, Lager K, Polson D, Dee S, Rossow K. Detection of porcine reproductive and respiratory syndrome virus in semen and serum of boars during the first six days after inoculation. J Swine Health Prod. 2006;14:35–41.
4. Reicks DL, Muñoz-Zanzi C, Rossow K. Sampling of adult boars during early infection with porcine reproductive and respiratory syndrome virus for testing by polymerase chain reaction using a new blood collection technique (blood-swab method). J Swine Health Prod. 2006;14:258–264.
*5. Rovira A, Muñoz-Zanzi C. Evaluation of PRRSV monitoring protocol for negative boar studs. Proc IPVS. Denmark. 2006;70.
6. Wasilk A, Callahan JD, Christopher-Hennings J, Gay TA, Fang Y, Dammen M, Reos ME, Torremorell M, Polson D, Mellencamp M, Nelson E, Nelson WM. Detection of U.S., Lelystad, and European-like porcine reproductive and respiratory syndrome viruses and relative quantitation in boar semen and serum samples by real-time PCR. J Clin Microbiol. 2004;42:4453–4461.
*7. Rovira A, Muñoz-Zanzi C. A quantitative approach to sampling studs. Proc Allen D. Leman Swine Conf. St Paul, Minnesota. 2005;52–53.
*8. Reicks DL. Boar stud PRRS monitoring – science and economics. Proc ISU Swine Dis Conf Swine Pract. Ames, Iowa. 2005;141–145.
9. Muirhead M. Blood sampling in pigs. In Pract. 1981;3:16–20.
*10. Reicks DL. An overview of blood collection strategies for boar studs. Proc Allen D. Leman Conf. St Paul, Minnesota. 2005;54–55.
*11. Rovira A, Muñoz-Zanzi C. Evaluation of the potential use of pooling in PRRSV monitoring protocols for boar studs. Proc IPVS. Denmark 2006;19.
12. Christopher-Hennings J, Faaberg KS, Murtaugh MP, Nelson ER, Roof MB, Vaughn EM, Yoon KJ, Zimmerman J. Porcine reproductive and respiratory syndrome (PRRS) diagnostics: Interpretation and limitations. J Swine Health Prod. 2002;10:213–218.
13. Hutet E, Chevallier S, Eloit M, Touratier A, Blanquefort P, Albina E. Porcine reproductive and respiratory syndrome antibody detection on filter discs. Rev sci tech Off Int Epi. 2003;22:1077–1085.
14. Spagnuolo-Weaver M, Walker IW, McNeilly F, Calvert V, Graham D, Burns K, Adair BM, Allan GM. The reverse transcription polymerase chain reaction for the diagnosis of porcine reproductive and respiratory syndrome: comparison with virus isolation and serology. Vet Microbiol. 1998;62:207–215.
*15. Yoon K-J, Patterson A, Karriker L. Alternatives to serum sampling for PRRS detection. Proc ISU Swine Dis Conf Swine Pract. Ames, Iowa. 2005;139–140.
*16. Dewey CE, Carman S. Practice tip: Blood sampling boars. J Swine Health Prod. 2006;14:267–268.
* Non-refereed references.