| Original research | Peer reviewed |
Cite as: Ravishankar C, Priya PM, Mini M, et al. First confirmed occurrence of classical swine fever in Kerala state, India. J Swine Health Prod. 2007;15(3):156–159.
Also available as a PDF.
SummaryThis case study describes the first detection of classical swine fever (CSF) in Kerala state, India. Two cases of acute mortality in small-scale pig farming units were investigated. In both cases, affected pigs had been maintained on uncooked waste and were not vaccinated against CSF. Anorexia, hyperaemia of the ears, pyrexia, and convulsions were the primary clinical signs observed in both cases. Clinical signs exhibited, necropsy lesions, and histopathological findings suggested a diagnosis of CSF. Diagnosis was confirmed by detection of CSF viral nucleic acid by reverse transcription-polymerase chain reaction in samples collected from both dead pigs. In Kerala, hereafter, differential diagnosis of acute mortality in pigs should include CSF. Suitable screening and control measures are to be implemented to prevent further outbreaks of the disease in this state. | ResumenEste estudio describe la primera detección de fiebre porcina clásica (CSF por sus siglas en inglés) en el estado de Kerala, India. Se investigaron dos casos de mortalidad aguda en granjas de cerdos de producción de pequeña escala. En ambos casos, los cerdos afectados se alimentaron con desecho no cocido y no se habían vacunado contra la CSF. La anorexia, hiperemia de las orejas, pirexia, y convulsiones fueron los signos clínicos principales observados en ambos casos. Los signos clínicos exhibidos, las lesiones a la necropsia y los hallazgos histopatológicos sugirieron un diagnóstico de CSF. Este diagnóstico fue confirmado con la detección del ácido nucleico viral de CSF a través de la prueba de trascriptaza reversa de la reacción en cadena de la polimerasa en muestras recolectadas de ambos cerdos muertos. De ahora en adelante, en Kerala, el diagnóstico diferencial de mortalidad aguda en cerdos debe incluir la CSF. Así mismo, deben implementarse medidas de control y monitoreo apropiadas para prevenir otros brotes de esta enfermedad en este estado. | ResuméCette étude décrit la détection des premiers cas de peste porcine classique (CSF) dans l’état de Kerala en Inde. Deux cas de mortalités aiguës dans une exploitation porcine de petite envergure ont été investigués. Dans les deux cas, les porcs atteints avaient été nourris avec des déchets de table non-cuits et n’étaient pas vaccinés contre la CSF. Les premiers signes cliniques observés dans les deux cas étaient de l’anorexie, de l’hyperémie des oreilles, de la pyrexie, et des convulsions. Les signes cliniques démontrés, les lésions macroscopiques, et les trouvailles à l’examen histopathologique étaient suggestifs d’un diagnostic de CSF. Ce diagnostic a été confirmé par la détection d’acide nucléique du virus de la CSF dans des échantillons prélevés sur les deux porcs morts par réaction d’amplification en chaîne par la polymérase avec la transcriptase réverse. À partir de maintenant, dans l’état de Kerala, la CSF est incluse dans le diagnostic différentiel lors de mortalité aiguë. Des mesures appropriées de contrôle et de criblage sont à mettre en place afin de prévenir de nouvelles épidémies de cette maladie dans cet état de l’Inde. |
Keywords: swine, classical
swine fever, mortality, reverse transcription-polymerase chain reaction,
India
Search the AASV web site
for pages with similar keywords.
Received: September
1, 2006
Accepted: January
5, 2007
Classical swine fever (CSF) is a highly contagious, potentially fatal disease of pigs and is classed as a List A disease by the Office International des Epizooties.1 The disease is currently endemic in most countries of continental Western Europe, South America, and the Far East.2 It is caused by CSF virus, a member of the genus Pestivirus, family Flaviviridae,3 and is closely related antigenically and structurally to bovine viral diarrhea virus and border disease virus.4 In India, outbreaks of the disease have been reported from the states of Uttar Pradesh,5 Maharashtra,6 Tamil Nadu,7,8 and Punjab,9 and the North East Indian states of Arunachal Pradesh, Manipur, Mizoram, Nagaland, and West Bengal.10 This study deals with the first occurrence of CSF in Kerala, a small state in South India.
Case descriptions
The two cases included in this study occurred in small-scale holdings of pigs, one owned by an individual and the other by a private institution. Case A occurred in March 2006 and Case B in May 2006. No animals in either site had been vaccinated for CSF.
Case A
Contagious disease, characterized by mortality, spread during a 3-week period in a group of pigs in a private farm in Muttil in Wayanad district of north Kerala. The unit consisted of 23 four-month-old pigs purchased in two batches from various locations within the state, with the second batch purchased 3 weeks after the first. The disease commenced 1 or 2 days after introduction of the second batch of pigs into the farm, and gradually spread in the herd, causing deaths of 20 pigs within 3 weeks.
Feed and housing. The pigs were housed in four adjacent pens with concrete floors, separated by walls approximately 1.2 m high, and fully roofed with asbestos sheets. No ventilation was provided other than the natural air flow through the pens. Plastic pipes conveyed waste from the pens to a biogas plant. The animals were maintained primarily on uncooked restaurant-bakery-poultry waste: no concentrate ration was fed. As part of the biosecurity measures, outside personnel were not allowed to enter the farm, vehicles used for transport and purchase of pigs were cleaned and disinfected, and the floors of the pens were cleaned twice a week. All pigs were vaccinated for foot-and-mouth disease. Production and mortality data for the 6 years since the farm had been established were not available.
Clinical signs. In the initial phase of the illness, the
condition was treated symptomatically with antibiotics (eg,
enrofloxacin and gentamicin) and multivitamin injections, but
mortality continued. Two pigs showing anorexia, hyperemia of the
ears, pyrexia, convulsions, and posterior paresis were submitted to
the Department of Veterinary Microbiology, College of Veterinary
and Animal Sciences, Pookot, Wayanad, for detailed investigation.
Of the two animals, one died on arrival and necropsy was conducted
in the Department of
Veterinary Pathology. The temperature of the other pig was
subnormal (38.5°C). It was maintained in the Department of
Veterinary Pathology on its regular feed (bakery waste), without
specific drug therapy, to study the course of the disease.
Approximately 4 days after it was admitted to the facility, the pig
showed improvement, though posterior incoordination persisted. Feed
and water intake became normal by approximately 7 days after
admission, and the animal was subsequently slaughtered.
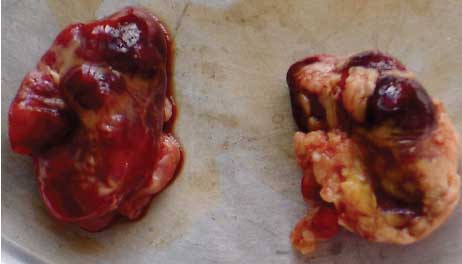
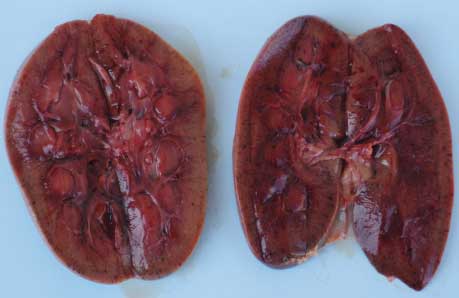
Necropsy findings. On necropsy, enlargement, focal congestion, hemorrhages in the thymus and retropharyngeal and mesenteric lymph nodes (Figure 1), and necrosis of the liver were observed. The spleen was slightly enlarged, with focal hemorrhagic areas on the surface. Reduction in white pulp was also noticed. Both kidneys were moderately enlarged with focal dark red areas visible through the capsule. When the capsule was removed from each kidney, these were revealed as hemorrhagic areas 0.1 to 0.4 cm in diameter. Sectioning revealed hemorrhagic streaks in the cortex (Figure 2). Hemorrhagic enteritis was also observed.
| Figure 1: Hemorrhages in lymph nodes
from a 4-month-old pig with classical swine fever.
|
| Figure 2: Hemorrhagic streaks in the cortices of
both kidneys of a 4-month-old pig with classical swine fever.
|
Histopathology. Histopathological examination of lymph nodes revealed congestion and depletion of lymphocytes in the paracortex, with moderate congestion in the medulla. Splenic white pulp was depleted of lymphocytes. Capillaries were moderately to severely engorged with marked sinusoidal dilation. Kidney sinuses were enlarged, and hemorrhages were evident in Bowman’s space and the proximal convoluted tubule. There was severe degeneration and necrosis of the collecting duct epithelium. In the liver, vacuolar degeneration of the hepatocytes was the predominant pathological change. The hepatic sinusoids were engorged with erythrocytes, and numerous cystic spaces were evident in the parenchyma.
Samples of lymph nodes, thymus, spleen, and pancreas were submitted to the Division of Pathology, Indian Veterinary Research Institute (IVRI), Izatnagar, Uttar Pradesh, for confirmatory diagnosis of CSF by reverse transcription-polymerase chain reaction (RT-PCR).
Diagnosis. On the basis of the clinical signs observed, the tentative diagnosis was CSF, a disease hitherto unreported in the state, and the matter was reported to the Department of Animal Husbandry of the state. The disease was confirmed as CSF when representative tissue samples from the dead pigs tested positive for CSF virus nucleic acid by RT-PCR.
Outcome. When the diagnosis of CSF was confirmed, the owner was required by the Department of Animal Husbandry to depopulate and disinfect his farm. A retrospective investigation on the outbreak in Muttil revealed that the second batch of pigs, believed to have introduced infection into the herd, came from a single source in the central part of Kerala. The exact location of this source could not be established conclusively.
There have been no fresh outbreaks of the disease in the Muttil farm since March 2006, and no outbreaks of the disease within a few miles of the case herd were reported. The owner of the affected farm suffered financial loss due to the outbreak; however, there was no apparent affect on the pig industry in the area.
Case B
The second outbreak of CSF occurred in two pigs reared at Holy Cross Convent in Mananthavady in the Wayanad district. The 4-month-old pigs had been purchased from nearby areas approximately 2 months before the outbreak. Whether the pigs came from the same source as in Case A is not known. Housing was similar to that described for Case A, except that the drain carrying waste from the pens was covered by concrete slabs. The pigs were not reared for commercial purposes and were maintained entirely on kitchen waste. No vaccines had been given.
Clinical signs. Anorexia, pyrexia, convulsions, and purplish discoloration of the ventral abdomen and ears were observed in one of the pigs. The animal succumbed to the illness in 48 hours. The other pig had died 4 days earlier, showing similar signs.
Necropsy findings. Necropsy findings included serosanguinous exudate in the thorax and pericardial sac, petechiae on the apex of the heart, congestion of the lungs, necrosis of the liver, and congestion of the spleen. Pus was observed in the renal pelvis and urinary bladder mucosa. Catarrhal enteritis and congestion of the mesentery were evident.
Laboratory examination. Histopathological examination of organs was not performed. Samples collected at necropsy were tested for CSF virus nucleic acid by RT-PCR at IVRI, Izatnagar, as in Case A.
Diagnosis. The tentative diagnosis of CSF was confirmed by RT-PCR, and the result was communicated to the Department of Animal Husbandry.
Discussion
Classical swine fever may occur in peracute, acute, subacute, and chronic forms, with the acute form occurring most commonly.2 In the acute form, high fever, depression, anorexia, and conjunctivitis appear 2 to 4 days post exposure, followed by vomiting, bacterial pneumonia, paresis, paralysis, tremor, and convulsions. In light-skinned pigs, hyperemia and purpura of the abdomen and ears may be observed. Nearly all pigs in a unit become affected within approximately 10 days, and mortality may reach 100%.3 This pattern of disease was evident in Case A, with the majority of pigs dying within 3 weeks, and in Case B, in which both animals died within 4 days. The chronic form of the disease occurs in areas where CSF is endemic, with growth retardation, chronic diarrhea, and secondary bacterial pneumonia the common signs.11
The CSF virus gains entry into a herd either via an inapparently infected carrier or in garbage containing infected pork scraps.3 In both Cases A and B, it is improbable that feed containing infected pork was fed to the animals. Hence, it is most likely that the virus was introduced by one or more carrier animals. This is evident in Case A, as mortality began after the second batch of pigs was introduced. Excretions and secretions from a carrier pig might have been the source of infection for other animals. However, in Case B, nearly 2 months had elapsed from the time the pigs were purchased until the disease occurred. It is possible that these pigs had already been infected with the CSF virus when they were introduced into the farm, and that the disease manifested after a long incubation period.
In acute CSF, submucosal and subserosal petechial hemorrhages, subcapsular kidney hemorrhage, and engorged lymph node cortical sinuses may be observed. Lymph nodes are usually enlarged, marginal splenic infarcts may occur, and liver, bone marrow, and lungs may be congested.2 The virus exerts its adverse effects on cells of the reticuloendothelial system, causing severe lymphocyte deficiency and occlusion of blood vessels.2 The histopathological findings in this study, ie, lymphocyte deficiency and changes in the spleen, kidney, and liver, are attributable to vascular dysfunction.
Classical swine fever may be diagnosed by the fluorescent antibody technique, immunoperoxidase assay using monoclonal antibodies, antigen-capture enzyme-linked immunosorbant assay, and RT-PCR.4 Of these tests, the RT-PCR test is most commonly used by researchers because of its accuracy and sensitivity in detecting CSF virus nucleic acid.12-14
Classical swine fever must be differentiated from salmonellosis, which is usually accompanied by enteritis and dyspnea; erysipelas, which causes characteristic skin lesions; pasteurellosis, which is a predominantly respiratory disease; African swine fever; and other encephalitides.2
Measures taken for control of CSF in India include control of animal movement within the country; modified “stamping out” (ie, application of only some of the measures described for stamping out,15 for example, slaughter of sick animals without other measures); vaccination; and periodic screening of herds.10 Recommended prevention and control measures for classical swine fever include sanitary and medical prophylaxis.16 Sanitary prophylaxis includes effective disease reporting, good communication between veterinary personnel and swine owners, a strict import policy for pigs and processed pig products, quarantine, efficient sterilization or prohibition of swill feeding to pigs, routine serological surveillance of breeding sows and boars, and good pig identification and recording systems. Medical prophylaxis consists of using modified-live-virus vaccine to prevent losses in countries where classical swine fever is enzootic, although this will not entirely eliminate infection.16
In outbreaks of CSF, all pigs on affected farms must be slaughtered and the carcasses and bedding properly disposed of. This should be followed by thorough disinfection of the farm and control of pig movement. The source of infection must be identified and herds on farms near the infected premise should be tested serologically.
In Kerala, mortality in pigs in large numbers is usually attributed to noninfectious causes, eg, heat stroke, stress, or poisoning, or to infectious bacterial diseases such as salmonellosis. Hence, detection of CSF in swine in the state is critical to introduction of suitable prevention and control measures. However, control strategies for Kerala should be planned and activated only after the prevalence of CSF in the state has been fully investigated.
Implications
- Differential diagnosis of acute pig mortality in Kerala should include CSF.
- Animals dying of unidentified causes should be tested to rule out CSF.
- Farmers must be educated about the possibility of CSF infection in their pigs and methods of management and control for this disease.
- Periodic serological screening of all pig herds for CSF must be undertaken.
- Suitable control measures must be carefully designed to prevent further outbreaks.
Video available
A video clip showing neurological signs in a 4-month-old pig from Case A is available with the Web version of this article (available to journal subscribers at www.aasv.org).
Acknowledgements
The authors gratefully acknowledge the help rendered by Dr G. Sai Kumar, Senior Scientist, Division of Pathology, Indian Veterinary Research Institute, Izatnagar, in confirming the disease as CSF by RT-PCR. The authors also thank Dr P. P. Balakrishnan, Associate Dean, College of Veterinary and Animal Sciences, Pookot, for providing facilities required for conduct of this study.
References
1. Quinn PJ, Markey BK, Carter ME, Donnelly WJ, Leonard FC. Veterinary Microbiology and Microbial Diseases. Oxford, United Kingdom: Blackwell Science Limited; 2002:431.
2. Radostits OM, Gay CC, Blood DC, Hinchcliff KW. Veterinary Medicine. 9th ed. Philadelphia, Pennsylvania: Saunders; 2003:1019–1027.
3. Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ. Veterinary Virology. 3rd ed. London, UK: Academic Press; 1999:567–568.
4. Office International des Epizooties. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Part 2. Section 2.1. Chapter 2.1.13. Classical swine fever (hog cholera). Available at: http://www.oie.int/eng/normes/mmanual/A_00036.htm. Accessed 26 Feb 2007.
5. Krishnamurthy D, Adlakha SC. A preliminary report on the swine fever epidemic in Uttar Pradesh. Indian Vet J. 1962;39:406–419.
6. Sapre SN, Moghe RG, Bhagwat SV, Choudhary PG, Purohit BL. Observation on swine fever in Maharashtra. Indian Vet J. 1962;39:527–529.
7. Damodaran S, Ramakrishnan R, Rahmathullakhan. Swine fever in Madras. Indian Vet J. 1971;48:1202–1207.
8. Govindarajan R, Vengadabady N, Albert A, Purushothaman V. Detection of hog cholera in desi pigs. Cheiron. 2003;32:47–48.
9. Saini SS, Dhand NK, Sharma DR, Sood SK. An outbreak of swine fever in Punjab. Indian J Vet Pathol. 2000;24:135–136.
10. Office International des Epizooties. India. Classical swine fever. Multiannual Animal Disease Status. Available at: http://www.oie.int/hs2/sit_pays_mald_pl.asp?c_pays=88&c_mald=14. Accessed 26 Feb 2007.
11. Maclachlan NJ, Stott JL. Togaviridae and Flaviviridae. In: Hirch DC, Maclachlan NJ, Walker RL, eds. Veterinary Microbiology. 2nd ed. Ames, Iowa: Blackwell Publishing; 2004:359–360.
12. Cho HS, Lee HI, Park NY. Method of discrimination between vaccine type and wild type viruses of classical swine fever. Indian Vet J. 2006;83:847–849.
13. Singh VK, Sai Kumar G, Paliwal OP. Detection of classical swine fever virus in archival formalin-fixed tissues by reverse transcription polymerase chain reaction. Res Vet Sci. 2005;79:81–84.
14. Katz JB, Ridpath JF, Bolin SR. Presumptive diagnostic differentiation of hog cholera virus from bovine viral diarrhea and border disease viruses by using a cDNA nested-amplification approach. J Clin Microbiol. 1993;31:565–568.
15. Office International des Epizooties. Definitions. Available at http://www.oie.int/hs2/help.asp#def. Accessed 21 Feb 2007.
16. Office International des Epizooties. Classical swine fever (hog cholera). Available at: http://www.oie.int/eng/maladies/fiches/a_A130.htm. Accessed 24 Dec 2006.