Original research |
Peer reviewed |
Planned exposure to porcine circovirus type 2 by serum injection is not effective at preventing porcine circovirus associated disease
La exposición planeada contra circovirus porcino tipo 2 por inyección de suero no es efectiva para prevenir la enfermedad asociada al circovirus porcino
L’exposition planifiée au circovirus porcin de type 2 par injection de sérum n’est pas utile pour prévenir une maladie associée au circovirus porcin
Peter J. Thomas, DVM, MS; Tanja Opriessnig, Dr med vet, PhD; Nicole M. Juhan, PhD; Xiang-Jin Meng, MD, PhD; Patrick G. Halbur, DVM, PhD
PJT, TO, PGH: Department of Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, Ames, Iowa. NMJ, XJM: Department of Biomedical Sciences and Pathobiology, Center of Molecular Medicine and Infectious Diseases, College of Veterinary Medicine, Virginia Polytechnic Institute and State University, Blacksburg, Virginia. Corresponding author: Dr Tanja Opriessnig, Department of Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, Ames, IA 50011; Tel: 515-294-1950; Fax: 515-294-3564; E-mail: tanjaopr@iastate.edu.
Cite as: Thomas PJ, Opriessnig T, Juhan NM, et al. Planned exposure to porcine circovirus type 2 by serum injection is not effective at preventing porcine circovirus associated disease. J Swine Health Prod. 2007;15(6):330–338.
Also available as a PDF.
SummaryObjective: To evaluate the efficacy of injecting serum containing porcine circovirus type 2 (PCV2) or PCV2 antibodies in preventing porcine circovirus associated disease (PCVAD). Materials and methods: Seventy pigs were each randomly assigned to one of 10 groups (n = 7). Two groups per treatment were injected intraperitoneally (Day 0) with saline or with serum collected from pigs at the acute or convalescent stages of PCV2 infection, or from pigs with high levels of passively acquired antibodies. The remaining two groups were vaccinated with an experimental live chimeric vaccine containing PCV types 1 and 2 (PCV1-2). Half of the groups were challenged with PCV2 intranasally and intramuscularly at Day 16 and the remaining groups at Day 35. All pigs were necropsied 21 days post challenge. Results: No significant differences were detected among groups challenged at Day 16. However, among groups challenged at Day 35, less severe lymphoid depletion (PÂ =Â .04) and lower levels of virus in serum (P < .05) were observed in the group vaccinated with PCV1-2. One pig treated with saline and one treated with serum developed clinical signs and systemic lesions consistent with severe PCVAD. Implications: Under the conditions of this study, serotherapy does not prevent PCV2 infection or development of PCV2-associated lesions or disease in pigs challenged 16 or 35 days post treatment. Pigs treated with serum containing live PCV2 are at risk to develop PCVAD. The live chimeric PCV1-2 vaccine used in this study is effective in controlling PCV2 viremia and minimizing PCV2-associated lesions. | ResumenObjetivo: Evaluar la eficacia de la inyección de suero conteniendo circovirus porcino tipo 2 (PCV2 por sus siglas en inglés) o anticuerpos de PCV2 para prevenir enfermedad asociada con circovirus porcino (PCVAD por sus siglas en inglés). Materiales y métodos: Se asignaron al azar setenta cerdos a uno de 10 grupos (n = 7). Dos grupos por tratamiento se inyectaron intraperitonealmente (Día 0) con solución salina o con suero recolectado de cerdos en estado agudo o convalesciente de la infección por PCV2, o de cerdos con altos niveles de anticuerpos adquiridos pasivamente. Los dos grupos restantes fueron vacunados con una vacuna experimental quimérica viva conteniendo PCV tipos 1 y 2 (PCV1-2 por sus siglas en inglés). La mitad de los grupos fueron retados con PCV2 intranasalmente e intramuscularmente el Día 16 y los grupos restantes el Día 35. Veintiún días después del reto, se realizó la necropsia a todos los cerdos. Resultados: No se detectaron diferencias significativas entre los grupos retados el Día 16. Sin embargo, entre los grupos retados el Día 35, se observó una depleción linfática menos severa (P = .04) y niveles más bajos del virus en suero (P < .05) en el grupo vacunado con PCV1-2. Un cerdo tratado con solución salina y otro tratado con suero desarrollaron signos clínicos y lesiones sistémicas consistentes con PCVAD severa. Implicaciones: Bajo las condiciones de este estudio, la sueroterapia no previene la infección de PCV2 o el desarrollo de lesiones asociadas con el PCV2 o la enfermedad en cerdos retados los días 16 o 35 post tratamiento. Los cerdos tratados con suero conteniendo PCV2 vivo están en riesgo de desarrollar PCVAD. La vacuna quimérica viva de PCV1-2 utilizada en este estudio es eficaz para controlar la viremia por PCV2 y para minimizar las lesions asociadas con el PCV2. | ResuméObjectif: Évaluer l’efficacité d’injecter du sérum contenant du circovirus porcin type 2 (PCV2) ou des anticorps dirigés contre le PCV2 à prévenir la maladie associée au circovirus porcin (PCVAD). Matériels et méthodes: Soixante-dix porcs ont été répartis de manière aléatoire à l’un des 10 groupes (n = 7). Deux groupes par traitement ont été injectés par voie intra-péritonéale (Jour 0) avec de la saline ou du sérum prélevé de porcs au stade aigu ou convalescent d’une infection par PCV2, ou de porcs avec des taux élevés d’anticorps acquis passivement. Les deux autres groupes ont été vaccinés avec un vaccin chimérique vivant expérimental contenant les types 1 et 2 du PCV (PCV1-2). La moitié des groupes a été inoculée avec PCV2 par voies intra-nasale et intramusculaire au Jour 16 et les autres groupes au Jour 35. Tous les porcs ont été soumis à une nécropsie 21 jours post-exposition. Résultats: Aucune différence significative n’a été détectée parmi les groupes inoculés au Jour 16. Toutefois, parmi les groupes inoculés au Jour 35, une déplétion lymphoïde moins sévère (P = .04) et une quantité moindre de virus dans le sérum (P < .05) étaient observées dans les groupes des animaux vaccinés avec PCV1-2. Un porc traité avec de la saline et un traité avec le sérum ont développé des signes cliniques et des lésions systémiques compatibles avec une forme sévère du PCVAD. Implications: Dans les conditions expérimentales de la présente étude, la sérothérapie n’a pas empêché une infection par PCV2 ou le développement de lésions associées à PCV-2 ou de maladie chez des porcs inoculés 16 ou 35 jours post-traitement. Les porcs traités avec du sérum contenant du PCV2 vivant sont à risque à développer PCVAD. Le vaccin chimérique vivant PCV1-2 utilisé dans la présente étude est efficace pour contrôler une virémie associée à PCV2 et minimise les lésions associées à l’infection par PCV-2. |
Keywords: swine, serotherapy,
vaccine, porcine circovirus type 2, PCV2, porcine circovirus associated disease,
PCVAD
Search the AASV web site
for pages with similar keywords.
Received: February
21, 2007
Accepted: June
28, 2007
Porcine circovirus (PCV), a member of the Circoviridae family, is a small, nonenveloped, icosahedral DNA virus with a circular single-stranded genome.1,2 Porcine circovirus type 1 (PCV1) was first discovered in 1974 as a contaminant of a porcine kidney cell line (PK-15)3 and was later found to be nonpathogenic to pigs by experimental infection.4,5 Porcine circovirus type 2 (PCV2) emerged in the early 1990s as the cause of postweaning multisystemic wasting syndrome (PMWS).6 Clinical disease caused by PCV2 is now referred to as PCV associated disease (PCVAD).7 Porcine circovirus associated disease has since become a global problem.2,8
Severe systemic PCVAD is characterized by progressive weight loss or failure to gain weight, illthrift, chronic respiratory illness, and lymph node enlargement.2,6,9 These clinical signs are coupled with the hallmark microscopic lesion, lymphoid depletion, with granulomatous inflammation and histiocytic replacement of lymph node follicles associated with PCV2 antigen or DNA.10 Porcine circovirus type 2 is also considered an important contributor to the porcine respiratory disease complex, typically in combination with other swine respiratory pathogens, or less frequently as a single infection.11,12 Other less common manifestations of PCVAD include abortions, enteritis, and porcine dermatitis and nephropathy syndrome.
Experimental challenge with PCV2 alone rarely causes severe clinical disease.13-18 Research has shown that experimental co-infection with porcine reproductive and respiratory syndrome virus (PRRSV),11,13,18,19 porcine parvovirus,15,17,20-22 or Mycoplasma hyopneumoniae23 increases the severity of clinical disease and lesions associated with PCV2 infection. Additionally, it is likely that co-infection of PCV2 with other organisms plays a role in disease potentiation.
As PCVAD continues to emerge globally, effective measures are needed to protect pigs against PCV2 infection and PCVAD. Immunizing pigs against PCV2 may be attempted through natural or planned exposure to the virus or accomplished through the use of commercial vaccines. The recent licensure of efficacious PCV2 vaccines for control of PCVAD is encouraging.
Success in controlling PCVAD using serotherapy has been documented in European field trials conducted by practicing veterinarians.24-26 In these trials, serum was collected from 100-kg, healthy pigs located on the same farm. Results demonstrated a significantly lower mortality rate for serum-injected pigs compared to untreated pigs in the same herd. However, these reports are abstracts describing uncontrolled field observations. The objective of this study was to evaluate the efficacy of serotherapy in protecting against PCV2 infection and development of associated lesions and clinical signs under controlled laboratory conditions.
Serum for serotherapy was collected from pigs at the acute and convalescent stages of experimental PCV2 infection and from pigs with high levels of passively acquired antibodies. Other groups were either vaccinated with an experimental chimeric PCV1-2 live vaccine or administered sterile saline. Half of the groups receiving each treatment were challenged with wild-type PCV2 at 16 days post treatment and the other half were challenged at 35 days post treatment to evaluate the effect of treatment timing relative to virus exposure.
Materials and methods
Experimental animals and housing
Seventy 14-day-old pigs were purchased from a high-health herd free of PRRSV and M hyopneumoniae as determined by regular serological monitoring. Pigs were delivered to the Iowa State University Livestock Infectious Disease Isolation Facility where they were housed for the duration of the study. All pigs received 0.5 mL (25 mg) of ceftiofur (Excenel; Pfizer, New York, New York) intramuscularly (IM) daily for 3 consecutive days after arrival. Animal care and use protocols used in this study were approved by the Iowa State University Animal Care and Use Committee.
Experimental design
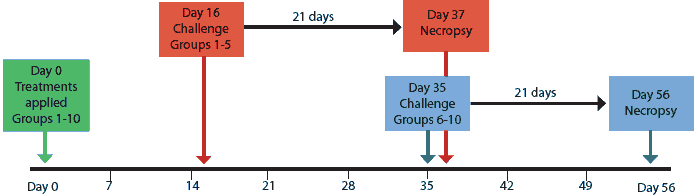
Blood samples were collected from all pigs upon arrival at 14 days of age, at 5 weeks of age (1 day before treatments were applied; Day -1), and then at weekly intervals for the duration of the study. Serum collected Day -1 was tested for PCV2-specific antibodies using a PCV2 open reading frame 2 (ORF2) capsid-protein-based enzyme-linked immunosorbent assay (ELISA) as previously described.27 All pigs were then blocked by sample-to-positive (S:P) ratio level, randomly assigned to treatment groups within a block, redistributed into 10 groups of seven, and administered their respective treatments (Day 0) as described in Table 1. Groups vaccine-16 and vaccine-35 were vaccinated with the PCV1-2 chimeric live vaccine as previously described.28,29 Study design is summarized in Figure 1.
Table 1: Treatments administered on Day 0 to groups of 5-week-old pigs (seven pigs per group) experimentally infected with porcine circovirus type 2 (PCV2) on Day 16 or Day 35 post treatment
* Pooled serum from early weaned piglets with high maternal antibody levels. † Pooled serum from pigs inoculated with PCV2 56 days previously.30 ‡ Pooled serum from pigs inoculated with PCV2 14 and 21 days previously.30 § Chimeric live vaccine.28,29 |
The maternal antibody and convalescent groups were placed in one room in separate pens that did not allow for direct contact. Each of the other treatment groups was placed in a separate room.
Pigs were weighed Day -13, Day 0, and weekly thereafter for the duration of the study. Rectal temperatures were recorded on alternate days. Pigs were monitored daily for signs of coughing, sneezing, lethargy, icterus, and wasting, beginning on Day 0 and continuing for the duration of the study. Respiratory disease scores were recorded on alternate days using a previously established scale ranging from 0 (normal) to 6 (severe) to monitor dyspnea and tachypnea.23 Evaluators were blinded to the treatment status of the pigs.
On Days 16 and 35, groups were challenged with PCV2 as shown in Figure 1. On each day of challenge, all inoculated pigs were moved into one room, and treatment groups were distributed randomly and evenly among six pens. All pigs were necropsied 21 days post challenge.
Figure 1: Experimental design for groups of pigs treated on Day 0 as described in Table 1, challenged with porcine circovirus type 2 on Day 16 or Day 35, and necropsied 21 days post challenge.
|
Serotherapy and vaccine treatment
Three serotherapy treatments were used in this study (Table 1). For the maternal antibody treatment (matAb), blood was collected at the farm of origin from five early weaned piglets (2 weeks of age) with high maternal antibody levels. Pooled serum was negative for PCV2 DNA at 40 cycles when tested by quantitative PCR and had an S:P ratio of 1.251 by PCV2 ORF2 ELISA. Serum for the “acute” treatments was collected from nine pigs 14 or 21 days post experimental PCV2 infection.30 The pooled serum contained a virus level of approximately 201,975 PCV2 DNA copies per mL and was negative by PCV2 ORF2 ELISA (S:P ratio, 0.188). Blood for the “convalescent” treatments was collected from four pigs 56 days post experimental PCV2 infection.30 The pooled serum contained approximately 2538 PCV2 DNA copies per mL and was positive by PCV2 ORF2 ELISA (S:P ratio, 1.032). Both the saline and PCV1-2 vaccine were negative for PCV2 ORF1 DNA and by PCV2 ORF2 ELISA. The real-time PCR assay16 used in the study targets the PCV2 ORF1 gene that is absent (replaced by the PCV1 ORF1) in the PCV1-2 chimeric vaccine.
Sera were stored at -80°C until used in serotherapy treatment. Sera were administered intraperitoneally (IP) at a dose of 8 mL per pig using a 24-gauge, 0.5-inch needle. Each pig in the control group and the vaccine group was administered 8 mL of saline buffer IP as a procedural control. Intraperitoneal injections were administered bilaterally, with 4 mL injected 1 cm on either side of the linea alba halfway between the 2nd and 3rd caudal-most nipples.
The vaccine used in the study was an experimental chimeric PCV1-2 live vaccine containing the immunogenic ORF2 capsid gene of PCV2 cloned into the genomic backbone of the nonpathogenic PCV1.28 This vaccine has been shown to induce protective immunity against PCV2 challenge in naive pigs.29 Pigs in the PCV1-2 vaccine group received 3 mL of vaccine IM in the right side of the neck. Pigs in the other groups received an IM injection of 3Â mL of saline buffer in the same location. All IM vaccinations and saline injections were administered on the same day as the IP serotherapy and saline injections.
All pigs received a 1.5-mL injection (75Â mg) of ceftiofur (Excenel; Pfizer, New York, New York) IM in the left side of the neck on the day of IP treatment in order to prevent bacterial infections.
Porcine circovirus type 2 inoculum
The challenge virus, PCV2 isolate 40895, was recovered in 1998 from a pig with PMWS in an Iowa herd. This isolate has since been cloned,14 and the virus stock generated from the infectious DNA clone was used as the challenge virus in this and several other studies.16,17,23,28-34 Inocula were prepared in PK-15 cells by direct transfection of the cells with the PCV2 DNA clone as previously described.14 The inoculum titer, determined by a previously described method,12,14 was 105.3 median tissue culture infective doses (TCID50) per mL. On each of the two challenge dates, the inoculum stock was thawed at room temperature and diluted to a titer of 104.5 TCID50 per mL using 45 mL of the stock virus and 155 mL of minimal essential medium. All pigs in each group received a total dose of 5 mL (1.58 × 105 TCID50) of the inoculum: 3 mL intranasally and an additional 2 mL IM in the right side of the neck.
Necropsy and histopathology
All animals were euthanized with an intravenous overdose of pentobarbital sodium and necropsied 21 days post challenge (Days 37 and 56). Lungs were scored for macroscopic lesion severity in a blinded fashion using a previously established scale of 0% to 100% of the lung exhibiting visible pneumonia.35 Enlargement of superficial inguinal, tracheobronchial, mediastinal, external iliac, and mesenteric lymph nodes was scored using a scale ranging from 0 to 3 (0 = normal size, 1 = twice normal size, 2 = three times normal size, and 3 = four times normal size).34
Thin sections of tissue were collected from each of the seven lung lobes, five lymph nodes (superficial inguinal, tracheobronchial, mediastinal, external iliac, and mesenteric), heart, tonsil, liver, kidney, spleen, thymus, ileum, and colon, and fixed in 10% neutral buffered formalin. Fixed tissues were processed by routine procedures and stained with hematoxylin and eosin. Slides were then evaluated and scored in a blinded manner by an experienced veterinary pathologist. Lung samples were scored using a range from 0 (normal lung) to 6 (severe diffuse lymphohistiocytic interstitial pneumonia).35 Liver, kidney, heart, and colon were examined for lesions and scored for severity of lymphohistiocytic inflammation from 0 (normal) to 3 (severe). Lymphoid tissues, including lymph nodes, tonsil, spleen, and Peyers patches, were evaluated for lymphoid depletion of follicles and given a score from 0 (normal) to 3 (severe lymphoid depletion). Lymphoid tissues were also scored for amount of histiocytic replacement of the lymphoid follicles from 0 (none) to 3 (severe).23
Immunohistochemistry
Detection of PCV2-specific antigen by immunohistochemistry (IHC) was performed using a rabbit polyclonal antiserum on sections of tissues embedded in paraffin blocks, as previously described.36 Lymphoid tissues were tested by IHC, including tonsil, spleen, thymus, and lymph nodes (superficial inguinal, tracheobronchial, mediastinal, external iliac, and mesenteric). The amounts of PCV2 antigen detected in the tissues were scored in a blinded fashion from 0 (no signal) to 3 (strong signal).23
Overall lymphoid lesion score
An overall microscopic lesion score (0 to 9) was calculated for each group by totaling the scores for lymphoid depletion, histiocytic replacement, and IHC for each tissue (tonsil, spleen, and five lymph nodes), and then dividing by seven (ie, the total number of tissues). This method has been used previously to determine and compare overall PCV2-associated lymphoid lesions.23
Serology
Blood samples were immediately processed to collect serum, which was aliquoted into individual 5-mL snap-top tubes and frozen at -80°C. Sera collected Day -1 (the day before challenge) and 21 days post challenge were tested for PCV2-specific antibodies using a PCV2-ORF2-based ELISA as previously described.27 Sample-to-positive ratios ≥ 0.2 were considered positive. Sera collected 21 days post challenge from three randomly selected pigs in each group were also tested for PRRSV-specific antibodies using a commercial PRRSV ELISA kit (HerdChek PRRS ELISA; Idexx Laboratories, Westbrook, Maine).
Quantitative PCR
Duration of PCV2 viremia and quantity of PCV2 DNA in the serum were determined by testing sera collected on the day of challenge and 7, 14, and 21 days post challenge by a quantitative real-time PCR specific for PCV2. Deoxyribonucleic acid was extracted using the QIAamp DNA Mini Kit according to manufacturer instructions (Quiagen, Valencia, California). The DNA extracts were subsequently tested by real-time PCR using a previously established protocol.16 This procedure measures PCV2 DNA genomic material within the sample, but does not distinguish between viable and nonviable virus.
Statistical analysis
Data was statistically analyzed using JMP 5.1 software (SAS, Cary, North Carolina). Analysis of variance (ANOVA) was performed on continuous data, including rectal temperature, average daily weight gain, PCR results, and serological test results. An initial residual analysis of the raw serum PCR data indicated heterogeneity of variances. This problem was corrected by log-transforming the raw data for statistical analysis. If the P value in an ANOVA was <Â .05, a Tukey-Kramer HSD test was used to determine which groups were significantly different. For nonparametric data (ie, respiratory scores, gross lesion scores, and histopathology scores), ANOVA was followed by pairwise Wilcoxon testing. Data were analyzed separately for groups inoculated on Days 16 and 35.
Results
Clinical disease
Clinical signs characterized by fever, mild dyspnea and tachypnea, sporadic sneezing, rough hair coats, and lethargy were observed in pigs in all groups after PCV2 inoculation. In the groups challenged at Day 35, fever was observed in several pigs per group on days 8 to 21 post challenge, with the exception of the vaccine-35 group. One pig in the acute-35 group demonstrated severe fever during the period between 7 and 21 days post challenge and had a rectal temperature of > 40.5°C for the final 10 days of the study. Another pig in the same group had severe respiratory disease (score of 6) 21 days post challenge. One pig in the saline-35 group gained only 1.13 kg during the period between 7 and 21 days post challenge, and the pig in the acute-35 group that exhibited protracted fever lost 0.18 kg during the period between 7 and 14 days post challenge. Although substantial clinical disease was observed in individual pigs, there were no significant differences (P < .05) in mean rectal temperature, respiratory disease, or average daily weight gain between groups.
Macroscopic lesions
One pig in the matAb-16 group, two pigs in the acute-16 group, and one pig in the conv-35 group had lung lesions characterized by mild-to-moderate, multifocal tan-red areas of lung consolidation (score range, 8% to 20% of the lung affected). No pigs in either the saline or the vaccine-treated groups had grossly visible lung lesions. Mean group macroscopic lung lesion scores did not differ at necropsy among groups challenged at Day 16 (P = .23) or those challenged at Day 35 (P = .41). Gross enlargement of the lymph nodes was observed in all groups (Table 2). Mean group lymph node scores did not differ at necropsy among groups challenged at Day 16 (P = .94) or those challenged at Day 35 (P = .07). Mean lymph-node enlargement scores ranged from 1.3 to 1.6 for all groups challenged with PCV2 at Day 16, but substantially more variation in mean lymph-node enlargement scores was observed among groups challenged at Day 35 (range 0.9 to 2.1; Table 2).
Table 2: Mean macroscopic and microscopic lymphoid lesion scores in pigs treated with serotherapy or vaccinated with a porcine circovirus types 1 and 2 (PCV1-2) vaccine (Day 0),* challenged with PCV2 Day 16 or Day 35, and necropsied 21 days post challenge
* Treatment groups described in Table 1. † Superficial inguinal, tracheobronchial, mediastinal, external iliac, and mesenteric lymph nodes (LNs) were examined. Lymph node scores: 0, normal size; 1, twice normal size; 2, three times normal size; 3, four times normal size. ‡ Lymphoid tissues (LNs, tonsil, spleen, and Peyers patches) were scored for lymphoid depletion of follicles and amount of histiocytic replacement of the lymphoid follicles. Scores for each evaluation: 0, none; 1, mild; 2, moderate; 3, severe. § PCV2 antigen detected by immunohistochemistry, scores: 0, none; 1, low; 2, moderate; 3, severe. ¶ Total of scores for microscopic lesions (lymphocytic depletion, histiocytic replacement, and PCV2 antigen): 0, none; 1-3, mild; 4-6, moderate; 7-9, severe. ab Values within a column with different superscripts differ (Wilcoxon test; P < .05). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Microscopic lesions and IHC
No significant differences were observed in lymphoid tissue histopathology or IHC scores among the groups challenged at Day 16 (Table 2). Among the groups challenged at Day 35, lymphoid depletion was significantly lower for the vaccine-35 group than the acute-35, conv-35, and matAb-35 groups (Table 2). Mean overall microscopic lymphoid lesion scores for the groups challenged at Day 35 were lowest in the vaccine-35 group, followed by saline-35, acute-35, matAb-35, and conv-35 groups (Table 2).
Mild multifocal bronchointerstitial pneumonia lesions were observed in all groups, characterized by mild peribronchiolar lymphoplasmacytic infiltrates and alveolar septal thickening with mixed mononuclear cells. Mild lymphohistiocytic inflammation was observed in the liver and kidney in pigs of all groups. Mild multifocal lymphohistiocytic myocarditis was present in one pig in the matAb-35 group and one in the conv-35 group. No significant differences were found between groups for liver, kidney, heart, and intestinal lesions.
Serology
Mean PCV2-ELISA S:P ratios for all groups were below the cutoff (S:P < 0.2) on Day 0 and on the day of challenge (Days 16 and 35). Among the groups challenged on Day 16, only the Vaccine-16 group had seroconverted at the time of necropsy, with a mean group S:P ratio of 0.24. All five groups challenged on Day 35 had seroconverted at the time of necropsy, with mean S:P ratios of 0.31, 0.43, 0.45, 0.52, and 0.61 for the saline-35, matAb-35, conv-35, acute-35, and vaccine-35 groups, respectively. Mean S:P ratios on Day 0, on the day of PCV2 challenge, or on the day of necropsy did not differ significantly among groups challenged on Day 35.
Quantitative PCR
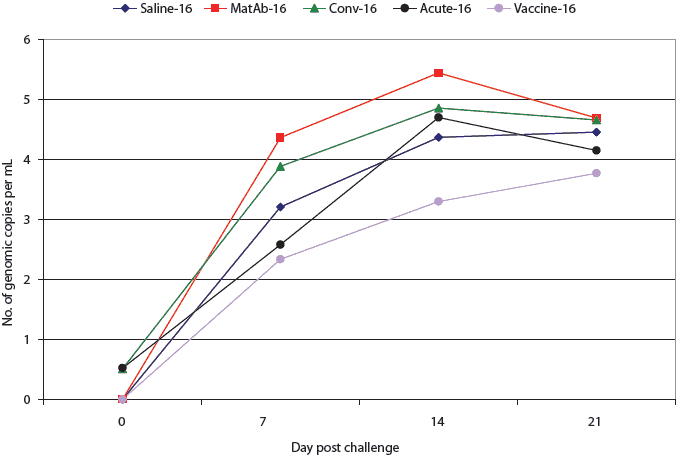
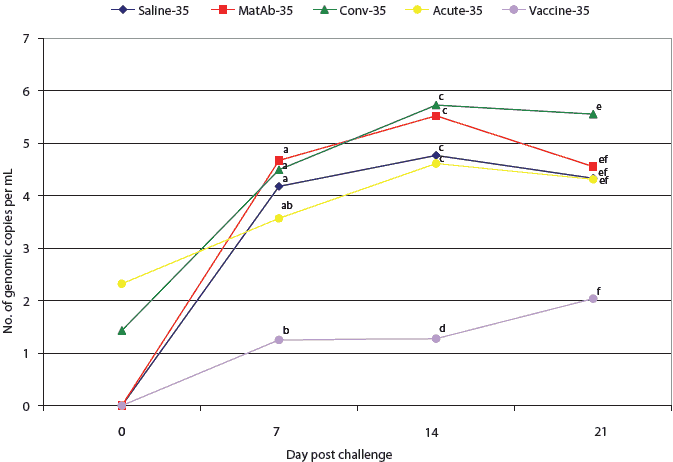
All pigs in the saline, maternal antibody, and vaccine groups were PCR-negative for PCV2 DNA at the time of challenge (Figures 2 and 3). One pig in the acute-16 group and one in the conv-16 group were PCR-positive at the time of challenge (Figure 2), as were two pigs in the conv-35 group and three pigs in the acute-35 group (Figure 3). Among groups challenged on Day 16, PCR results did not differ significantly from the time of challenge through necropsy (Figure 2). However, at 7,14, and 21 days post challenge, virus levels detected by PCR differed among the groups challenged at Day 35 (Figure 3). Four of the seven pigs in the vaccine-35 group remained PCV2-PCR negative for the duration of the study.
| Figure 2: Mean number of genomic copies (log transformed)
of porcine circovirus type 2 (PCV2) DNA per mL in serum of pigs treated
as described in Table 1 and challenged with PCV2 16 days later. There were
no significant differences among groups (P > .05; analysis of
variance).
|
| Figure 3: Mean number of genomic copies (log transformed)
of porcine circovirus type 2 (PCV2) DNA per mL of serum, determined by
quantitative real-time polymerase chain reaction, in pigs challenged with
PCV2 35 days after application of treatments described in Table 1. Mean
numbers of genomic copies of PCV2 per mL of serum were compared among groups
by analysis of variance followed by pairwise testing using Tukey’s
adjustment. Data points with no common superscript letter differ significantly:
ab, P < .01; cd, P < .001; ef, P = .03.
|
PCVAD diagnosis
Severe systemic PCVAD was diagnosed in two pigs. In the acute-35 group, one pig gained 4.8 kg between 0 and 7 days post challenge, but then gained only 1.1 kg between 7 and 21 days post challenge. This pig also had a persistent fever (40.2°C to 40.9°C) during this period. On gross examination, the lymph nodes of this pig were three times normal size. Microscopic lesions included severe lymphoid depletion and histiocytic replacement of follicles in lymph nodes associated with large amounts of PCV2 antigen. Similar lesions were observed in the tonsil, spleen, and Peyer’s patches, and lymphohistiocytic inflammation was observed in the liver and kidney. The other pig with severe systemic PCVAD was in the saline-35 group. This pig lost 0.18 kg of body weight between 7 and 14 days post challenge and had a persistent fever between 16 and 21 days post challenge, reaching a maximum of 40.5°C. Gross and microscopic lesions were similar in the two pigs.
Discussion
Porcine circovirus associated disease has become an important global problem.2,8 European field trials have provided some evidence that immunization using serotherapy is effective in controlling PCVAD.24-26 The apparent success of serotherapy in those trials might have been due to the protective effects of anti-PCV2 antibodies, immunization through exposure to PCV2 antigen remaining in the serum, or other factors.
In this study, we compared the efficacy of serum collected from pigs at either the acute or convalescent stage of PCV2 infection and serum from pigs with high levels of passively acquired antibodies to protect pigs against clinical disease, lesions, and viremia when challenged with PCV2. We compared these three serotherapy treatments to vaccination with an experimental chimeric PCV1-2 live vaccine that has been shown to induce protective immunity in pigs.29 Timing of treatment relative to virus exposure was tested by challenging the pigs with PCV2 either at Day 16 or 35 post treatment.
Passive transfer of high levels of PCV2-antibodies via serum administration might potentially protect against PCV2 infection and disease. Since the half-life of PCV2-specific antibodies is 19 days,37 we anticipated that pigs challenged at 16 days post treatment would be better protected than those challenged at 35 days post treatment. However, results of ORF2 ELISA testing showed that no pigs in either the matAb-16 or matAb-35 groups had seroconverted by the day of PCV2 challenge. After challenge, both groups became infected with PCV2 and developed macroscopic and microscopic lymph node lesions associated with PCV2 antigen. Thus, IP injection of serum containing high levels of passively-acquired anti-PCV2 antibodies failed to protect against PCV2 infection or PCV2-associated lesions. This may have been due to incomplete absorption of antibodies into the circulation and dilution of antibodies that were absorbed.
We hypothesized that immunization by administration of serum from pigs at the convalescent stage of PCV2 infection, which contained low levels of PCV2 (2538 genomic copies per mL), would provide adequate antigen to induce a protective immune response without causing clinical disease. Previous research shows that pigs challenged with PCV2 developed detectable levels of anti-PCV2 antibodies 21 to 42 days post inoculation.16,17,23 Therefore, pigs challenged at Day 35 should have had enough time to develop some level of protection. However, ELISA results showed that no pigs in the conv-16 group and only two pigs in the conv-35 group had an S:P ratio above the cut-off of 0.2 prior to challenge. Quantitative PCR results demonstrated that pigs in both the conv-16 and conv-35 groups were viremic prior to challenge, presumably as a result of serotherapy, with no difference in PCV2 load between these groups and the saline treatment control groups.
None of the six serotherapy treatment protocols utilized in this study were effective in reducing the amount of PCV2 present in serum, preventing the development of PCV2-associated lymphoid lesions, or inducing a measurable antibody response. Our results do not agree with the results reported in the field trials,24-26 nor do they provide evidence of a mechanism that might have produced the results seen in the field trials.
The experimental PCV1-2 chimeric live vaccine minimized PCV2 viremia and lymphoid depletion in pigs challenged with PCV2 on Day 35. In addition, mean PCV2-antibody ELISA S:P ratios were highest in the vaccine-16 and vaccine-35 groups at all time points after challenge. Unlike serotherapy, the chimeric PCV1-2 vaccine induced a protective immune response, preventing lesions in vaccinated pigs following challenge with wild-type PCV2.
Recently, killed PCV2 vaccines have been approved for use and are currently available commercially in Europe and North America. Although supplies of the commercial products are limited and extensive evaluation in the field is still in progress, preliminary indications are that the commercial vaccines are effective.38-40 Recent research has indicated that there are different genotype groups of PCV2 in North America.41 It has been suggested that the recent outbreaks of PCVAD in Canada may be attributable to a different and more virulent strain of PCV2.42 Commercial vaccines may not be effective at inducing sufficient immunity to new isolates or strains. The serotherapy techniques examined in this experiment were not effective and thus cannot be recommended.
Management strategies such as disinfection of facilities using Virkon-S (Antec International, Sudbury, Suffolk, United Kingdom);43 minimizing crowding; maximizing pig comfort; segregated early weaning; all-in, all-out flow; good hospital-pen management; and strict biosecurity protocols all may help to reduce PCV2 infection and PCVAD. Successful immunologically based methods are also needed to reduce the risk of PCV2 infection and development of PCVAD. Further research in the area of PCVAD prevention is warranted.
Implications
- Under the conditions of this study, serotherapy protocols are not effective at preventing PCV2 infection or development of PCV2-associated lesions or disease.
- Pigs treated with serum containing live PCV2 are at risk to develop PCVAD.
- The live chimeric PCV1-2 vaccine used in this study is effective in controlling PCV2 viremia and minimizing PCV2-associated lesions.
Acknowledgements
This study was funded by a grant from the Iowa Livestock Health Advisory Council. We thank the staff at the Iowa State University Livestock Infectious Disease Isolation Facility for animal care, Dr Igor Morozov at Fort Dodge Animal Health, Inc, for PCV2 inocula preparation, and Josh Bowden and Brian VanderLon for assistance with animal handling.
References
1. Tischer I, Gelderblom H, Vettermann W, Koch MA. A very small porcine virus with circular single-stranded DNA. Nature. 1982;295:64–66.
2. Allan GM, Ellis JA. Porcine circoviruses: a review. J Vet Diagn Invest. 2000;12:3–14.
3. Tischer I, Rasch R, Tochtermann G. Characterization of papovavirus-and picornavirus-like particles in permanent pig kidney cell lines. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene, 1. Abteilung, Originale A. 1974;226:153–167.
4. Tischer I, Mields W, Wolff D, Vagt M, Griem W. Studies on epidemiology and pathogenicity of porcine circovirus. Arch Virol. 1986;91:271–276.
5. Allan GM, McNeilly F, Cassidy JP, Reilly GA, Adair B, Ellis WA, McNulty MS. Pathogenesis of porcine circovirus; experimental infections of colostrum deprived piglets and examination of pig foetal material. Vet Microbiol. 1995;44:49–64.
6. Harding J, Clark E. Recognizing and diagnosing postweaning multisystemic wasting syndrome (PMWS). Swine Health Prod. 1997;5:201–203.
7. American Association of Swine Veterinarians. Porcine circovirus associated disease (PCVAD) case definition. Available at: http://www.aasp.org/aasv/position-PCVAD.htm. Accessed 4 Feb 2007.
8. Segalés J, Allan GM, Domingo M. Porcine circovirus diseases. Anim Health Res Rev. 2005;6:119–142.
9. Ellis J, Hassard L, Clark E, Harding J, Allan G, Willson P, Strokappe J, Martin K, McNeilly F, Meehan B, Todd D, Haines D. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can Vet J. 1998;39:44–51.
*10. Sorden SD. Update on porcine circovirus and postweaning multisystemic wasting syndrome (PMWS). Swine Health Prod. 2000;8:133–136.
11. Harms PA, Halbur PG, Sorden SD. Three cases of porcine respiratory disease complex associated with porcine circovirus type 2 infection. J Swine Health Prod. 2002;10:27–30.
12. Kim J, Chung HK, Chae C. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet J. 2003;166:251–256.
13. Allan GM, McNeilly F, Ellis J, Krakowka S, Meehan B, McNair I, Walker I, Kennedy S. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch Virol. 2000;145:2421–2429.
14. Fenaux M, Halbur PG, Haqshenas G, Royer R, Thomas P, Nawagitgul P, Gill M, Toth TE, Meng XJ. Cloned genomic DNA of type 2 porcine circovirus is infectious when injected directly into the liver and lymph nodes of pigs: characterization of clinical disease, virus distribution, and pathologic lesions. J Virol. 2002;76:541–551.
15. Krakowka S, Ellis JA, Meehan B, Kennedy S, McNeilly F, Allan G. Viral wasting syndrome of swine: experimental reproduction of postweaning multisystemic wasting syndrome in gnotobiotic swine by coinfection with porcine circovirus 2 and porcine parvovirus. Vet Pathol. 2000;37:254–263.
16. Opriessnig T, Yu S, Gallup JM, Evans RB, Fenaux M, Pallares F, Thacker EL, Brockus CW, Ackermann MR, Thomas P, Meng XJ, Halbur PG. Effect of vaccination with selective bacterins on conventional pigs infected with type 2 porcine circovirus. Vet Pathol. 2003;40:521–529.
17. Opriessnig T, Fenaux M, Yu S, Evans RB, Cavanaugh D, Gallup JM, Pallares FJ, Thacker EL, Lager KM, Meng XJ, Halbur PG. Effect of porcine parvovirus vaccination on the development of PMWS in segregated early weaned pigs coinfected with type 2 porcine circovirus and porcine parvovirus. Vet Microbiol. 2004;98:209–220.
18. Rovira A, Balasch M, Segalés J, Garcia L, Plana-Dúran J, Rosell C, Ellerbrok H, Mankertz A, Domingo M. Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2. J Virol. 2002;76:3232–3239.
19. Harms PA, Sorden SD, Halbur PG, Bolin SR, Lager KM, Morozov I, Paul PS. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet Pathol. 2001;38:528–539.
20. Allan GM, Kennedy S, McNeilly F, Foster JC, Ellis JA, Krakowka SJ, Meehan BM, Adair BM. Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J Comp Pathol. 1999;121:1–11.
21. Kennedy S, Moffett D, McNeilly F, Meehan B, Ellis J, Krakowka S, Allan GM. Reproduction of lesions of postweaning multisystemic wasting syndrome by infection of conventional pigs with porcine circovirus type 2 alone or in combination with porcine parvovirus. J Comp Pathol. 2000;122:9–24.
22. Kim J, Choi C, Han DU, Chae C. Simultaneous detection of porcine circovirus type 2 and porcine parvovirus in pigs with PMWS by multiplex PCR. Vet Rec. 2001;149:304–305.
23. Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng XJ, Halbur PG. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol. 2004;41:624–640.
*24. Ferreira D, Sansot B, Laval A. Attempt to use serotherapy to control mortality in PMWS. Proc Conf ssDNA Viruses, Plants, Birds, Pigs, and Primates. 2001;144.
*25. Waddilove AEJ, Marco E. Assessing serotherapeutic control of PMWS in the field. Proc IPVS. Ames, Iowa. 2002;17:204.
*26. Marco E. PMWS control – European style. Proc Swine Dis Conf Swine Pract. 2002;10:83–90.
27. Nawagitgul P, Harms PA, Morozov I, Thacker BJ, Sorden SD, Lekcharoensuk C, Paul PS. Modified indirect porcine circovirus (PCV) type 2-based and recombinant capsid protein (ORF2)-based enzyme-linked immunosorbent assays for detection of antibodies to PCV. Clin Diagn Lab Immunol. 2002;9:33–40.
28. Fenaux M, Opriessnig T, Halbur PG, Meng XJ. Immunogenicity and pathogenicity of chimeric infectious DNA clones of pathogenic porcine circovirus type 2 (PCV2) and nonpathogenic PCV1 in weanling pigs. J Virol. 2003;77:11232–11243.
29. Fenaux M, Opriessnig T, Halbur PG, Elvinger F, Meng XJ. A chimeric porcine circovirus (PCV) with the immunogenic capsid gene of the pathogenic PCV type 2 (PCV2) cloned into the genomic backbone of the nonpathogenic PCV1 induces protective immunity against PCV2 infection in pigs. J Virol. 2004;78:6297–6303.
30. Opriessnig T, McKeown NE, Harmon KL, Meng XJ, Halbur PG. Porcine circovirus type 2 infection decreases the efficacy of a modified live porcine reproductive and respiratory syndrome virus vaccine. Clin Vaccine Immunol. 2006;13:923–929.
31. Fenaux M, Opriessnig T, Halbur PG, Xu Y, Potts B, Meng XJ. Detection and in vitro and in vivo characterization of porcine circovirus DNA from a porcine-derived commercial pepsin product. J Gen Virol. 2004;85:3377–3382.
32. Fenaux M, Opriessnig T, Halbur PG, Elvinger F, Meng XJ. Two amino acid mutations in the capsid protein of type 2 porcine circovirus (PCV2) enhanced PCV2 replication in vitro and attenuated the virus in vivo. J Virol. 2004;78:13440–13446.
33. Opriessnig T, Halbur PG, Yu S, Thacker EL, Fenaux M, Meng XJ. Effects of the timing of the administration of Mycoplasma hyopneumoniae bacterin on the development of lesions associated with porcine circovirus type 2. Vet Rec. 2006;158:149–154.
34. Opriessnig T, Fenaux M, Thomas P, Hoogland MJ, Rothschild MF, Meng XJ, Halbur PG. Evidence of breed-dependent differences in susceptibility to porcine circovirus type-2-associated disease and lesions. Vet Pathol. 2006;43:281–293.
35. Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, Lum MA, Andrews JJ, Rathje JA. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32:648–660.
36. Sorden SD, Harms PA, Nawagitgul P, Cavanaugh D, Paul PS. Development of a polyclonal-antibody-based immunohistochemical method for the detection of type 2 porcine circovirus in formalin-fixed, paraffin-embedded tissue. J Vet Diagn Invest. 1999;11:528–530.
37. Opriessnig T, Yu S, Thacker EL, Halbur PG. Derivation of porcine circovirus type 2-negative pigs from positive breeding herds. J Swine Health Prod. 2004;12:186–191.
*38. Connor J, Elsener J. Field efficacy of Suvaxyn® PCV2 One Dose in pigs. Proc AASV. Orlando, Florida. 2007;38:151–152.
*39. De Grau A, Jorgensen J, Thacker B, Fransisco C, Wilson W, Schlueter R, Eggen A. Field performance of a conditionally licensed vaccine: Canadian experience. Proc AASV. Orlando, Florida. 2007;38:159–161.
*40. Desrosiers R, Clark E, Tremblay D, Tremblay D, Polson D. Preliminary results with Ingelvac® CircoFLEX™ to protect multiple ages of Quebec pigs against PCVAD. Proc AASV. Orlando, Florida. 2007;38:143–145.
41. Cheung AK, Lager KM, Kohutyuk OI, Vincent AL, Henry SC, Baker RB, Rowland RR, Dunham AG. Detection of two porcine circovirus type 2 genotypic groups in United States swine herds. Arch Virol. 2007;152:1035–1044.
*42. DeLay J, McEwen B, Carman S, van Dreumel T, Fairles J. Porcine circovirus type 2-associated disease is increasing. AHL Newsletter. 2005;9:22.
43. Royer RL, Nawagitgul P, Halbur PG, Paul PS. Susceptibility of porcine circovirus type 2 to commercial and laboratory disinfectants. J Swine Health Prod. 2001;9:281–284.
* Non-refereed references.