Case study |
Peer reviewed |
Effect of treatment with phytosterols in three herds with porcine respiratory disease complex
Efecto del tratamiento con fitoesteroles en tres hatos con complejo respiratorio porcino
Effet d’un traitement aux phytostérols dans trois troupeaux aux prises avec le complexe des maladies respiratoires porcines
Lorenzo J. Fraile, DVM, PhD; Elisa Crisci, DVM; Joan Weenberg, DVM; Montse Armadans, DVM; Lorenzo Mendoza, DVM; Lara Ruiz, DVM; Santi Bernaus, DVM; María Montoya, MSc, PhD
LJF, EC, MM: Centre de Reçerca en Sanitat Animal (CReSA), Edifici V, Universitat Autónoma de Barcelona, Bellaterra, Spain. LJF, MM: Institut de Recerca i Tecnologies Agroalimentaries (IRTA), Barcelona, Spain. JW, MA, LM, LR: Pinsos Baucells, Seu central Tona, Barcelona, Spain. SB: Avinguda Alcalde Rovira Roure, 110, Lleida, Spain. Corresponding author: Dr Lorenzo J. Fraile, CReSA, Edifici V, Universitat Autónoma de Barcelona, 08193 Bellaterra, Spain; Tel: +34 93 581 44 95; Fax: +34 93 581 44 90; E-mail: lorenzo.fraile@cresa.uab.es.
Cite as: Fraile LJ, Crisci E, Weenberg J, et al. Effect of treatment with phytosterols in three herds with porcine respiratory disease complex. J Swine Health Prod. 2009;17(1):32-41.
Also available as a PDF.
SummaryThis case study includes three pig production systems belonging to two companies in Spain. Mortality, percent culls, average daily gain (ADG), and feed efficiency in Production Systems One and Two were incorporated into a database program and analyzed using statistical process control (SPC) techniques to assess changes in performance before and after phytosterols, natural substances that act as immunomodulators, were added to the feed. Inmunicin Maymo (Maymo Laboratories SA, Barcelona, Spain), a commercial phytosterol product, was administered in feed during the nursery and finishing periods, from 4 weeks before until 4 weeks after the predicted date of an outbreak of porcine respiratory disease complex (PRDC). In Production System Three, data obtained for batches treated or not treated with Inmunicin Maymo were compared using a one-way ANOVA, with the level of significance set at .05. In all three production systems, finisher mortality and percent culls were lower and production parameters were best when the immunomodulator was applied. Differences were statistically significant for all parameters evaluated, except feed conversion ratio, when assessed using SPC criteria in Systems One and Two and one-way ANOVA in System Three. Phytosterols may be useful to control endemic PRDC under field conditions. | ResumenEste estudio de casos incluye tres sistemas de producción de cerdos que pertenecen a dos compañías en España. En un programa de base de datos se integraron la mortalidad, el porcentaje de desechado, ganancia diaria promedio (ADG por sus siglas en inglés), y eficiencia alimenticia de los Sistemas de Producción Uno y Dos y se analizaron utilizando la técnica de control estadístico del proceso (SPC por sus siglas en inglés) para evaluar cambios en el desempeño antes y después de que los fitoesteroles, sustancias naturales que actúan como inmunomoduladores, se añadieran al alimento. Inmunicin Maymo (Laboratorios Maymo SA, Barcelona, España) un producto comercial a base de fitoesteroles, se administró en el alimento durante los periodos de destete y finalización, desde 4 semanas antes hasta 4 semanas después de la fecha predicha de un brote de complejo respiratorio porcino (PRDC por sus siglas en inglés). En el Sistema de Producción Tres, la información obtenida de grupos tratados y no tratados con el Inmunicin Maymo se comparó utilizando un ANOVA de una vía, con un nivel de significancia establecido a .05. En los tres sistemas de producción, la mortalidad en las engordas y el porcentaje de desechos fueron más bajos y los parámetros de producción fueron mejores cuando se aplicó el inmunomodulador. Las diferencias fueron estadísticamente significativas en todos los parámetros evaluados, excepto en el índice de conversión alimenticia, cuando se evaluaron utilizando los criterios del SPC en los Sistemas Uno y Dos y la ANOVA de una vía en el Sistema Tres. Los fitoesteroles pueden ser útiles para controlar el PRDC endémico bajo condiciones de campo. | ResuméLa présente étude de cas inclus trois systèmes de production appartenant à deux compagnies en Espagne. Les donnés sur les mortalités, le pourcentage de réforme, le gain quotidien moyen (ADG), et l’efficacité alimentaire pour les Systèmes de Production Un et Deux ont été incorporées dans un programme de base de données et analysées à l’aide de techniques utilisant un processus de contrôle statistique (SPC) afin d’évaluer les changements dans les performances avant et après que des phytostérols, substances naturelles qui agissent comme des immunomodulateurs, aient été ajoutés aux aliments. Immunicin Maymo (Maymo Laboratories SA, Barcelone, Espagne), un produit phytostérol commercial, a été administré dans l’alimentation durant les périodes en pouponnière et en finition, d’une période allant de 4 semaines avant à 4 semaines après la date prévue d’une éclosion du complexe des maladies respiratoires porcines (PRDC). Dans le Système de Production Trois, les données obtenues pour les lots traités ou non-traités avec Immunicin Maymo ont été comparées à l’aide d’une analyse de variance univariée (ANOVA), avec un seuil significatif établi à .05. Dans les trois systèmes de production, la mortalité chez les finisseurs et le pourcentage d’animaux réformés étaient plus faibles et les paramètres de production étaient meilleurs lorsque l’immunomodulateur était appliqué. Les différences étaient statistiquement significatives pour tous les paramètres évalués, sauf le taux de conversion alimentaire, en utilisant les critères SPC dans les Systèmes Un et Deux et une ANOVA univariée dans le Système Trois. Les phytostérols pourraient être utiles pour maîtriser les PRDC endémiques dans des conditions de terrain. |
Keywords: swine, phytosterols, porcine respiratory
disease complex, inmunomodulation, statistical process control
Search the AASV web site
for pages with similar keywords.
Received: November 16, 2007
Accepted: February 6, 2008
Porcine respiratory disease complex (PRDC) seems to have evolved with modern swine production. It is characterized clinically by dyspnea, coughing, acute depression, anorexia, fever, and nasal discharge, most often affecting growing to finishing pigs.1 The interaction of multiple factors contributes to PRDC. Both viral and bacterial organisms play a role, as well as environmental conditions and various management practices. In the right combination, these factors can compromise respiratory defense mechanisms sufficiently to cause severe respiratory disease.2
The most common viral pathogens associated with PRDC are porcine reproductive and respiratory syndrome virus (PRRSV), swine influenza virus (SIV), and porcine circovirus type 2 (PCV2).3 The most commonly associated bacterial pathogens include Mycoplasma hyopneumoniae, Actinobacillus pleuropneumoniae, Bordetella bronchiseptica, Pasteurella multocida, Haemophilus parasuis, and Streptococcus suis.
Measures used to cope with PRDC include strict management policies, environmental monitoring, pig flow changes, implementation of strategic vaccination programs focused mainly on viral infectious agents (PRRSV, PCV2, and SIV), and antibiotic medication.4 Antibiotics are used as prevention, therapeutic treatment, or both in swine medicine. This use has been associated with a significant increase in the resistance pattern of some microorganisms to antibiotics used in human and veterinary medicine.4 For this reason, many alternatives to antibiotic use have been considered by the swine industry, including natural substances (immunomodulators) that may modulate the immune system, helping to overcome common infectious diseases. Many categories of immunomodulators have been investigated in animals, but only a few have been licensed for use in food animals, both in the United States and Europe.5 However, this is an active field of research, not only with the goals of enhancing survival and clinical parameters for common infectious diseases, but also for improving the response to vaccines in many species.6-12 Use of immunomodulators as an alternative to antibiotic use in livestock is highly supported by the European Commission’s Seventh Framework Programme for research and technical development.13
Use of immunomodulators might be a useful approach to enhance immune responses after vaccination with PRRSV modified live vaccines or to overcome infectious diseases in swine. Recently, Inmunicin Maymo (Maymo Laboratories SA, Barcelona, Spain), a product containing plant phytosterols with immunomodulating activity,14 has become commercially available in Spain. Its exact composition is protected under European patent, but the main component is beta-sitosterol (BSS). In animals, BSS and its glucoside have exhibited anti-inflammatory, antineoplastic, antipyretic, and immune-modulating activity15 in a number of studies, including in vitro studies, animal models, and human clinical trials.16 This phytosterol complex seems to target specific T-helper lymphocytes, increasing Th1 activity and resulting in improved T-lymphocyte and natural killer cell activity.17 Taking into account the pathogenic mechanisms of PRRSV, SIV, and PCV2 infections, it is possible that an increase in Th1 activity would improve the immune response, helping to minimize the negative production consequences in herds where PRDC occurs endemically.18-20
Porcine respiratory disease complex causes immune dysfunction in affected animals, interfering with the capacity to overcome infectious challenges.21 Our laboratory has preliminary experimental data on the use of a phytosterol mixture administered to pigs in feed to treat respiratory diseases that cause immune dysfunction.22 This case study describes growth-production results in three production systems when phytosterols were administered in feed during the period when herd records showed that an outbreak of PRDC was likely to occur.
Production systems
The three pig-production systems described in this study belonged to two companies located in northeastern Spain. All animals were fed, housed, and handled with due concern for their welfare. The three facilities operated under the guidelines of the animal care and use committee of the Universidad Autónoma de Barcelona. No specific authorization was required for this study as Inmunicin is an authorized product in Spain (ie, it is not an experimental product).
Production System One
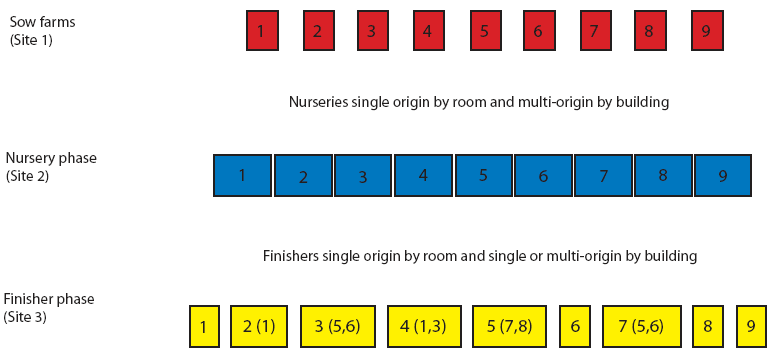
This 7000-sow multi-site production system included nine sow farms. Pig flow is shown in Figure 1. Briefly, pigs born in different sow farms were weaned at 21 days of age and moved to nurseries (Site 2) that were multi-origin by site and single origin by room. Nurseries were managed all-in, all-out by room. Pigs moved from the nurseries to the finishing units (Site 3) at 8 to 10 weeks of age. Finisher buildings 1, 6, 8, and 9 were single-origin ie, housed only pigs from sow farms 1, 6, 8, and 9, respectively. Finisher buildings 2, 3, 4, 5, and 7 were multi-origin, with pigs from two to three farms of origin per building. All finisher buildings were managed all-in, all-out and housed approximately 1000 pigs each.
| Figure 1: Pig flow for Production System One, a 7000-sow
multi-site system in Spain. Finisher buildings 1, 6, 8, and 9
housed only pigs from sow farms 1, 6, 8, and 9, respectively.
Finisher buildings 2, 3, 4, 5, and 7 each housed pigs from two to
three farms of origin. Numbers in parentheses represent additional
sow farms of origin. All finisher buildings were managed all-in,
all-out (approximately 1000 pigs per barn).
|
During 2005, this system experienced> 10% mortality in late nursery pigs, early finishing pigs, or both, despite treatment with broad-spectrum antibiotics in feed and water and by injection (data from 108,000 pigs). During 2006, clinical signs compatible with PRDC were less severe and mortality decreased, but data from 120,000 pigs compared unfavorably with average finisher mortality for pigs in Spain (6.1%) during the same year (J. Font, SIP Consultors, oral communication, 2007). Both in 2005 and 2006, clinical signs compatible with PRDC were observed in pigs 8 to 9 weeks of age. For this reason, the company decided to use Inmunicin Maymo to improve performance in the system. This product was administered to pigs 4 to 12 weeks of age (end of the nursery period to the early finishing period) beginning in March 2006. Pigs in finishing closeouts beginning in August 2006 received this treatment (data for 120,000 pigs).
Production System Two
This multi-site production system included 1000 sows in a 3-week batch system. Pigs were moved to a nursery at a weaning age of 21 days. The nursery was single-origin by site, single-aged by room, and managed all-in, all-out by room. The finishing units were filled with pigs from this nursery (9 weeks of age) and were managed all-in, all-out by building (between 1000 and 1500 pigs per barn). Each closeout was from one finisher barn.
During 2005, the system experienced > 10% mortality in the finishing period (data from 21,589 pigs in 21 barns) and clinical signs compatible with PRDC were observed when pigs were 13 weeks of age. Mortality did not improve significantly during 2006 (data from 11,922 pigs in eight barns). For this reason, the company decided to use Inmunicin Maymo to improve performance in pigs 9 to 17 weeks of age, with treatment beginning in January 2006. Pigs in finishing closeouts beginning in May 2006 (batch 29) received this treatment (data from 16,694 pigs in 14 barns).
Production System Three
This 2135-sow multi-site production system included three farms, with 500 to 900 sows per farm. Pigs born in different sow farms were moved to nurseries (Site 2) at a weaning age of 21 days. Nurseries were multi-origin by site and single origin by room, and were managed all-in, all-out by room. The finishing units (1350 to 4220 pigs per barn) were filled with pigs from these nurseries (8 to 10 weeks of age) and were managed all-in, all-out by building.
During 2006, the system experienced high mortality in the finishing period because of PRRS outbreaks in some sow farms. Clinical signs characteristic of PRDC were observed when pigs were 13 weeks of age. The company decided to use Inmunicin Maymo to improve performance, with treatment administered to pigs 9 to 17 weeks of age in some finisher batches beginning in June 2006. Others batches were not treated (controls). Control and treated batches originating from the same sow herd included closeouts of 28,252 and 12,902 pigs from 10 and four finisher farms, respectively.
Treatment with Inmunicin Maymo
In each production system, Inmunicin Maymo was administered according to the label instructions (2 kg of Inmunicin Maymo per tonne of feed) during the period from 4 weeks before until 4 weeks after the predicted date of a PRDC outbreak, according to clinical experience in that system. No changes in gilt acclimation, genetic background, vaccinations, semen extenders, boar management, or weaning age of pigs were made during the treatment period.
Parameters evaluated
Criteria evaluated included average daily gain (ADG), feed efficiency, mortality, and percent culls during the finisher phase. Average daily gain was calculated as the difference between final weight at closeout and initial weight of all pigs, divided by the length of the finisher period. Mortality was calculated as the number of pigs that had died by closeout divided by the number of pigs that had entered the finisher. Percent culls was calculated as the number of culls at closeout divided by the number of pigs that had entered the finisher. Feed efficiency was calculated by dividing feed consumption (including feed wastage) at barn level during the finisher period by the difference between final weight at closeout and initial weight of all pigs that had entered the finisher in the three production systems.
Diagnostic testing
Diagnostic testing was performed in each production system at several time points. Blood samples from 12 animals that exhibited signs of PRDC (dyspnea, coughing, anorexia, and fever) were collected and tested for PRRSV genomes by reverse transcriptase polymerase chain reaction (RT-PCR).23 Samples were collected on the day when clinical signs were first noticed (Day 0) and from the same ear-tagged animals 21 days later (Day 21). Extraction and amplification of PRRSV DNA was performed on pools of Day 0 samples (four samples per pool). Day 0 and Day 21 sera were tested for PRRSV antibodies by ELISA (HerdChek PRRS 2XR; Idexx Laboratories, Barcelona, Spain).
Necropsies were performed by the herd veterinarian during the PRDC outbreak. To avoid misinterpretation of pathological findings, only fresh specimens (ie, no autolyzed carcasses) were examined. The main purpose of necropsy was to determine whether or not postweaning multisystemic wasting syndrome (PMWS) was a significant contributor to disease and mortality. Tissue samples (lung, superficial inguinal lymph node, spleen, kidney, and liver) were submitted to the histopathology department, Universidad Autonoma de Barcelona (Barcelona, Spain), for histopathology and testing for PCV2 infection by in situ hybridization.24
No microbiological isolation was attempted, as many animals were being treated with antimicrobials prophylactically or therapeutically during the PRDC outbreak. Pigs were treated with tiamulin (200 g per tonne) and chlortetracycline (400 g per tonne) in the feed at the end of the nursery period and early in the finishing period (5 weeks total).
Statistical analyses
Data from Systems One and Two were incorporated into a database program and analyzed using statistical process control (SPC) techniques25 to assess changes in performance before and after addition of the immunomodulator to the feed. If the process remained in control, future measurements would continue to follow the same probability distribution as previously. All analyses were performed with the QI Macros2007 SPC for Excel (KnowWare international Inc; www.excel-spc-software.com/excel-spc-software.html). System changes were considered significant if one or several of the following conditions existed: one single point more than 3 s away from the mean; at least two of three successive points 2 s away and on the same side of the mean; at least nine successive points on the same side of the mean; at least four of five successive points 1 s away and on the same side of the mean.
A control chart was constructed for each analyzed parameter and the control limit, upper control limit, and lower control limit were calculated from the inherent variation using the software described. The chart was selected according to the type of analyzed data and whether or not the data was normally distributed.25
Production parameters (ADG, feed efficiency, and mortality) of control and treated batches in System Three were compared in a one-way ANOVA, as data for controls and treated groups were generated concurrently rather than in successive groups as in Systems One and Two. Level of significance was established at < .05. All analyses were performed in NCSS 2004 and PASS 2005 (NCSS, Kavysville, Utah).
Results of diagnostic testing
Diagnostic results are described in Table 1. Infections with both PRRSV and PCV2 were diagnosed in System One, while PMWS alone was diagnosed in System Two and PRRS alone was diagnosed in System Three on a single occasion. Diagnostic testing for PMWS was not performed in System Three. In Production Systems One and Two there was a clinical diagnosis of PRDC (respiratory signs as described) and a laboratory diagnosis of PRRSV infection, PCV2 infection, or both during the Unstable, Stable, and Stable with immunomodulator periods (defined in Table 2 and described in Figure 2). No additional diagnostic testing was performed for other pathogens.
Table 1: Results of diagnostic testing for two agents associated with porcine respiratory disease complex in finisher pigs in three production systems in Spain
* Blood samples were collected from the same 12 animals on Day 0 (first observation of dyspnea, coughing, anorexia, and fever) and Day 21. Day 0 samples were tested for PRRSV by reverse-transcriptase PCR. Day 0, and Day 21 samples were tested for PRRSV antibodies by ELISA (HerdChek PRRS 2XR; Idexx Laboratories, Barcelona, Spain), defining a positive result as sample:positive ratio (S:P) > 0.4. Seroconversion was defined as an S:P in the Day 21 sample that was at least three times that of the Day 0 sample. † PMWS was diagnosed using fresh specimens and internationally accepted criteria19 for clinical signs and histopathology lesions, and porcine circovirus type 2 was detected by in situ hybridization. PRRSV = porcine reproductive and respiratory syndrome virus; PCR = polymerase chain reaction; PMWS = postweaning multisystemic wasting syndrome; ND = not done. |
|||||||||||||||||||||
Table 2: Beginning and ending dates for periods when parameters analyzed using statistical process control methods were clearly different in two production systems with endemic PRDC treated by administration of an immunomodulator*
* The Unstable time period corresponds to an outbreak of PRDC
(epidemic phase of PRRS, PCV2, or both), when the highest levels of
mortality and percent culls, and worst production parameters, were
observed, and treatment with antimicrobials began. The Stable
period was associated with the endemic phase of PRRS, PCV2, or
both. During the third period (Stable with immunomodulator), 4
weeks before until 4 weeks after the predicted date of a PRDC
outbreak, the immunomodulator Inmunicin Maymo (Maymo Laboratories
SA, Barcelona, Spain) was administered (2 kg per tonne of
feed). |
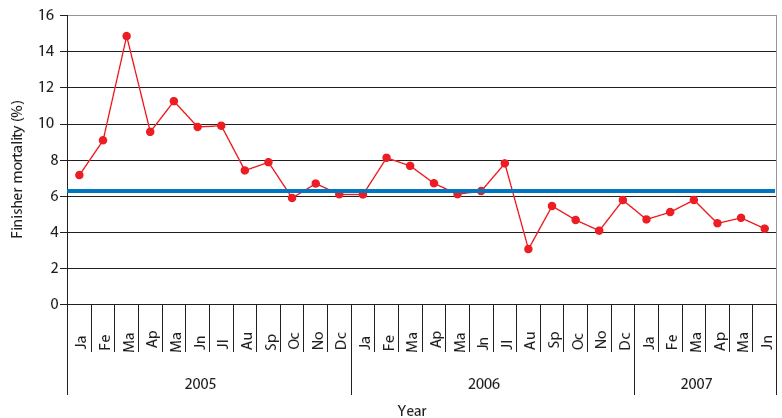
| Figure 2: Finisher mortality in Production System One.
Each monthly average represents closeouts of 12 finisher barns
(approximately 1000 pigs per barn). Unstable, Stable, and Stable
with immunolmodulator periods described and defined in Table 2. The
blue line represents the average value for finisher mortality in
pigs in Spain (J. Font, SIP Consultors, oral
communication, 2007).
|
Mortality, percent culls, and production parameters
The mean values of the studied parameters in Systems One and Two are represented in the XmedianR charts (Figures 2, 3, 4, and 5). This chart was chosen because the mean values for the studied parameters were normally distributed (NCSS 2004 and PASS 2005 software). From these mean values, in both production systems, three periods could be clearly defined: Unstable, Stable, and Stable with immunomodulator. The dates of the beginning and end of each period are shown in Table 2. Highest mortality and percent culls and worst production parameters were observed during the first period (Unstable) in both production systems, which corresponded with the epidemic phase of PRRS, PMWS, or both in each production system. In both systems, the outbreak of PRDC was first noticed during this period, and treatment with antimicrobials began. It was not possible to calculate chart limit values during the Unstable period, because SPC may be applied only in a stable situation.26 During the following period (Stable), all studied parameters improved.
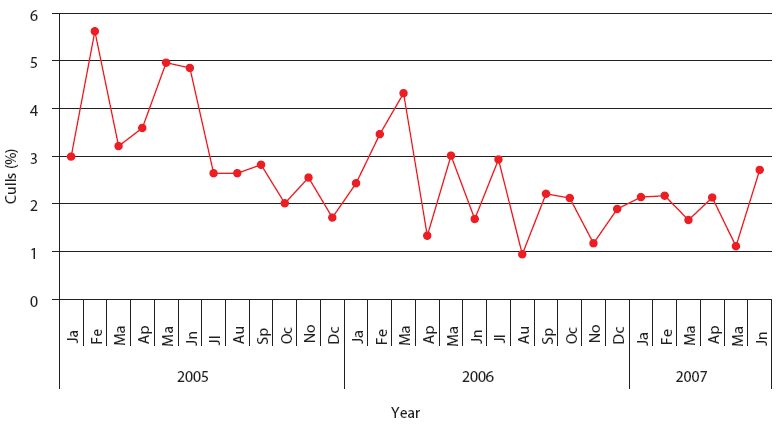
| Figure 3: Percent culls in Production System One
(described in Figure 1). Each monthly average represents closeouts
of 12 finisher barns (12,000 pigs). Unstable, Stable, and Stable
with immunomodulator periods are described in Figure 2.
|
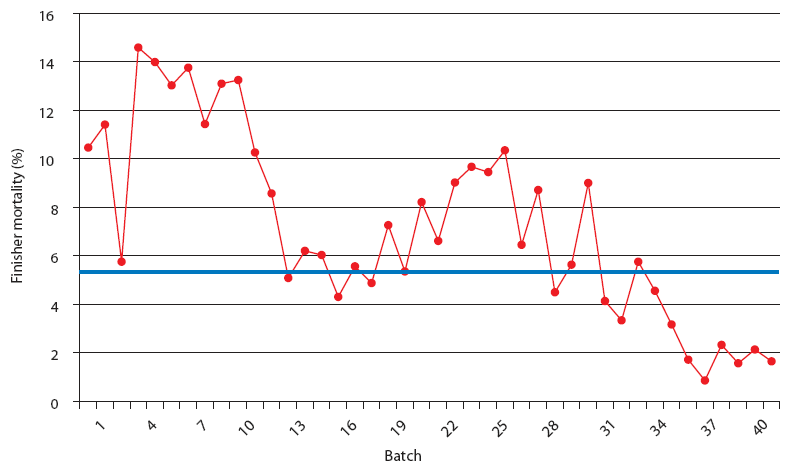
| Figure 4: Finisher mortality in Production System Two, a
1000-sow multi-site system working in a 3-week batch system. Each
closeout is from one finisher barn (1000 to 1500 pigs per barn).
The Unstable (data from 21 barns), Stable (data from 8 barns), and
Stable with immunomodulator (data from 14 barns) periods are
described in Figure 2. The blue line represents the average value
for finisher mortality in pigs in Spain (J. Font, SIP Consultors,
oral communication, 2007).
|
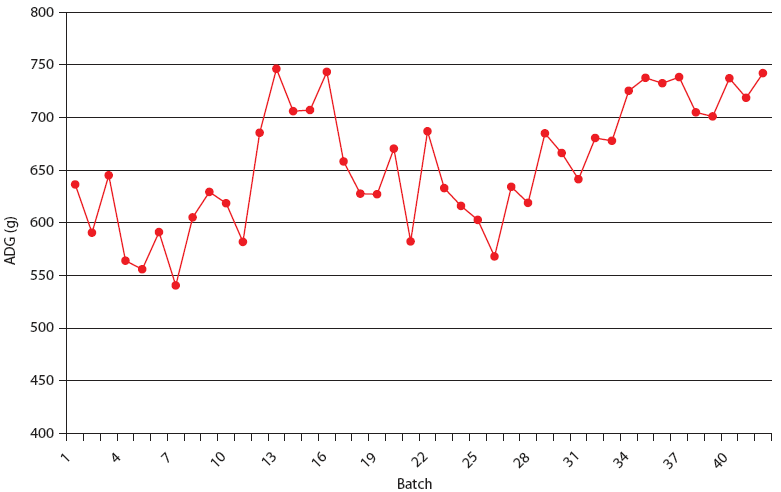
| Figure 5: Average daily gain (ADG) in Production System
Two. The Unstable (data from 21 barns), Stable (data from 8 barns),
and Stable with immunomodulator (data from 14 barns) periods are
described in Table 2. Each value is calculated from closeouts of
each finisher barn (1000 to 1500 pigs per barn).
|
This stable phase was associated with the endemic phase of PRRS, PMWS, or both, but production parameters were always inferior to those accepted as average in Spain (J. Font, SIP consultors [www.sipconsultors.com], oral communication, 2007). During the stable period, natural variation inherent in a process is expected to occur according to the underlying statistical distribution. Production parameters in both production systems were best during the period when the immunomodulator was administered (Figures 2, 3, 4, and 5). Moreover, in both production systems, according to SPC criteria, the changes in the system were statistically significant for all parameters except feed efficiency (Table 3).
Table 3: Chart limits for two production systems in Spain* calculated applying statistical process control
* Production System One (described in Figure 1) and Production System Two in which endemic porcine respiratory disease complex was treated by administration of an immunomodulator. In Production System Two, nurseries were single-origin by site and managed all-in, all-out, and finishing units were filled from a single nursery and managed all-in, all-out by building. Average daily gain (ADG) for System One and percent culls for System Two were not analyzed because of missing values. † Process control periods described in Figure 2. ‡ Percent culls = (number of culls at closeout ÷ number of pigs that entered the finisher) × 100. § Feed efficiency at barn level = feed consumption during the finishing period ÷ (final weight of all pigs at closeout – initial weight of all pigs that entered the finisher). ¶ Mortality = (number of dead pigs at closeout ÷ number of pigs that entered the finisher) × 100. ** Average daily gain (ADG) = (final weight of all pigs at closeout -- initial weight of all pigs that entered the finisher) ÷ length of the finishing period. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
System Three experienced high mortality in the finisher during 2006 because of PRRS outbreaks in some sow farms. The immunomodulator was administered to pigs from 9 to 17 weeks of age in some batches, and other batches were not treated. Lower mortality and better production parameters were observed in the group treated with the immunomodulator (Table 4). These differences were statistically significant (P < .05) for all studied parameters except feed efficiency.
Table 4: Means (± standard deviation) for ADG, feed efficiency, and mortality in Production System Three for batches of finishers either treated with an immunomodulator* or not treated (Controls)
* Inmunicin Maymo (Maymo Laboratories SA, Barcelona, Spain) administered in feed to pigs 9 to 17 weeks of age in some finisher batches beginning in June 2006. Control and treated batches originating from the same sow herd included closeouts of 28,252 and 12,902 pigs from 10 and four finisher farms, respectively. † Variables compared using one-way ANOVA. ‡ Average daily gain (ADG) = (final weight of all pigs at closeout – initial weight of all pigs that entered the finisher) ÷ length of the finishing period. § Feed efficiency at barn level = feed consumption during the finishing period ÷ (final weight of all pigs at closeout – initial weight of all pigs that entered the finisher). ¶ Mortality = (number of dead pigs at closeout ÷ number of pigs that entered the finisher) × 100. |
Discussion
The objective of immunomodulation in food-producing animals is to control an immune response for the benefit of the animal and for production efficiency. Substances that exert this control are called immunomodulators.5 Broad categories of immunomodulators include cytokines, pharmaceuticals, microbial products, nutraceuticals, and traditional medicinal plants. Many categories of immunomodulators have been investigated in food-producing animals, but only a few have been licensed for use in food animals by regulatory authorities, not only in the United States, but also in Europe. Many authorized products were licensed after clinical studies demonstrated efficacy of the products by measuring improvements in clinical or production parameters or both.27 In the three production systems described in this study, growth-production parameters, mortality, and percent culls were examined to assess whether a phytosterol mixture administered in the feed could aid in control of endemic PRDC under field conditions.
Formal studies are designed to determine the efficacy of a product to treat a disease or disease complex. These studies are usually performed using a small number of animals under experimental conditions. Extrapolation of results to practical situations has been extensively discussed.28,29 Using a formal study with concurrent control and treated groups, Pearson et al10 showed that low-dose dietary supplementation with ginseng (a traditional medicinal plant) may be a useful adjunct to vaccination against equid herpesvirus 1 in horses. A simpler approach than a formal trial may be performed under field conditions. For example, it is possible to compare the performance of a process by using statistical process control to examine data collected before and after a change has been introduced. This tool has been widely used in pig production to assess the efficacy of vaccine protocols and feed additives under field conditions, where formal studies (using concurrent control and treated groups) were not suitable.30-32
Immunomodulators licenced in Europe for use in swine are usually administered by the parenteral route, either alone or combined with vaccines.33-35 However, when the objective is to administer a product to a large population, the oral route is much more practical. For this reason, it is very easy to understand that nutraceuticals are the fastest growing category of immunomodulators.5 A nutraceutical is a food that provides medical or health benefits, including prevention or treatment of disease.36 The oral route has been used to administer immunomodulators to fish. For example, Kumari and Sahoo8 showed that the introduction of β-1,3 glucan, levamisole, lactoferrin, and vitamin C (pharmaceutical and nutraceutical immunomodulators) into the diet of fish grown in farms under immunosuppressive or stressful conditions enhances protection against infection and offers economic benefits.
The mechanisms of action of phytosterols in swine remain elusive. Few reports in the literature describe the mechanisms of action of immunomodulators. Schierack et al11 showed that feed supplementation with the probiotic Bacillus cereus var toyoi (a microbial product) improved the outcome of vaccination against Mycoplasma hyopneumoniae and influenza virus by modulating the composition and activities of blood immune cells in treated piglets. Data reported by Yuk et al37 suggest that beta-sitosterol, the main component of Inmunicin Maymo, may be a potential therapeutic molecule in asthma because in this respiratory disease, Th1/Th2 balance is switched towards Th2 (antibody production).38 Beta-sitosterol seems to target specific T-helper lymphocytes, increasing Th1 activity and resulting in improved T-lymphocyte and natural killer cell activity (cellular immunity).17 Recently, Lee et al39 showed that daucosterol, a beta-sitosterol glycoside, has an immunomodulating activity that mediates induction of Th1-dominant cytokine production from activated CD4+ T-cells. This Th-1 response is involved in protection of mice against disseminated candidiasis. In this disease, the dominance of Th2 responses correlates with severity of the fungal infection, and Th1-type dominance can reduce severity.39 Unpublished data from our laboratory agree with these results, showing that beta-sitosterol treatment enhanced immune responses in pigs. Lymphocyte function, assessed as ability to proliferate in the presence of different concentrations of phytohemagglutinin (PHA), was measured in porcine blood mononuclear cells 2 days after vaccination with an MLV PRRS vaccine. Surprisingly, PRRS MLV vaccination induced a decrease in PHA proliferation responses during the first 2 days after vaccination in animals fed a standard diet. In contrast, when treatment with Inmunicin Maymo was administered in the diet, PHA proliferation responses were normal. In addition, when IL-6 levels were measured to evaluate tissue damage during the acquired phase of the immune response, 33 days post administration of the PRRS MLV vaccine, levels were generally lower in pigs fed phytosterols than in pigs fed a standard diet. These results suggest that immunomodulation was apparent not only 2 days after vaccination with a PRRS MLV (innate phase of the immune response), but also during the acquired phase of the immune response such that these responses might aid in control of infectious diseases that contribute to PRDC.22
In this study, lower finisher mortality and percent culls and the best production parameters were observed in all three production systems when the inmunomodulator was applied. These system changes were statistically significant for all parameters except feed efficiency, according to SPC criteria for Systems One and Two and ANOVA criteria for System Three. Feed efficiency depends on feed consumption and weight gain during a period of time. The mortality observed in Systems One and Two occurred during the first month of the finisher phase, so the impact on feed efficiency might be minimal, as observed in this case.
It can be argued that the observed improvement in most of the studied parameters during the third period in Systems One and Two is a direct consequence of the natural evolution of the PRRSV and PCV2 outbreak, with development of herd level active immunity against these agents,19,40 and not a result of treatment with the immunomodulator. A similar argument could be used for Production System Three, because each finisher batch was not divided into immunomodulator-treated and control groups, although consecutive batches were divided into treated and untreated groups. Thus, it is not clear whether treatment with the immunomodulator was linked to the better production measures observed in the three studied pig-production systems, because there were no controls within each batch. Nevertheless, similar results were observed in three different production systems belonging to two different pig-production companies, involving a large number of animals in a long follow-up study. It is unlikely that these results are explained by “natural evolution” or chance in all three cases. Therefore, the observed enhancement of production values was most probably linked with the use of the immunomodulator.
Implications
- Statistical process control may be used to assess the efficacy of products in pig production when formal studies are not feasible.
- Phytosterols are immunomodulators that may reduce the negative impact of PRDC under field conditions.
Acknowledgements
This work was partly funded by one project of Laboratorios Maymo SA and by Spanish Ministry of Education and Science, project AGL2005-07073-C02-02.
References
1. Dee SA. The PRDC: Are subpopulations important? Swine Health Prod. 1996;4:147–149.
2. Christensen G, Sorensen V, Mousing J. Diseases of the respiratory system. In: Straw BE, D`Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:913–941.
3. Thacker EL. Lung inflammatory responses. Vet Res. 2006;37:469–486.
4. Henry SC, Apley MD. Therapeutics. In: Straw BE, D`Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:1155–1163.
5. Blecha F. Immunomodulators for prevention and treatment of infectious diseases in food-producing animals. Vet Clin North Am Food Anim Pract. 2001;17:621–623.
6. Cook ME. Nutritional effects on vaccination. Adv Vet Med. 1999;41:53–59.
7. Eicher SD, Mckee CA, Carroll JA, Pajor EA. Supplemental vitamin C and yeast cell wall β-glucan as growth enhancers in newborn pigs and as immunomodulators after an endotoxin challenge after weaning. J Anim Sci. 2006;84:2352–2360.
8. Kumari J, Sahoo PK. Dietary immunostimulants influence specific immune response and resistance of healthy and immunocompromised Asian catfish Clarias batrachus to Aeromonas hydrophila infection. Dis Aquat Organ. 2006;70:63–70.
9. Selvaraj V, Sampath K, Sekar V. Adjuvant and immunostimulatory effects of β-glucan administration in combination with lipopolysaccharide enhances survival and some immune parameters in carp challenged with Aeromonas hydrophila. Vet Immunol Immunopathol. 2006;114:15–24.
10. Pearson W, Omar S, Clarke AF. Low-dose ginseng (Panax quinquefolium) modulates the course and magnitude of the antibody response to vaccination against equid herpesvirus 1 in horses. Can J Vet Res. 2007;71:213–217.
11. Schierack P, Wieler LH, Taras D, Herwig V, Tachu B, Hlinak A, Schimdt MFG, Scharek L. Bacillus cereus var. toyoi enhanced systemic immune response in piglets. Vet Immunol Immunopathol. 2007;118:1–11.
12. Shan T, Wang Y, Wang J, Liu J, Xu Z. Effect of lactoferrin on the immune functions and serum iron level of weanling piglets. J Anim Sci. 2007;85:2140–2146.
13. Seventh Framework Programme for research and technical development. 2007. Available at http://cordis.europa.eu/en/home.html. Accessed 13 March 2008.
14. Bouic PJD. The role of phytosterols and phytosterolins in immune modulation: a review of the past 10 years. Curr Opin Clin Nutr Metab Care. 2001;4:471–475.
*15. Lamprecht JH. A comparison of the survival benefit provided by putative immune modulators in the FIV (feline immunodeficiency virus) infected laboratory cat model. Proc 13th Int AIDS Conf. Durban, South Africa. 2000;21–24.
16. Breytenbach U, Clark A, Lamprecht J, Bouic P. Flow cytometric analysis of the Th1-Th2 balance in healthy individuals and patients infected with the human immunodeficiency virus (HIV) receiving a plant sterol/sterolin mixture. Cell Biol Int. 2001;25:43–49.
*17. Bouic PJD. Sterols and sterolins: new drugs for the immune system? Drug Discovery Today. 2002;7:775–778.
18. Darwich L, Segales J, Mateu E. Pathogenesis of postweaning multisystemic wasting syndrome caused by porcine circovirus 2: An immune riddle. Arch Virol. 2004;149:857–874.
19. Segales J, Allan GM, Domingo M. Porcine circovirus diseases. Anim Health Res Rev. 2005;6:119–142.
20. Mateu E, Diaz I. The challenge of PRRS immunology. Vet J. 2008;177:345–351.
21. Thacker EL. Immunology of the porcine respiratory disease complex. Vet Clin North Am Food Anim Pract. 2001;17:551–565.
*22. Fraile L, Gimeno M, Crisci E, Diaz I, Mateu E, Montoya M. Immunomodulation in PRRS MLV vaccination. Proc 5th Int Symp Emerg Re-emerg Pig Dis. Krakow, Poland. 2007;159.
23. Mateu E, Martín M, Vidal D. Genetic diversity and phylogenetic analysis of glycoprotein 5 of European-type porcine reproductive and respiratory virus strains in Spain. J Gen Virol. 2003;84:529–534.
24. Rosell C, Segalés J, Plana-Durán J, Balasch M, Rodríguez-Arrioja GM, Kennedy S, Allan GM, McNeilly F, Latimer KS, Domingo M. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J Comp Pathol. 1999;120:59–78.
25. Reneau JK, Lukas J. Using statistical process control methods to improve herd performance. Vet Clin North Am Food Anim Pract. 2006;22:171–193.
26. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458–464.
27. European Food Safety Authority. Feed Additives Application. Available at: http://www.efsa.europa.eu/EFSA/ScientificPanels/FEEDAP/efsa_locale-1178620753812_FeedAdditivesApplications.htm. Accessed 13 March 2008.
*28. Kendall D. Evaluation and implementation of new products in a large production system. Proc 25th Ann Feed Ingredient Conf. Cave City, Kentucky. 2005;6.
*29. Boyd D, Mellencamp MA, Donavan T. Structured process for evaluating new products for production systems: Animal science perspective. Allen D. Leman Swine Conf. St Paul, Minnesota. 2007;75–78.
*30. Campbell J, Donavan T, Boyd D, Rousell L, Crenshaw J. Use of statistical process control analysis to evaluate the effects of spray-dried plasma in gestation and lactation feed on sow productivity in a PRRS-unstable farm. Proc AASV. Kansas City, Missouri. 2006;139–142.
*31. Maala CUM, Bulay AC, Lising RT, Ballesteros CB. A case of PRRS and PCV2 control in the Philippines. Proc 19th IPVS Cong. Copenhagen, Denmark. 2006;2.
*32. Diaz EF, Chevez JC. Evaluation of a protocol to control Lawsonia intracelllularis using live vaccine in farm in Mexico. Proc 19th IPVS Cong. Copenhagen, Denmark. 2006;198.
33. Kyriakis SC, Alexopoulos C, Giannakopoulos K, Tsinas AC, Saoulidis K, Kritas SK, Tsiloyiannis V. Effect of a paramunity inducer on reproductive performance of gilts. Zentralblatt fur Veterinarmedizin Rejhe A. 1996;43:483–487.
34. Kyriakis SC, Tzika ED, Lyras DN, Tsinas AC, Saoulidis K, Sarris K. Effect of an inactivated Parapoxvirus based immunomodulator (Baypamun) on post weaning diarrhoea syndrome and wasting pig syndrome of piglets. Res Vet Sci. 1998;64:187–190.
35. Fachinger V, Schlapp T, Strube W, Schmeer N, Saalmüller A. Poxvirus-induced immunostimulating effects on porcine leukocytes. J Virol. 2000;74:7943–7951.
36. Hardy G. Nutraceuticals and functional foods: Introduction and meaning. Nutrition. 2000;16:688–689.
37. Yuk JE, Woo JS, Yun CY, Lee JS, Kim JH, Song GY, Yang EJ, Hur IK, Kim IS. Effects of lactose-beta-sitosterol and beta-sitosterol on ovalbumin-induced lung inflammation in actively sensitized mice. Int Immunopharmacol. 2007;7:1517–1527.
38. Kay AB. Allergy and allergic diseases – First of two parts. N Engl J Med. 2001;344:30–37.
39. Lee JH, Lee JY, Park JH, Lee JS, Jung HS, Kim JS, Kang SS, Kim YS, Han Y. Immunoregulatory activity by daucosterol, a β-sitosterol glycoside, induces protective Th1 immune response against disseminated candidiasis in mice. Vaccine. 2007;25:3834–3840.
40. Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green AL, Zimmerman JJ. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. JAVMA. 2005;227:385–392.
*Non-refereed references.