Original research |
Peer reviewed |
A comparison of the safety, cross-protection, and serologic response associated with two commercial oral Salmonella vaccines in swine
Comparación de la seguridad, protección cruzada, y respuesta serológica asociada a dos vacunas comerciales orales de Salmonella en cerdos
Comparaison de l’innocuité, de la protection croisée, et de la réponse sérologique associées à deux vaccins commerciaux oraux contre Salmonella chez le porc
Jeffrey A. Husa, DVM; Roy A. Edler, MS; Donald H. Walter, DVM; J. Tyler Holck, DVM, MS, MBA; Ryan J. Saltzman, DVM
JAH, RAE, DHW, JTH: Boehringer Ingelheim Vetmedica, Inc, Ames, Iowa. RJS: Veterinary Resources, Inc, Ames, Iowa. Corresponding author: Dr Jeff Husa, PO Box 50, Sioux Center, IA 51250; Tel: 712-722-8711; Fax: 712-722-8701; E-mail: jeff.husa@boehringer-ingelheim.com. Dr Husa, Roy Edler, and Drs Walter and Holck were employed by Boehringer Ingelheim Vetmedica, Inc, while this study was being conducted.
Cite as: Husa JA, Edler RA, Walter DH, et al. A comparison of the safety, cross-protection, and serologic response associated with two commercial oral Salmonella vaccines in swine. J Swine Health Prod. 2009;17(1):10–21.
Also available as a PDF.
SummaryObjectives: To compare safety, cross-protection, and serologic response associated with two Salmonella serovar Choleraesuis vaccines. Materials and methods: Eighty 4-week-old pigs, seronegative and culture-negative for Salmonella, were assigned to four groups of 20. The nonvaccinated challenged control group (NVC) was inoculated with virulent Salmonella serovar Typhimurium. Two groups received either Enterisol SC-54 (SC-54; Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri) or Argus SC/ST (Argus; Intervet Inc, Millsboro, Delaware) avirulent live Salmonella serovar Choleraesuis vaccines (Day 0) and were challenged (Day 43) with Salmonella serovar Typhimurium. The strict control group (NVNC) was nonvaccinated and nonchallenged. Individual body weights, clinical scores, rectal temperatures, and necropsy observations were recorded. Salmonella serum antibodies were measured using an indirect ELISA (Idexx Laboratories, Westbrook, Maine). Results: After vaccination, the Argus group showed more severe and frequent pyrexia and lower average daily gain (ADG) and Day 43 body weights than the SC-54 and NVC groups (P < .05). Vaccinates demonstrated cross-protection against Salmonella Typhimurium, with less severe and frequent pyrexia and lower individual clinical scores (P < .05). Prevalence of enteric lesions and total clinical scores were lower with SC-54 (P < .05). Vaccinal seroconversion was not detected pre-challenge, despite demonstrated cross-protection. By Day 52, 95% to 100% of all challenged pigs seroconverted. Implications: Enterisol SC-54 causes no adverse effects. Argus SC/ST induces significant deleterious responses. Both vaccines confer Salmonella Typhimurium cross-protection, with greater cross-protection by SC-54. As vaccinal seroconversion is not detected, monitoring programs using this ELISA are unlikely to be confounded by vaccination. | ResumenObjetivos: Comparar la seguridad, protección cruzada, y repuesta serológica asociada con dos vacunas de Salmonella serovar Choleraesuis. Materiales y métodos: Ochenta cerdos de 4 semanas de edad, seronegativos y negativos al cultivo de Salmonella, se asignaron a cuatro grupos de 20. El grupo control retado, no vacunado (NVC por sus siglas en inglés) fue inoculado con Salmonella serovar Typhimurium virulenta. Dos grupos recibieron vacunas contra Salmonella Choleraesuis viva, no virulenta ya fuera Enterisol SC-54 (SC-54; Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri) ó Argus SC/ST (Argus; Intervet Inc, Millsboro, Delaware) (Día 0) y se retaron con Salmonella Typhimurium (Día 43). El grupo control negativo (NVNC por sus siglas en inglés) no fue vacunado ni retado. Se registraron los pesos individuales, evaluación clínica, temperatura rectal, y observaciones a la necropsia. Se midieron los anticuerpos en suero contra Salmonella utilizando una ELISA indirecta (Idexx Laboratories, Westbrook, Maine). Resultados: Después de la vacunación, el grupo Argus presentó una pirexia más severa y frecuente y menor ganancia diaria (ADG pos sus siglas en inglés) y peso corporal en el Día 43 que los grupos vacunados con la SC-54 y NVC (P < .05). Los cerdos vacunados demostraron una protección cruzada contra Salmonella Typhimurium, con una pirexia menos severa y frecuente y una mejor evaluación clínica individual (P < .05). La prevalencia de lesiones entéricas fue menor y la evaluación clínica fue mejor con la vacuna SC-54 (P < .05). No se detectó serconversión contra la vacuna antes del reto, a pesar de que se demostró la protección cruzada. Para el Día 52, 95% a 100% de los cerdos retados seroconvirtieron. Implicaciones: La vacuna Enterisol SC-54 no causa efectos adversos. Argus SC/ST induce reacciones deletéreas significativas. Ambas vacunas confieren protección cruzada contra Salmonella Typhimurium, con una mayor protección cruzada de la SC-54. Al no detectarse la seroconversión contra la vacuna, es poco probable que la vacunación confunda los programas de monitoreo utilizando la prueba de ELISA. | ResuméObjectifs: Comparer l’innocuité, la protection croisée, et la réponse sérologique associées avec deux vaccins contenant Salmonella serovar Cholerasuis. Matériels et méthodes: Quatre-vingts porcs âgés de 4 semaines, négatifs pour Salmonella par culture et par sérologie, ont été assignés à quatre groupes de 20 porcs. Le groupe témoin non-vacciné infecté (NVC) a été inoculé avec une souche virulente de Salmonella serovar Typhimurium. Deux groupes ont été vaccinés (Jour 0) soit avec Enterisol SC-54 (SC-54; Boehringer Ingelheim Vetmedica Inc, St-Joseph, Missouri) ou Argus SC/ST (Argus; Intervet Inc, Millsboro, Delaware), des vaccins constitués d’une souche vivante avirulente de Salmonella Cholerasuis, et inoculés (Jour 43) avec Salmonella Typhimurium. Le groupe témoin négatif (NVNC) était non-vacciné et non-inoculé. Des données individuelles sur le poids corporel, les pointages cliniques, la température rectale, et les observations à la nécropsie ont été notées. Les anticorps anti-Salmonella ont été mesurés à l’aide d’une épreuve ELISA indirecte (Idexx Laboratories, Westbrook, Maine). Résultats: Après la vaccination, les animaux du groupe Argus ont montré une pyrexie plus sévère et plus fréquente ainsi qu’un plus faible gain journalier moyen (ADG) et poids corporel au Jour 43 que ceux des groupes SC-54 et NVC (P < .05). Une protection croisée envers Salmonella Typhimurium, manifestée par une pyrexie moins sévère et fréquente ainsi que par des pointages cliniques individuels plus bas, a été observée chez les animaux vaccinés (P < .05). La prévalence des lésions entériques et les pointages cliniques totaux étaient plus faibles avec SC-54 (P < .05). Une séroconversion vaccinale n’a pas été détectée avant l’inoculation défi, malgré l’évidence d’une protection croisée. Au Jour 52, 95% à 100% de tous les animaux soumis à une infection défi ont présenté une séroconversion. Implications: Le vaccin Enterisol-54 n’a pas causé d’effets adverses. Le vaccin Argus SC/ST a induit de sérieuses réactions adverses. Les deux vaccins confèrent une protection croisée contre Salmonella Typhimurium, avec une meilleure protection croisée associée à SC-54. Étant donné qu’une séroconversion vaccinale n’est pas détectée, les programmes de surveillance utilisant cet ELISA ne sont pas sujets à être confondus par la vaccination. |
Keywords: swine, Salmonella serovar Typhimurium, vaccine, safety, efficacy
Search the AASV web site
for pages with similar keywords.
Received: November 30, 2007
Accepted: July 2, 2008
Salmonella enterica has been recognized as a disease threat to animals since the late 1800s, when Dr D. E. Salmon and others initially investigated this bacterium as the suspected cause of hog cholera.1-7 Salmonellosis significantly reduces swine performance due to clinical and subclinical disease, and farms with 15% seroprevalence or higher have been shown to produce 7.3 kg less pork per square meter of building floor space per year.5,7-10 Studies cite prevalence as high as 62.6% on an individual pig basis, with up to 94% of herds found positive.11-15 Veterinary diagnostic laboratories continue to report Salmonella as a primary cause of enteritis and septicemia, and recently as a cofactor in porcine circovirus associated disease.6,16-18
Salmonellae can also infect humans, and may be passed between animals and man as a zoonosis.19-26 Numerous human cases are reported globally, of which 95% are estimated to be food-borne.27 In 2005, the US Centers for Disease Control reported 36,184 Salmonella isolates from human sources.28 Annual socio-economic costs attributed to food-borne salmonellosis are estimated at $2.3 billion to $12.8 billion in the United States, and more specifically, at $81.53 million for cases associated with pork.27,29 The threat of food-borne illness has prompted the adoption of national Salmonella reduction programs in countries such as Denmark, Great Britain, and the United States. These programs rely on effective control measures and reliable monitoring methods from pre-harvest through post-harvest.30-35
Pre-harvest control of Salmonella enterica serovars can be achieved through vaccination, sanitation, medication, and management of known risk factors.4,5,7,9,10,36-75 Several vaccines are licensed for control of Salmonella in swine. Before these licenses were granted, the manufacturers were required to prove acceptable safety in pigs.7,40,59,71 The United States Department of Agriculture (USDA) Animal and Plant Health Inspection Service defines “safe” as “freedom from properties causing undue local or systemic reactions when used as recommended or suggested by the manufacturer.”76 These pre-licensure evaluations are commonly based upon clinical appearance and growth performance. Vaccines may employ a wide variety of immunity-stimulating mechanisms with varying safety attributes and risks. Inactivated (killed) vaccines commonly include adjuvants to augment an immune response. Avirulent live culture vaccines consist of altered bacteria or virus populations that interact with the pig’s immune system. The variety of materials or methods used in manufacturing vaccines may result in different levels of safety or side-effects. Highly reactive vaccines may reduce animal performance as a result of stress, hypersensitivity, and other reactions.74 If, for instance, vaccines are administered to unhealthy pigs or are administered incorrectly, they may induce undesirable side-effects. In some studies, reactive Salmonella vaccines have even caused pig death.77
Few swine vaccines have documented effectiveness against multiple Salmonella serovars.7,39-41,58,59,71,78 Of the more than 2500 serovars recognized, Salmonella Choleraesuis and Salmonella Typhimurium are the ones most commonly associated with disease in swine.5,6,11,17,36,47,73,79
Various diagnostic tools are available to evaluate Salmonella control measures and to monitor food safety assurance programs. Ante-mortem serum antibody tests are available which provide insights into Salmonella exposure, prevalence, and onset of infection.8,9,80-85 To allow control-program monitoring while utilizing vaccine, these tests are most useful when they differentiate vaccine-induced antibody from that generated by natural infection.
For maximum benefit, Salmonella control measures, including vaccines, should cause minimal negative effects and should be effective against multiple serovars and compatible with monitoring and reduction initiatives. To assess the compatibility of two commercial swine Salmonella vaccines using these criteria, this study was conducted to compare the safety, cross-protection, and serologic response associated with Enterisol SC-54 (Boehringer Ingelheim Vetmedica, Inc, St Joseph, Missouri) and Argus SC/ST (Intervet Inc, Millsboro, Delaware).
Materials and methods
Animals
Eighty pigs were randomly assigned to four treatment groups at 28 ± 3 days of age (n = 20 per group; Table 1). All pigs were both negative for Salmonella by fecal culture (Iowa State University Veterinary Diagnostic Laboratory, Ames, Iowa)86 and seronegative by the HerdChek Swine Salmonella Antibody Test Kit (Idexx Laboratories, Inc, Westbrook, Maine), an indirect ELISA test which detects a broad range of Salmonella serogroups, with a negative test defined as a sample:positive ratio < 0.25. The size of each treatment group was based upon the maximum number of pigs that Veterinary Resources, Inc (VRI; Ames, Iowa) could house in accordance with their Institutional Animal Care and Use Committee (IACUC) and biosecurity standard operating procedures. As this was the first published study to compare swine Salmonella vaccines in a controlled challenge, reference data was not available and no pre-study power calculation could be performed. Commercial, crossbred, mixed-sex pigs weighing 9.13 ± 0.28 kg (95% CI; Table 2) were used in this study.
Table 1: Treatment groups and event timeline for groups of pigs vaccinated or not vaccinated with live avirulent Salmonella serovar Choleraesuis vaccines on Day 0 and challenged or not challenged with virulent Salmonella serovar Typhimurium on Day 43
* NVC = Nonvaccinated controls, not vaccinated on Day 0 (33 ± 3 days of age), challenged Day 43; SC-54 = Vaccinated with Enterisol † NT = no treatment; V = vaccinated; C = challenged with virulent Salmonella Typhimurium. ‡ N1 = one-half of pigs in each treatment group necropsied; N2 = remaining pigs in each treatment group necropsied. |
||||||||||||||||||||||||||||||||||||||||||||
Table 2: Mean body weight and ADG (± SE) and CVs of body weight and ADG during the vaccination safety phase (Days 0 through 43)* of a study in which nursery pigs were either vaccinated or not vaccinated Day 0 with live avirulent Salmonella serovar Choleraesuis vaccine and challenged or not challenged Day 43 with virulent Salmonella serovar Typhimurium
* Treatment groups and timeline described in Table 1. Least squares means reported. SE = standard error; CV = coefficient of variation; ADG = average daily gain. ab Values within a row with different superscripts differ significantly (Tukey honestly significant difference test; P < .05) |
Housing, biosecurity, feeding
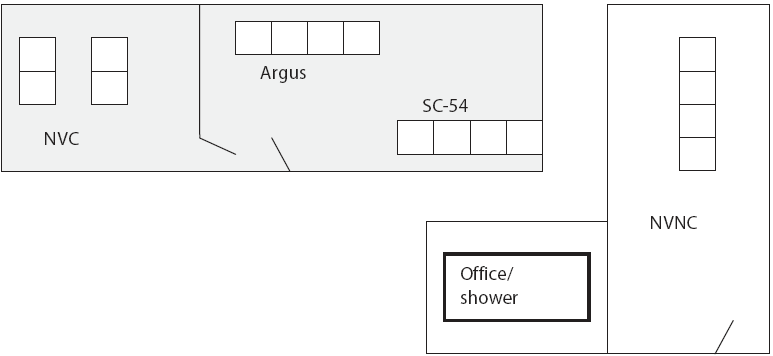
Pigs were housed and managed by an independent research firm (VRI). Internal and external site biosecurity was maintained according to VRI standard operating procedures, with an emphasis on Salmonella transmission control. All pigs were housed by treatment group in solid-walled plastic tubs designed to minimize the risk of lateral transmission of Salmonella vaccine or challenge organisms via fecal-oral or nose-to-nose transfer (Figure 1). Each tub held five pigs, with a raised, fenestrated plastic floor (1.2 m by 1.5 m) above a self-contained waste pit. Water and feed containers were dedicated to each tub for the duration of the study. Tub walls were 1.07 m in height above the raised deck. Additional measures against cross-contamination between treatments included housing the nonvaccinated nonchallenged controls (NVNC) in a separate building; separation of the nonvaccinated controls (NVC) by a solid wall and door from the SC-54 and Argus groups; separation of the SC-54 and Argus groups within the same room by a distance of more than 5.7 m; waste handling equipment, rectal thermometer, snare, and other tools required daily dedicated by treatment group; and requirements for all personnel to wash hands and change boots, coveralls, and gloves prior to any movement between treatment groups. Figure 2 shows the site diagram.
| Figure 1: Cohort housing for nursery-age pigs (five pigs per unit), designed to minimize risk of lateral Salmonella transmission between treatment groups.
|
| Figure 2: Site diagram of a facility used to house nursery-age pigs in a Salmonella vaccine study. Distances and barriers were designed to minimize risk of lateral Salmonella transmission among treatments. Pigs were either vaccinated or not on Day 0 (approximately 33 days of age) and either challenged or not on Day 43. Vaccines were oral avirulent live culture Salmonella serovar Choleraesuis: Enterisol SC-54 (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri) and Argus SC/ST (Intervet Inc, Millsboro, Delaware). The challenge organism was virulent Salmonella serovar Typhimurium. NVC = Nonvaccinated challenged controls; SC-54 = Vaccinated with Enterisol SC-54, challenged; Argus = Vaccinated with Argus SC/ST, challenged; NVNC = Nonvaccinated nonchallenged controls (biosecurity sentinels).
|
Feed and water rations provided throughout this trial were suitable for the size, age, and condition of the test animals according to acceptable industry standards. As an additional safeguard against inadvertent Salmonella exposure, all groups of pigs were fed a ration including 55 g per tonne of carbadox (Mecadox; Phibro Animal Health Corporation, Ridgefield Park, New Jersey) from Day -10 through Day -4. All feed and water available from Day -3 through study termination were free of antimicrobials. Animal care and euthanasia during this study were conducted in accordance with VRI’s IACUC guidelines. This IACUC maintains compliance with all standards set forth in the USDA Code of Federal Regulations (9 CFR 2 Subpart C)76 and the 2000 Report of the American Veterinary Medical Association Panel on Euthanasia.87
Study design
On Day -5, pigs were assigned a uniquely numbered identification tag (ID) and then individually weighed. The resulting list of pigs was next sorted by sex and by weight, and a random number was generated and assigned to each animal (Microsoft Excel; Microsoft Corporation, Redmond, Washington). The list of pigs was then blocked by sex and weight, and treatment group (1 through 4) was assigned from lowest to highest random number within block. Pigs were allocated to pen location by sorting the list by ascending treatment, and assigning pen such that pens 1 through 4 contained pigs in Treatment Group 1, pens 5 through 8 contained pigs in Treatment Group 2, pens 9 through 12 contained pigs in Treatment Group 3, and pens 13 through 16 contained pigs in Treatment Group 4. This method succeeded in achieving uniform starting weights among groups (Table 2) and ensured unbiased pen allocation. Treatments were assigned to groups as described in Table 1. By convention, where there are two sets of controls, our protocols utilize the nonvaccinated challenged controls for data comparisons, and the nonvaccinated nonchallenged controls to verify a lack of uncontrolled field infection. In this study, the NVNC group acted as sentinels to validate the controlled challenge model, and their data is not included in comparative analyses.
This trial was divided into two consecutive phases: a vaccination safety phase followed by a heterologous cross-protection phase (Table 1). On Day 0, the SC-54 and Argus groups were vaccinated by oral drench (study initiation). Administration of virulent Salmonella Typhimurium (heterologous challenge) on Day 43 marked the delineation between the vaccination safety phase and the cross-protection phase (Table 1).
To eliminate bias, the investigator making clinical and necropsy observations was blinded to treatment by his absence during pen allocation and treatment administration. Rectal temperatures were measured on Days -2, -1, 0, 1 through 21, 28, and 43 through 58. Additionally, rectal temperatures were measured on Day 0 at 0, 4, 8, and 12 hours after vaccination. Pigs in all groups were individually weighed on Days -5, 0, 2, 7, 14, 21, 28, 35, 43, 50, 57, 64, and 71. Serum samples were collected from all pigs on Days 0, 7, 14, 21, 28, 35, 43, 52, 57, 64, and 70. All sera were tested for Salmonella antibodies using the Idexx HerdChek Swine Salmonella Antibody Test Kit.82
On Day 57, one-half of the pigs in each treatment group were randomly selected for euthanasia. To make these selections, a random number was assigned to each pig using Microsoft Excel. Pigs were then sorted by this number within pen, and pigs with the lower one-half of these numbers were euthanized. On Day 71, all remaining animals were euthanized.
Clinical observation scoring
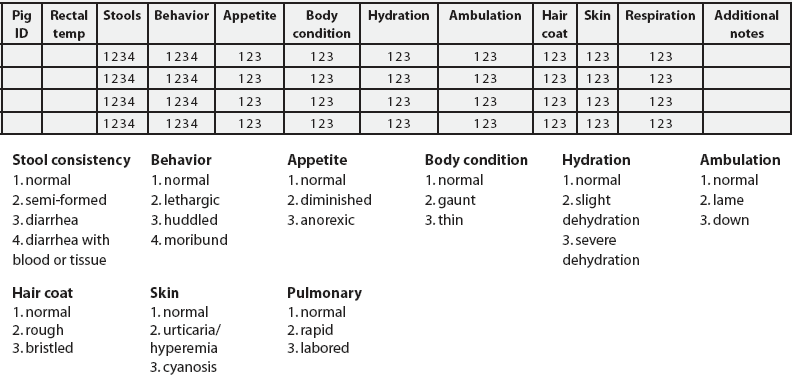
Observations were made on Days -2 through 7, 14, 21, 28, 35, 43 through 58, 61, 63, 65, 68, and 70, as well as at 0, 4, 8, and 12 hours post vaccination. Clinical observations were recorded using qualitative scoring with a numeric grading scale (Figure 3). A daily summation of individual parameter scores was calculated for each pig, with normal being a score of 9 and a maximum total score possible of 29.
| Figure 3: Clinical observations record sheet for the study described in Table 1. A separate record sheet was completed each observation day. Each pig’s score for each parameter was circled according to the scale shown at the bottom of the form: normal individual parameter score 1, normal total daily score, 9.
|
Challenge
On Day 43, 2 mL of virulent Salmonella Typhimurium (strain BIVI 02-04) was administered intranasally to the SC-54, Argus, and NVC groups. Each challenge dose contained 1.22 × 1010 colony forming units. This high-dose inoculum was chosen for its documented ability to cause clinical signs and lesions.78
Off-test procedures
At necropsy, the investigator blindly assessed all pigs for gross enteric lesions consistent with Salmonella Typhimurium infection. Post-mortem samples of tonsil, lung, liver, spleen, ileum, cecum, mesenteric lymph node, and ileocecal lymph node were collected from each animal and submitted to the Iowa State University Veterinary Diagnostic Laboratory for qualitative Salmonella culture.
Calculations and statistical analysis
The experimental unit in this study was the individual pig. All statistical analyses were performed using JMP 6.0 (SAS, Cary, North Carolina). Rectal temperatures of the SC-54, Argus, and NVC treatment groups for the time periods from 4 hours post vaccination through Day 43 (vaccination safety phase) and Days 44 through 58 (cross-protection phase) were analyzed using parametric and nonparametric methods. Parametric analyses included multivariate analysis of variance (ANOVA), with time as the repeated measure. One-way ANOVA was used as a second parametric analysis to compare differences among the three treatment groups. Tukey honestly significant difference (HSD) test was used to determine the response by treatment differences for mean rectal temperature. Nonparametric analysis was also performed using Fisher’s exact test to compare the number of normal versus abnormal rectal temperatures by treatment group. The minimum value for fever (abnormal temperature) for each study phase was determined using methods described by Vincent et al.88 Abnormal temperature for the vaccination safety phase was calculated to be ≥ 40.77°C, based upon two standard deviations above the mean rectal temperature for all pigs in the three challenged groups on Day -2, Day -1, and Day 0 prior to vaccination. For the cross-protection phase, these calculations determined an abnormal temperature to be ≥ 40.37°C, based upon mean rectal temperature for all challenged pigs on Days 21, 28, and 43. For the vaccination safety phase, average daily gain (ADG) comparisons among the SC-54, Argus, and NVC groups during the period from Day 0 through 43, and the Day 43 body weights, were analyzed using one-way analysis of covariance (ANCOVA), with Day 0 body weight as a covariate. Day 0 body weight comparisons used one-way ANOVA. For the cross-protection phase, Day 57 and 71 body weights, Days 44 through 57 ADG, and Days 58 through 71 ADG, ANCOVA calculations used Day 43 body weight as a covariate to account for weight-gain differences arising after vaccination and prior to challenge. Multiple comparisons among means were tested using Tukey HSD. Clinical observation scores, segregated by vaccination safety phase or cross-protection phase, were analyzed using Kruskal-Wallis rank sums test. Rank sums were compared among treatment groups using Tukey HSD. Nonparametric analysis was also performed using Fisher’s exact test to compare the number of normal versus abnormal clinical observations by treatment group. An abnormal binomial was assigned for any score above baseline (ie, above individual parameter score 1 or total score 9). The proportion of pigs exhibiting gross enteric lesions consistent with Salmonella Typhimurium infection at necropsy was compared among groups using Fisher’s exact test.
Results
Prior to challenge administration, one pig from the NVC group and one from the NVNC group died due to causes unrelated to treatments.
Vaccination safety phase
During Days 1 to 43, both frequency of abnormally elevated rectal temperatures and mean rectal temperature were significantly higher in pigs vaccinated with Argus than in the SC-54 and NVC pigs (P < .05; Table 3). No significant differences in rectal temperature measurements were observed between the SC-54 vaccinates and the nonvaccinated controls.
Table 3: Rectal temperature results for vaccination safety phase (Days 0 through 43)* in a study in which nursery pigs were either vaccinated or not vaccinated Day 0 with live avirulent Salmonella serovar Choleraesuis vaccine and challenged or not challenged Day 43 with virulent Salmonella serovar Typhimurium
* Treatment groups and timeline described in Table 1. Abnormal rectal temperature defined as ≥ 40.77°C. ab Values within a row with different superscripts differ significantly (Fisher’s exact test; P < .05). cd Values within a row with different superscripts differ significantly (Tukey honestly significant difference test; P < .05). |
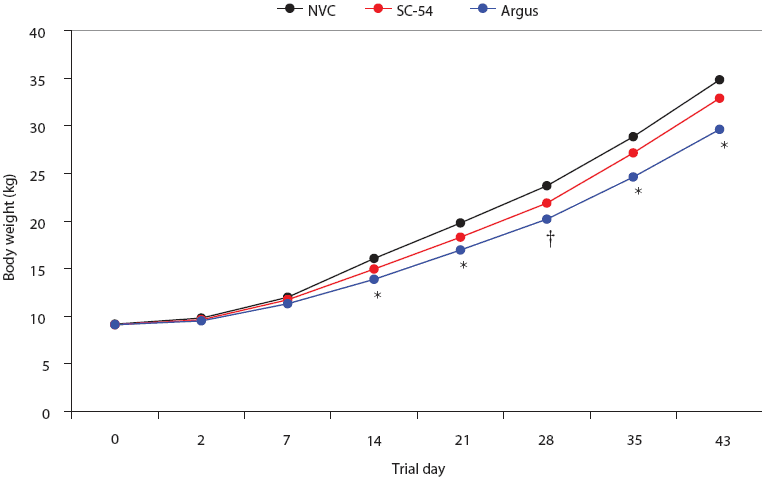
No significant difference in ADG was detected between the SC-54 and NVC groups, but ADG was significantly lower in the Argus group than in the SC-54 and NVC groups (P < .05, Table 2). Mean body weights were not significantly different for the SC-54 group than for the NVC group (Figure 4). However, at some data points, mean body weights were significantly lower in the Argus group than the SC-54 and NVC groups (Figure 4). On Day 43, Argus pigs were 3.28 kg lighter than SC-54 vaccinates and 4.86 kg lighter than NVC pigs (Figure 4).
| Figure 4: Mean group body weights during the vaccination safety phase of the study described in Table 1. The symbol * indicates time point when mean body weights for SC-54 and NVC groups differed from those for the Argus group (ANCOVA; P < .05); † indicates time point when mean body weight for the Argus group differed from that of the NVC group (ANCOVA; P < .05).
|
The influence of vaccination on group pig weights and ADG variability was also assessed by calculation of the coefficient of variation (CV) for these parameters within each group. Less individual ADG variation was exhibited in the SC-54 group than in the NVC or Argus groups (Table 2). Day 43 body-weight variation was also less for the SC-54 group than for the Argus group (Table 2).
Differences in clinical observation scores included significantly lower total rank sums and frequency of abnormal total scores in the SC-54 group than in the NVC and Argus groups (P < .05; Table 4). Lower hair-coat rank sums and frequency of abnormal hair-coat scores were observed in SC-54 pigs than in NVC pigs. Lower appetite, hair-coat rank sums, and frequency of abnormal total scores were observed in SC-54 and Argus groups than in the NVC group (P < .05; Table 4). No statistical differences among groups were found in other clinical parameters. Throughout the study, no abnormal scores or differences among groups were found for hydration, ambulation, or skin condition (data not shown).
Table 4: Clinical observation results for vaccination safety phase (Days 0-43)* of a study in which nursery pigs were either vaccinated or not vaccinated Day 0 with live avirulent Salmonella serovar Choleraesuis vaccine and challenged or not challenged Day 43 with virulent Salmonella serovar Typhimurium
* Treatment groups and timeline described in Table 1. Clinical observation scoring described in Figure 3: normal individual parameter score = 1.00, normal total daily score = 9.00. ab Values within a row with no common superscript differ significantly (Tukey honestly significant difference test; P < .05). cd Values within a row with no common superscript differ significantly (Fisher’s exact test; P < .05). |
Cross-protection phase
Both Salmonella Choleraesuis vaccines conferred varying degrees of protection against Salmonella Typhimurium challenge during the post-challenge period (Days 44 through 71). Both frequency of abnormally elevated rectal temperatures and mean rectal temperature were significantly lower after inoculation with Salmonella Typhimurium in the SC-54 and Argus groups than in the NVC group (P < .05; Table 5).
Table 5: Rectal temperature for cross-protection phase (Days 44-71)* of a study in which nursery pigs were either vaccinated or not vaccinated Day 0 with live avirulent Salmonella serovar Choleraesuis vaccine and challenged or not challenged Day 43 with virulent Salmonella serovar Typhimurium
* Treatment groups described in Table 1. Rectal temperatures measured through Trial Day 58, with abnormal temperature defined as ab Values within a row with no common superscript differ significantly (Fisher’s exact test; P < .05). cd Values within a row with no common superscript differ significantly (Tukey honestly significant difference test; P < .05) |
No significant ADG or body-weight differences were detected among groups during the post-challenge period (Table 6). The ANCOVA for ADG from Day 44 to 57, and mean body weight on Day 57, suggest a difference between means (probability of obtaining greater F statistic, P = .04) for vaccinates versus the NVC group. However, when the data was assessed using Tukey HSD at α = .05, no pair-wise differences between groups were detected (Table 6).
Table 6: Body weight and ADG results for cross-protection phase (Days 44-71)* of a study in which nursery pigs were either vaccinated or not vaccinated Day 0 with live avirulent Salmonella serovar Choleraesuis vaccine and challenged or not challenged Day 43 with virulent Salmonella serovar Typhimurium
* Treatment groups and timeline described in Table 1. † Least squares means reported. |
Clinical observation scores also showed evidence of challenge effectiveness and vaccine-induced heterologous protection. Total rank sums and frequency of abnormal total scores were significantly lower in the SC-54 group than in the NVC and Argus groups (P < .05; Table 7). Rank sums and frequency of abnormal stool and respiration scores were significantly lower in the SC-54 and Argus groups than in the NVC group (P < .05; Table 7). Behavior rank sums, frequency of abnormal behavior, and frequency of abnormal body condition scores were significantly lower in the SC-54 and NVC groups than in the Argus groups (P < .05; Table 7).
Table 7: Clinical observation and enteric lesion results for cross-protection phase (Days 44-71)* of a study in which nursery pigs were either vaccinated or not vaccinated Day 0 with live avirulent Salmonella serovar Choleraesuis vaccine and challenged or not challenged Day 43 with virulent Salmonella serovar Typhimurium
* Treatment groups described in Table 1. Clinical observation scoring described in Figure 3: normal individual parameter score = 1.00; normal total daily score = 9.00. ab Values within a row with no common superscript differ significantly (Tukey honestly significant difference test; P < .05). cd Values within a row with no common superscript differ significantly (Fisher’s exact test; P < .05). |
A significantly lower proportion of pigs with enteric lesions consistent with Salmonella Typhimurium infection was found in the SC-54 group than in the NVC group
(P < .05), but this proportion did not differ between the Argus and NVC groups (Table 7). Gross lesions noted included micro-abscesses at the ileocecal junction, inflammation of ileum, cecum, and large intestine, adhesions, and thickening of intestinal tissues. Qualitative Salmonella Typhimurium isolation rate did not differ among the three challenged groups, as expected due to the high challenge dose. Salmonella isolates recovered after challenge inoculation were confirmed to be serogroup B by the Iowa State University Veterinary Diagnostic Laboratory.
No abnormal clinical signs or positive Salmonella cultures were observed in the NVNC group.
Serologic response
One animal from each vaccinated group (5% per group) seroconverted immediately prior to challenge. Following challenge with virulent Salmonella Typhimurium, high rates of seroconversion were observed in all challenged groups by 9 days post challenge (Day 52: seroconversion in 100% of NVC and SC-54 groups and 90% of the Argus group). By Day 70, 100% of the pigs in the three challenged groups were seropositive. Salmonella seroconversion was never observed in the NVNC group.
Discussion
During the vaccination safety phase of this study, differences in post-vaccination pyrexia, growth rates, and clinical scores significantly favored the safety of Enterisol SC-54 over that of Argus SC/ST. Both vaccines contain attenuated live culture Salmonella Choleraesuis isolates, but the methods of attenuation used to create them were distinctly different. Repeated passage in neutrophils caused natural deletion of the 50-kb plasmid from the Enterisol SC-54 isolate. This Salmonella Choleraesuis plasmid is important for virulence and intestinal invasiveness.89 Argus SC/ST is an isogenic Δcya Δcrp derivative from Salmonella Choleraesuis, using transposon-mediated deletion mutagenesis.57,77 These differences in attenuation methods may account for the significant differences in safety of the two vaccines observed in this study.
As demonstrated in previous clinical and field studies, Enterisol SC-54 39,58,61,62,78 and Argus SC/ST 41 confer heterologous protection against Salmonella Typhimurium. In the cross-protection phase of this study, both frequency of abnormally elevated rectal temperature and mean rectal temperature after challenge were lower in vaccinated pigs than in nonvaccinated controls. Clinically, stool and respiratory scores of both vaccinated groups were significantly lower than those of the NVC group, while total observation scores and enteric-lesion prevalence were significantly lower only in the SC-54 group. In the ANOVA model, ADG Days 44 to 57 and mean body weight on Day 57 were significantly greater in the vaccinated groups than in the NVC group. However, assessment of the data using Tukey HSD at α = .05 detected no pairwise differences between groups. A retrospective power calculation suggests that at least 30 pigs were needed in each of the challenged groups (NVC, SC-54, and Argus) in order to achieve P < .05 at 80% power, and to clarify the potential significance of these post-challenge differences in ADG. A larger field or clinical study is needed to investigate this trend.
Bacterial culture for this study was limited to qualitative methods (positive-negative) and was not designed to evaluate a difference in tissue colonization as reported in earlier studies.9,39,41,58,59,61,71,78 In all parameters measured in this study, protection induced against Salmonella Typhimurium challenge by the SC-54 vaccine was equal to or greater than that induced by the Argus vaccine.
The Idexx HerdChek Swine Salmonella Antibody Test Kit clearly differentiated Salmonella Typhimurium-exposed pigs from non-exposed pigs regardless of vaccination status with either SC-54 or Argus. Only one animal in each of the vaccinated groups seroconverted after vaccination and prior to challenge. These singleton results fall within reported false-positive (specificity) test-performance characteristics.82 At 9 days after challenge, all groups inoculated with virulent Salmonella Typhimurium (NVC, SC-54, and Argus groups) demonstrated 95% to 100% group seroconversion. The differential capability of this assay could allow pre-harvest Salmonella control programs to utilize these Salmonella vaccines without confounding the interpretation of serologic monitoring. When applied as part of a regularly scheduled audit, this assay could be used to monitor the effect of Salmonella reduction programs in clinically and subclinically affected herds.
It is notable that vaccinates demonstrated significant protective immunity without producing detectable levels of ELISA antibodies. This implies that ELISA antibodies are not indicative of protection, and that this test is not suitable as a vaccination compliance-monitoring tool.
An additional application for this Salmonella serum ELISA can be inferred from these results. The seroconversion of > 95% of pigs within 9 days after Salmonella Typhimurium challenge indicates rapid antibody detection after the onset of infection. This enables practitioners to serologically profile herds and then schedule preventive vaccination at an appropriate interval before wild-type Salmonella exposure. Thus, adequate time may be provided for onset of vaccinal immunity prior to exposure. The onset of immunity from SC-54 vaccination has been demonstrated within 14 days, and this vaccine has a proven duration of immunity of at least 20 weeks.39,59,71 Accounting for farm-to-farm variation in transmission factors, the authors recommend administration of this vaccine at least 4 weeks prior to the onset of group seroconversion (2 weeks prior to the onset of exposure).
Implications
- Under the conditions of this study, vaccination with Enterisol SC-54 does not adversely affect pig growth and clinical appearance, but vaccination with Argus SC/ST does induce significant deleterious biologic responses.
- In pigs infected with virulent Salmonella Typhimurium, pyrexia is less frequent and less severe, and stool and respiration scores are lower, in pigs previously vaccinated with either Enterisol SC-54 or Argus SC/ST.
- In pigs infected with virulent Salmonella Typhimurium, the prevalence of enteric lesions is lower in pigs previously vaccinated with Enterisol SC-54 than in non-vaccinated controls, and the magnitude of total observation scores is lower in pigs previously vaccinated with Enterisol SC-54 than in Argus SC/ST vaccinates and nonvaccinated controls.
- As the indirect Salmonella ELISA assay used in this study (Idexx HerdChek) differentiates pigs exposed to wild-type Salmonella Typhimurium from non-exposed pigs regardless of vaccination status, this test can be used together with vaccination in Salmonella control and monitoring programs.
- Seroconversion as measured using the Idexx HerdChek Swine Salmonella ELISA is not a suitable indicator of vaccination compliance or protection.
Acknowledgements
We wish to acknowledge the important contributions of Kelly Burkhart, Wayne Chittick, Bill Duckworth, Tom Fangman, Isabel Turney Harris, Joann Kinyon, John Kolb, Bob Morrison, Axel Neubauer, Klaas Okkinga, John Prickett, Mike Roof, Jeff Zimmerman, the ISU Veterinary Diagnostic Laboratory, and the BIVI Health Management Center in the conduct of this study.
References
1. Salmon D. Investigations of Swine Plague and Fowl Cholera. Contagious Diseases of Domestic Animals. Department of Agriculture Special Report No. 34. Washington, DC: Government Printing Office; 1881:13–80.
2. Hubbert W, Hagstad H, Spangler E, Hinton M, Hughes K. Food Safety and Quality Assurance. Foods of Animal Origin. 2nd ed. Ames, Iowa: Iowa State University Press; 1996.
3. Merchant IA, Packer RA. The genus Salmonella. In: Veterinary Bacteriology and Virology. 5th ed. Ames, Iowa: Iowa State University Press; 1956:341–369.
4. Barnes D, Sorensen D. Salmonellosis. In: Dunne HW, Leman AD, eds. Diseases of Swine. 4th ed. Ames, Iowa: Iowa State University Press; 1975:554–564.
5. Griffith R, Schwartz K, Meyerholz D. Salmonella. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Publishing Professional; 2006:739–754.
*6. Schwartz K. Diagnostic update: Grow finisher diseases. Proc AASV. Orlando, Florida. 2007;355–362.
*7. Flores J, Dufresne L, Kolb J. Effect of Enterisol SC-54 vaccination on pig growth performance. Proc IPVS. Ames, Iowa. 2002;2:151.
*8. Baum D, Nolan D, Fleck R, Worstell J, Kolb J, Kahle D. Use of process behavior charts (SPC) for the purpose of continuous improvement in commercial swine production. Proc AASP. Indianapolis, Indiana. 2000;219–223.
9. Baum D. Vaccine and Epidemiologic Studies of Salmonella Infections in Swine [PhD dissertation]. Ames, Iowa: Iowa State University; 1997.
10. Schwartz KJ. Salmonellosis in swine. Comp Cont Educ Pract. 1991;13:139–147.
*11. Rostagno M, Hurd H, McKean J. Salmonella enterica prevalence and serotype distribution in swine at slaughter. Proc Safepork. Verona, Italy. 2007;153–155.
*12. Turner M, Funk J, Gebreyes W, Altier C, Davies P. Salmonella prevalence, serotypes, and patterns of antimicrobial resistance in cohorts of nursery and finishing pigs. Proc AASP. St Louis, Missouri. 1999;53–56.
13. van der Wolf P, Elbers A, van der Heijden H, van Schie F, Hunneman W, Tielen M. Salmonella seroprevalence at the population and herd level in pigs in The Netherlands. Vet Microbiol. 2001;80:171–184.
14. Larsen S, Hurd H, McKean J, Griffith R, Wesley I. Effect of short-term lairage on the prevalence of Salmonella enterica in cull sows. J Food Protect. 2004;67:1489–1493.
15. Bahnson P, Damman D, Isaacson R, Miller G, Weigel R, Troutt F. Prevalence and serovars of Salmonella enterica isolated from ileocolic lymph nodes of market pigs reared in selected Midwest US swine herds. J Swine Health Prod. 2006;14:182–188.
*16. Schwartz K. Common infectious agents: diagnostic laboratory perspective. Proc ISU Swine Disease. Ames, Iowa. 2001;7–23.
*17. Stevenson G. Diarrheal diseases in the post-weaned pig: Salmonellosis and viral enteritis. Proc AASV. Des Moines, Iowa. 2004;527–531.
*18. Kolb J, Okkinga K, Henke N, Anderson G. Protocol to investigate the impact of PCV2-associated disease in growing pigs. Proc AASV. Orlando, Florida. 2007;141–142.
19. Acha PN, Szyfres B. Salmonellosis. In: Zoonoses and Communicable Diseases Common to Man and Animals. Vol 1 Bacterioses and Mycoses. 3rd ed. Washington, DC: Pan American Sanitary Bureau, Regional Office of the World Health Organization. 2001:233–246.
20. Bell C, Kyriakides A. Salmonella. A Practical Approach to the Organism and its Control in Foods. Oxford, England: Blackwell Science. 2002.
21. Centers for Disease Control and Prevention. Preliminary FoodNet Data on the incidence of infection with pathogens transmitted commonly through food – 10 states, 2006. MMWR Weekly. 2007;56:336–339. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5614a4.htm. Accessed 5 November 2008.
22. National Antimicrobial Resistance Monitoring System. NARMS Retail Meat Annual Report, 2004. Available at: http://www.fda.gov/cvm/NARMSReport2004.htm. Accessed 5 November 2008.
23. Duffy E, Belk K, Sofos J, Bellinger G, Pape A, Smith G. Extent of Microbial Contamination in United States Pork Retail Products. J Food Protect. 2001;64:172–178.
24. Rigney C, Salamone B, Anandaraman N, Rose B, Umholtz R, Ferris K, Parham D, James W. Salmonella serotypes in selected classes of food animal carcasses and raw ground products, January 1998 through December 2000. JAVMA. 2004;224:524–530.
25. Wong T, Nicol C, Cook R, MacDiarmid S. Salmonella in uncooked retail meats in New Zealand. J Food Protect. 2007;70:1360–1365.
26. Sørensen L, Wachmann H, Alban L. Estimation of Salmonella prevalence on individual level based upon pooled swab samples from swine carcasses. Vet Microbiol. 2007;119:213–220.
*27. Frenzen PD, Riggs TL, Buzby JC, Breuer T, Roberts T, Voetsch D, Reddy S, FoodNet Working Group. Salmonella cost estimate updated using FoodNet data. FoodReview. 1999;22:10–15.
28. Centers for Disease Control and Prevention. Salmonella Surveillance: Annual Summary, 2005. Atlanta, Georgia: US Department of Health and Human Services, CDC. 2006. Available at: http://www.cdc.gov/ncidod/dbmd/phlisdata/salmtab/2005/SalmonellaAnnualSummary2005.pdf. Accessed 5 November 2008.
29. Miller G, Liu X, McNamara P, Barber D. Influence of Salmonella in pigs preharvest and during pork processing on human health costs and risks from pork. J Food Protect. 2005;68:1788–1798.
30. World Health Organization. Food and Agriculture Organization of the United Nations. Food safety risk analysis. A guide for national food safety authorities. 2006. FAO Food and Nutrition Paper 87. Available at: ftp://ftp.fao.org/docrep/fao/009/a0822e/a0822e00.pdf. Accessed 5 November 2008.
31. Davies P. Food safety and its impact on domestic and export markets. J Swine Health Prod. 1997;5:13–20.
32. Mousing J, Jensen P, Halgaard C, Bager F, Feld N, Nielsen B, Nielsen J, Bech-Nielsen S. Nation-wide Salmonella enterica surveillance and control in Danish slaughter swine herds. Prev Vet Med. 1997;29:247–261.
33. Krarup L. The Salmonella “Programme” in Denmark: Structure and ways out of the infection. Pig J. 2002;49:170–175.
*34. O’Reilly K, Miller A, Snary E, Cook A. Zoonoses Action Plan for Salmonella in slaughter-age pigs: how will changes in sampling methods influence estimates of Salmonella? Proc Safepork. Verona, Italy. 2007;305–308.
35. United States Department of Agriculture – Food Safety and Inspection Service. Progress report on Salmonella testing of raw meat and poultry products, 1998–2006. Available at: http://www.fsis.usda.gov/science/Progress_Report_Salmonella_Testing/index.asp. Accessed 5 November 2008.
36. Alsop J. An outbreak of salmonellosis in a swine finishing barn. J Swine Health Prod. 2005;13:265–268.
37. American Association of Swine Veterinarians. Salmonellosis. Swine Disease Manual. 3rd ed. Perry, Iowa: AASV; 2004:39–41.
*38. Bahnson P, Troutt H, Weiel R, Miller G, Isaacson R. Risk factors for the detection of Salmonella in ileocolic lymph nodes in US slaughtered pigs. Proc Safepork. Verona, Italy. 2007;73–76.
*39. Baum D, Harris D, Roof M, Nielsen B, Holck J, Polson D, Baik J. Use of SC-54 for the reduction of Salmonella in swine. Proc IPVS. Birmingham, England. 1998;3:124.
*40. Baum D, Roof M, Harris D. Efficacy and safety of SC-54 vaccine administered to pigs at one day of age. Proc IPVS. Bologna, Italy. 1996;176.
41. Charles S, Abraham A, Trigo E, Jones G, Settje T. Reduced shedding and clinical signs of Salmonella Typhimurium in nursery pigs vaccinated with a Salmonella Choleraesuis vaccine. J Swine Health Prod. 2000;8:107–112.
42. Collins F. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974;38:371–402.
43. Davies P. Fecal shedding of Salmonella by pigs housed in buildings with open-flush gutters. J Swine Health Prod. 1998;6:101–106.
*44. Denagamage T, O’Connor A, Sargeant J, Rajic A, McKean J. Vaccination against Salmonella and the association with measures of Salmonella prevalence in live and slaughtered swine – A systematic review. Proc Safepork. Verona, Italy. 2007;283–286.
*45. Dorr P, Lowman H, Gebreyes W. The role of truck wash practices in dissemination of Salmonella and Campylobacter in commercial swine production. Proc Safepork. Rohnert Park, California. 2005;161–163.
46. Erdman M, Harris I, Torremorell M, Wilt V, Harris D. Occurrence of Salmonella serotype Typhimurium DT104 on a commercial swine farm before, during, and after depopulation and repopulation. JAVMA. 2005;227:460–466.
47. Erdman M, Wedel S, Harris D. Genotypic and phenotypic comparison of swine Salmonella isolates from farm and abattoir. J Swine Health Prod. 2003;11:169–172.
*48. Farzan V, Friendship R, Dewey C, Delange C, Poppe C, Muckle A. The effect of dry versus liquid feeding systems on the presence of Salmonella spp. Proc IPVS. Hamburg, Germany. 2004;2:682.
49. Fedorka-Cray P, Hogg A, Gray J, Lorenzen K, Velasquez J, Von Behren P. Feed and feed trucks as sources of Salmonella contamination in swine. Swine Health Prod. 1997;5:189–193.
50. Foster N, Lovell M, Marston K, Hulme S, Frost A, Bland P, Barrow P. Rapid protection of gnotobiotic pigs against experimental salmonellosis following induction of polymorphonuclear leukocytes by virulent Salmonella enterica. Infect Imm. 2003;71:2182–2191.
51. Funk J, Gebreyes W. Risk factors associated with Salmonella prevalence on swine farms. J Swine Health Prod. 2004;12:246–251.
*52. Godsey B, Skjolaas K, Minton J. Pre-exposure to Bacillus licheniformis reduces interleukin 8 response of swine intestinal epithelial cells to Salmonella enterica serovar Typhimurium. Proc Midwest Am Soc Anim Sci. 2007;108.
*53. Gramm B. In vitro susceptibility of Salmonella Choleraesuis and Escherichia coli to carbadox. Proc AASP. Kansas City, Missouri. 1993;85.
*54. Hansen C, Jørgensen L, Dahl J, Kjeldsen N. Effect of formic acid in drinking water on the incidence of Salmonella in growing-finishing pigs. Proc Intl Symp Epid Cont Salmonella Pork. 1999;299–301.
*55. Hurd H, McKean J. Control of Salmonella in swine with a special emphasis on transportation and lairage. Proc George A Young Swine Health Manage Conf. South Sioux City, Nebraska. 2004;33–37.
*56. Jones R, Kolb J, Cline G, Philips R. Controlling PCV2 and co-infections to reduce the impact of PCVAD in growing pigs. Proc AD Leman. 2007;14.
57. Kennedy M, Yancey R, Sanchez M, Rzepkowski R, Kelly S, Curtiss R. Attenuation and immunogenicity of &#Delta;cya&#Delta;crp derivatives of Salmonella choleraesuis in pigs. Infect Imm. 1999;67:4628–4636.
*58. Kolb J, Roof M, Burkhart K. Reduction of Salmonella in carcasses using Enterisol SC-54 vaccination. Proc IPVS. Ames, Iowa. 2002;2:14.
59. Kramer T, Roof M, Matheson R. Safety and efficacy of an attenuated strain of Salmonella choleraesuis for vaccination of swine. Am J Vet Res. 1992;53:444–448.
*60. Lee N, Harris D. The effect of bacteriophage treatment as a preharvest intervention strategy to reduce the rapid dissemination of Salmonella typhimurium in pigs. Proc AASV. Nashville, Tennessee. 2001;555–557.
61. Letellier A, Messier S, Lessard L, Quessy S. Assessment of various treatments to reduce carriage of Salmonella in swine. Can J Vet Res. 2000;64:27–31.
62. Letellier A, Messier S, Lessard L, Chénier S, Quessy S. Host response to various treatments to reduce Salmonella infections in swine. Can J Vet Res. 2001;65:168–172.
63. Low J, Angus M, Hopkins G, Munro D, Rankin S. Antimicrobial resistance of Salmonella enterica Typhimurium DT104 isolates and investigation of strains with transferable apramycin resistance. Epidemiol Infect. 1997;118:97–103.
*64. Mack A, Funk J, Bowman A. The effect of stringent cleaning and subtherapeutic chlortetracycline on the prevalence of Salmonella in commercial swine farms. Proc AASV. Kansas City, Missouri. 2006;27.
65. Mathew A, Beckmann M, Saxton A. A comparison of antibiotic resistance in bacteria isolated from swine herds in which antibiotics were used or excluded. J Swine Health Prod. 2001;9:125–129.
66. Mathew A, Jackson F, Saxton A. Effects of antibiotic regimens on resistance of Escherichia coli and Salmonella serovar Typhimurium in swine. J Swine Health Prod. 2002;10:7–13.
67. Ministry of Agriculture, Fisheries and Food, and the Scottish Executive Rural Affairs Department. Code of practice for the prevention and control of Salmonella on pig farms. 2000. London, England. Available at: http://www.defra.gov.uk/animalh/diseases/zoonoses/zoonoses_reports/pig.pdf. Accessed 5 November 2008.
68. Morehouse L. Salmonellosis in swine and its control. JAVMA. 1972;160:593–602.
69. Nietfeld J, Feder I, Kramer T, Schoneweis D, Chengappa M. Preventing Salmonella infection in pigs with offsite weaning. Swine Health Prod. 1998;6:27–32.
*70. O’Connor A, Schultz-Kaster C, Kocher M, Cast W, Norgrant A, Rostagno M, McKean J, Hurd H. Effect of pellet vs. mash corn-soy diets on Salmonella prevalence. Proc 6th Int Symp Epidemiol Control Foodb Path Pork. Rohnert Park, California. 2005;153.
71. Roof M, Doitchinoff D. Safety, efficacy, and duration of immunity induced in swine by use of an avirulent live Salmonella choleraesuis-containing vaccine. Am J Vet Res. 1995;56:39–44.
*72. Rosendal T, Friendship R. Salmonella in the finishing pig – does size matter? Proc AASV. Kansas City, Missouri. 2006;435–437.
*73. Schneider P. Salmonella. Proc AASV. Nashville, Tennessee. 2001;377–380.
74. Tizard I, Schubot R. Vaccination and vaccines. In: Tizard IR. Veterinary Immunology: An Introduction. 7th ed. Philadelphia, Pennsylvania: WB Saunders; 2004:265–271.
75. Wilcock B, Olander H. Influence of oral antibiotic feeding on the duration and severity of clinical disease, growth performance, and pattern of shedding in swine inoculated with Salmonella typhimurium. JAVMA. 1978;172:472–477.
76. USDA APHIS. Viruses, serums, toxins, and analogous products; organisms and vectors. 9th Code of Federal Regulations. 101.5. Washington, DC: US Government Printing Office; 2007:622. Available at: http://www.aphis.usda.gov/animal_health/vet_biologics/vb_cfr.shtml. Accessed 5 November 2008.
77. Stabel T, Mayfield J, Morfitt D, Wannemuehler M. Oral immunization of mice and swine with an attenuated Salmonella choleraesuis [∆cya-12 ∆(crp-cdt)19] mutant containing a recombinant plasmid. Infect Imm. 1993;61:610–618.
*78. Neubauer A, Roof M. Enterisol SC-54 cross-protection against a virulent S. typhimurium strain. Proc AASV. Toronto, Ontario. 2005;245–248.
79. Carlson A, Blaha T. In-herd prevalence of Salmonella in 25 selected Minnesota swine farms. J Swine Health Prod. 2001;9:7–10.
80. Baum D, Ward S, Baum C, Lee N, Polson D, Harris D, Nielsen B. Statistical process control methods used to evaluate the serologic responses of pigs infected with three Salmonella serovars. J Swine Health Prod. 2005;13:304–313.
81. Nielsen B, Baggesen D, Bager F, Haugegaard J, Lind P. The serological response to Salmonella serovars typhimurium and infantis in experimentally infected pigs. The time course followed with an indirect anti-LPS ELISA and bacteriological examinations. Vet Micro. 1995;47:205–218.
*82. Rossi A, Ballagi A, Goetz C. Use of ELISA HerdChek Swine Salmonella for evaluation and monitoring Salmonella in swine herds. Proc Safepork. Verona, Italy. 2007;489–492.
*83. Turney Harris I. Serologic basis for assessment of subclinical Salmonella infection in swine: Part 1. J Swine Health Prod. 2003;11:247–251.
*84. Turney Harris I. Serologic basis for assessment of subclinical Salmonella infection in swine: Part 2. J Swine Health Prod. 2003;11:300–303.
85. van der Heijden H. First international ring trial of ELISAs for Salmonella-antibody detection in swine. Berliner und Munchener Tierarztliche Wochenschrift. 2001;114:389–392.
86. Amass S, Arighi M, Kinyon J, Hoffman L, Schneider J, Draper D. Effectiveness of using a mat filled with a peroxygen disinfectant to minimize shoe sole contamination in a veterinary hospital. JAVMA. 2006;228:1391–1396.
87. Beaver B, Reed W, Leary S, McKiernan B, Bain F, Schultz R, Bennett B, Pascoe P, Shull E, Cork L, Francis-Floyd R, Amass K, Johnson R, Schmidt R, Underwood W, Thornton G, Kohn B. 2000 report of the AVMA panel on euthanasia. JAVMA. 2001;218:669–696.
88. Vincent A, Wenjun M, Lager K, Janke B, Webby R, García-Sastre A, Jürgen R. Efficacy of intranasal administration of a truncated NS1 modified live influenza virus vaccine in swine. Vaccine. 2007:25:7999–8009.
89. Roof M, Kramer T, Kunesh J, Roth J. In vivo isolation of Salmonella choleraesuis from porcine neutrophils. Am J Vet Res. 1992;53:1333–1336.
* Non-refereed references.