| Case report | Peer reviewed |
Cite as: García A, Ayuso D, Benítez JM, et al. Clostridium novyi infection causing sow mortality in an Iberian pig herd raised in an outdoor rearing system in Spain. J Swine Health Prod. 2009;17(5):264–268.
Also available as a PDF.
SummaryClostridium novyi was the suspected cause of death of two mature gestating Iberian-breed sows, on evidence of a gas-filled necrotic liver, rapid decomposition and tympany of the carcasses, and the absence of any other detectable cause of death. Anaerobic cultures yielded large numbers of Clostridium-like organisms, and C novyi type B was identified using a multiplex polymerase chain reaction (PCR) assay. In cases of unexpected mortality in gestating sows, veterinarians need to be aware of the most common causes of death, including C novyi infection. In order to achieve a correct diagnosis, it is essential to perform a postmortem examination and collect samples as soon as possible after death. In addition, use of PCR procedures may allow rapid identification of C novyi and the types implicated. | ResumenEl Clostridium novyi fue la causa sospechada de muerte de dos hembras gestantes de raza Ibérica, basándose en la evidencia de hígado necrótico lleno de gas, una rápida descomposición y timpanismo de las canales, así como la ausencia de otra causa detectable de muerte. Los cultivos anaeróbicos rindieron grandes cantidades de organismos similares a Clostridium, y se identificó el C novyi tipo B utilizando una prueba multiplex de reacción en cadena de la polimerasa (PCR por sus siglas en inglés). En casos de mortalidad inesperada en hembras gestantes, los veterinarios deben estar conscientes de las causas más comunes de muerte, incluyendo la infección por C novyi. Para lograr un diagnóstico correcto, es esencial realizar un examen post mortem y colectar muestras después de la muerte tan pronto como sea posible. Además, el uso de la prueba de PCR puede permitir la rápida identificación del C novyi y los serotipos implicados. | ResuméClostridium novyi était la cause suspectée de la mort de deux truies matures en gestation de race ibérique, sur la base d’un foie nécrotique empli de gaz, d’une décomposition rapide et de tympanisme des carcasses, et de l’absence d’autres causes détectables de mortalité. Des cultures anaérobiques ont permis la croissance d’un grand nombre de micro-organismes apparentés à des clostridies, et C novyi type B a été identifié à l’aide d’une réaction d’amplification en chaîne par la polymérase (PCR) multiplex. Dans les cas de mortalité soudaine chez des truies en gestation, les vétérinaires doivent être au fait des causes les plus courantes de la mort, incluant l’infection par C novyi. Afin d’arriver à un diagnostic correct, il est essentiel d’effectuer un examen post-mortem et de prélever des échantillons aussitôt que possible après le décès. De plus, l’utilisation de méthodes PCR pourrait permettre une identification rapide de C novyi et des types impliqués. |
Keywords: swine, Clostridium novyi, Iberian breed pig, sudden death
Search the AASV web site
for pages with similar keywords.
Received: April 6, 2009
Accepted: May 28, 2009
Sow culling and mortality are among the most important determinants of financial well-being in pig-breeding units.1 Financial losses associated with high sow mortality include the value of lost sows and pigs, the cost of early female replacement, and depletion of sow-herd quality as culling is less intentional.2,3
The Iberian pig is a unique autochthonous breed, perfectly adapted to the Mediterranean natural ecosystem in the southwest of the Iberian Peninsula. Traditionally, they are reared outdoors in long production cycles (12 to 18 months), with the finishing pigs grown by making use of natural resources, mainly acorns from evergreen oaks (Quercus ilex and Quercus rotundifolia) and pasture.4 At least 1 hectare (2.47 acres) of healthy oak-wooded meadow is needed to raise a single pig (extensive production system).
Nowadays, the Iberian pig breed is popular because its meat and meat products have very little in common with those obtained from selected pigs raised under intensive conditions. The high acceptance of these products in the Spanish market has allowed the flourishing of a niche market of increasing importance and very high profits.
Case description
Two mature gestating sows died unexpectedly in mid-January in an Iberian pig-breeding unit during their confinement in farrowing crates. At present, on many Iberian pig farms, traditional outdoor sow gestation is followed by indoor farrowing in modern premises (semi-extensive management). These buildings are equipped with slatted flooring and natural ventilation. The case farm had 160 sows fed a commercial feed and with access to the natural meadowland resources of pasture and acorns. Sows farrowed twice a year and pregnant sows were confined in farrowing crates 1 week before giving birth.
Although postmortem examinations were performed only 2 or 3 hours after the death of the sows and ambient temperature was approximately 7°C, the carcasses were grossly distended and there was purple discoloration of the skin. Necropsy findings revealed generalized subcutaneous edema, a foul odor when the carcass was opened, enlarged and congested lymph nodes, bloodstained fluid in pleural, pericardial, and peritoneal cavities, serosal hemorrhages, and enlarged spleen. In each case, the stomach was full, the lungs were congested, and the liver was enlarged, friable, and dark, with gas bubbles uniformly infiltrated, thereby presenting a spongy appearance on the cut surface (Figure 1).
Figure 1: The abdominal cavity of a mature gestating Iberian breed sow at necropsy. A: Full stomach and gas bubbles in the liver; B: The liver uniformly infiltrated with gas bubbles, presenting a spongy appearance on the cut surface, probably the most distinguishing feature of sudden death in sows caused by Clostridium novyi. 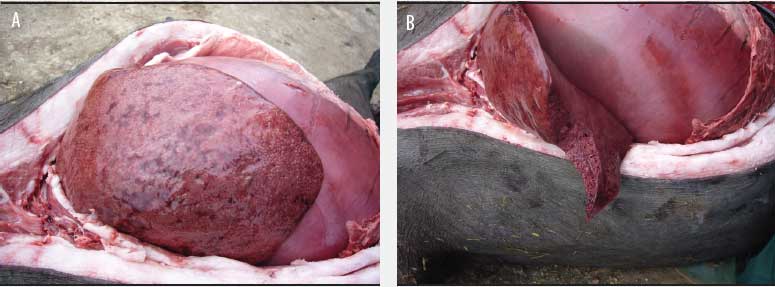 |
Necropsy samples were submitted to the School of Veterinary Sciences at the University of Extremadura (Cáceres, Spain) for examination of smears, culture, histopathology examination, and fecal flotation and sedimentation assays.
Histopathological examination of the liver of one sow revealed intrahepatic spherical non-staining cavities (gas bubbles) (Figure 2) and moderate multifocal lymphohistocytic hepatitis with hepatocellular degeneration and necrosis.
Figure 2: Histopathology section of the liver of an Iberian sow stained with hematoxilin and eosin showing hepatocellular degeneration and intrahepatic spherical non-staining cavities (gas bubbles) associated with Clostridium novyi infection (magnification ×100). 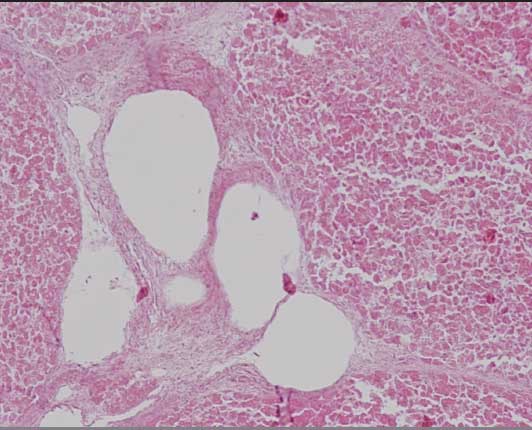 |
Large numbers of gram-positive rods were observed in Gram-stained smears from the heart, lungs, kidneys, spleen, and liver of each sow (Figure 3). Anaerobic cultures yielded large number of Clostridium-like organisms. To make a rapid identification of pathogenic clostridia, the multiplex polymerase chain reaction (PCR) procedure described by Sasaki et al5 was performed. A BLAST homology search (program available at www.ncbi.nlm.nih.gov/BLAST) revealed that the 427-bp nucleotide sequence of the amplified product (Figure 4) matched the partial flagellin (fliC) gene of Clostridium novyi type B ATCC 25758 (DDBJ accession no. AB058936) (Figure 5).
Figure 3: Smears from the liver of an Iberian sow that died of Clostridium novyi infection, showing large gram-positive rods with oval to cylindrical subterminal spores (magnification ×1000). 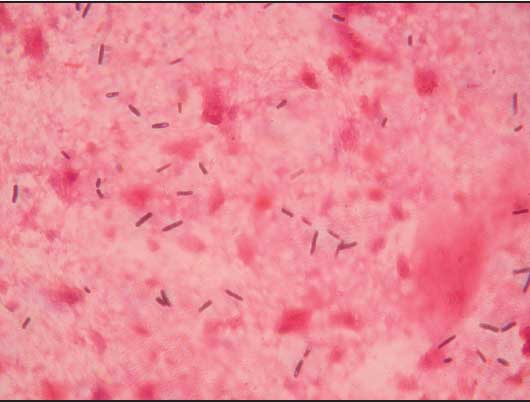 |
Figure 4: Gel electrophoresis (2% agarose gel stained with ethidium bromide) showing a species-specific 427-pb band identifying Clostridium novyi type B. The band was amplified using the multiplex polymerase chain reaction system described by Sasaki et al.5 Lanes 1 and 6: molecular weight marker ladder 1 kb (Bioline GmbH, Luckenwalde, Germany); lanes 2 to 5, PCR amplification products from duplicate positive cultures from the livers of two Iberian sows that died of C novyi infection. 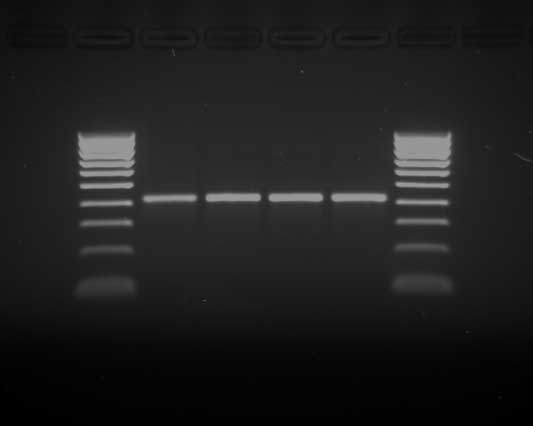 |
Figure 5: The amplification product obtained from the multiplex PCR in Figure 4 was sequenced. A BLAST homology search (program available at www.ncbi.nlm.nih.gov/BLAST) showed that the nucleotide sequence matched the partial flagellin (fliC) gene of Clostridium novyi type B ATCC 25758.  |
Discussion
Clostridium novyi is an anaerobic, spore-forming, gram-positive rod that varies in size.6 The organism produces highly potent exotoxins (A to D)7 of which the lethal, necrotizing alpha toxin is considered to be the principal toxin of the type B strain in pigs.8 This toxin causes necrosis, increases permeability of the cell barrier, and disrupts intercellular junctions.6,9,10
Clostridium novyi is the causative agent responsible for gas gangrene in humans and infectious necrotic hepatitis (black disease) in sheep, cattle, goats, and horses.7 Both C novyi types A and B have been isolated from reported cases of sudden death in sows.7,8
Although C novyi infections are unusual in pigs,8 cases of sudden death in sows have been reported in intensive swine-breeding units in Europe7,11 and in outdoor pig units in eastern Europe.12,13 Nevertheless, to our knowledge, mortality of sows caused by C novyi has not been reported in Iberian pigs reared under extensive or semi-extensive conditions, an important livestock subsector in the southwest of the Iberian Peninsula.
The pathogenesis of C novyi sudden death in sows has not been elucidated. Clostridium novyi is a normal inhabitant of the large intestine and liver in pigs, although the route by which the organism reaches the liver has not been documented. If disease develops, spores in the liver become vegetative and produce potent exotoxins, responsible for the severe necrotizing and edematous tissue damage.7 In sheep with infectious necrotic hepatitis, previous damage to the liver parenchyma, usually by migrating liver flukes, is required for proliferation of C novyi.14 In this case, we did not find lesions of parasitic or larval migration in the liver, and the fecal flotation for intestinal nematodes was negative for each sow. However, an outbreak of swine dysentery had occurred in the herd. Some studies have reported that other concomitant low-grade infectious processes (eg, metritis, cystitis, enteritis) may predispose sows to proliferation of C novyi in the liver.7 Furthermore, the peripartum period is a particularly stressful stage; much of the sow mortality associated with C novyi appears to occur at or near the critical farrowing period. In our opinion, general functional changes in the sow’s immune system during gestation may also play an important role in occurrence of C novyi hepatopathy.
Diagnosis of C novyi infection in swine is difficult, since suspect cases are usually found dead, and the interval between death and necropsy introduces the possibility of postmortem invasion. Demonstration of C novyi in the liver of a sow dead for > 24 hours does not alone constitute sufficient evidence of infectious necrotic hepatitis or clostridial hepatopathy.7.15 It is then essential to perform a postmortem examination and collect samples as soon as possible after death. Difficulties in determining whether necropsy findings constitute postmortem degeneration, particularly in the summer, have probably caused under-reporting of this cause of sow mortality.7
In one study, fluorescent antibody (FA) tests on liver smears were more sensitive than culture for identification of C novyi.7 However, the frequency of false-positive FA tests results increased with the interval between death and necropsy.8 Furthermore, the FA test can be confounded by cross-reactions between C novyi and Clostridium botulinum.16 Culture for C novyi must be performed in a specialized laboratory, as not all laboratories are skilled in its isolation. Clostridium novyi type B has very demanding anaerobic and nutritional requirements; thus, a negative culture may not necessarily rule out C novyi infection.13
Although great care must be taken in interpreting bacteriological findings, identification of clostridial organisms, along with the history of sudden death, rapid postmortem decomposition of internal organs, and the presence of gas bubbles in the liver, suggests death due to clostridial infection. Probably the most distinguishing feature of sudden sow mortality due to C novyi is an enlarged, friable, gas-filled liver, with liver lobes filled with pockets of gas that produce a honeycomb-like appearance (similar to a chocolate bar filled with air bubbles).7,8
Disease caused by C novyi can be controlled by reducing the incidence of pneumonia, metritis, and enteritis in affected groups of pigs. Several studies have reported the use of zinc bacitracin to reduce mortality, and disposal of carcasses by incineration or deep burial may reduce the contamination of the environment by spores.6,17,18 Prevention may also be achievable by the use of bacterin-toxoids or toxoids, and second-generation vaccines may be based upon native or recombinant alpha19 or beta toxoids. In contrast, vaccination of sows at risk with a multivalent clostridial vaccine does not seem to be an effective control measure.11,17
Implications
- Clostridium novyi infection is a common cause of death in gestating sows.
- If carcasses are not examined soon after death and depending on weather and other postmortem conditions, it may not be possible to reach a diagnosis of C novyi infection.
- A timely postmortem examination and sample collection, along with microbial isolation and the use of PCR procedures, may allow a correct diagnosis of C novyi infection in swine.
Acknowledgments
This work is part of the Research project PRI08A062 supported by the Regional Government Junta de Extremadura and the European Social Fund.
References
1. Bilkei G, Bölcskei A. Production related culling strategy in a large pig production unit. Pig J. 1995;35:140–149.
*2. Sanz M, Roberts J, Almond G, Alvarez R, Donovan T, Perfumo C. What we see with sow mortality. Proc AD Leman Swine Conf. St Paul, Minnesota. 2002;181–184.
*3. Deen J, Xue J. Sow mortality in the US: An industry-wide perspective. Proc AD Leman Swine Conf. St Paul, Minnesota. 1999;26:91–94.
4. López-Bote C. Sustained utilization of the Iberian pig breed. Meat Sci. 1998;49:17–27.
5. Sasaki Y, Kojima A, Aoki H, Ogikubo T, Takikawa N, Tamura Y. Phylogenetic analysis and PCR detection of Clostridium chauvoei, Clostridium haemolyticum, Clostridium novyi types A and B, and Clostridium septicum based on the flagellin gene. Vet Microbiol. 2002;86:257–267.
*6. Schultz R, Dau D, Höfling D, Duran O, Carson T, Becton L, Woodward C, Pollard K, Busker K, Kaster D, Steidinger M. A sow mortality study - the real reason sows die. Identifying causes and implementing actions. Proc AASV. Ames, Iowa. 2001;387–395.
7. Duran CO, Walton JR. Clostridium novyi sudden death in sows: Toxaemia or post mortem invader? Pig J. 1997;39:37–53.
8. Taylor DJ. Clostridial infections. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:405–406.
*9. Reeves DE, Harmon B. Clostridium novyi associated mortality in the sow. Proc AASV. Kansas City, Missouri. 2002;153–154.
10. Songer JG. Clostridial diseases of animals. In: Rood JI, McClane BA, Songer JG, Titball RW, eds. The Clostridia: Molecular Biology and Pathogenesis. New York, New York: Academic Press; 1997:153–184.
*11. Walton JR, Duran CO. Sow deaths due to Clostridium novyi infection. Proc IPVS Cong. The Hague, Netherlands. 1992;296.
12. Almond P, Bilkei G. Clostridium novyi caused outdoor sow mortality in Croatia. Berliner und Münchener Tierärtzliche Wochenschrift. 2005;118:296–299.
13. Friendship D, Bilkei G. Mortality caused by Clostridium novyi in outdoor sows in Slovakia. Vet Rec. 2006;158:601.
14. Bagadi HO, Sewell MMH. Experimental studies on infectious necrotic hepatitis (Black Disease) of sheep. Res Vet Sci. 1973;15:53–61.
15. Batty I, Kerry JB, Walker PD. The incidence of Clostridium oedematiens in postmortem material. Vet Rec. 1967;80:32.
16. Friendship CR, Bilkei G. Concurrent swine erysipelas and Clostridium novyi infections associated with sow mortality in outdoor sows in Kenya. Vet J. 2007;173:694–696.
17. Marco E. Sudden deaths in sows. Pig J. 1995;35:157–163.
*18. Kavanaugh NT, Spillane P. Cost benefit studies of zinc bacitracin for control of Clostridium novyi infection in intensively housed sows. Proc IPVS Cong. Birmingham, England. 1998;2:241.
19. Amimoto K, Sasaki O, Isogai M, Kitajima T, Oishi E, Okada N, Yasuhara H. The protective effect of Clostridium novyi type B alpha-toxoid against challenge with spores in guinea pigs. J Vet Med Sci. 1998;60:681.
* Non-refereed references.
