| Original research | Peer reviewed |
SummaryObjective: To characterize the potential of pig-associated Diptera flies to carry Lawsonia intracellularis. Materials and methods: On 15 British farms, invertebrate communities were trap-collected (14 per year), counted, and sorted into species groups. Lawsonia serological tests were conducted and total DNA was extracted from pig feces; DNA was also extracted from adult flies, pupae, and larvae samples and viscera of Blatta species. Each DNA sample was tested for L intracellularis by polymerase chain reaction; positive samples were subtyped via specific variable number tandem repeat analysis. Results: The pig-associated fly community was generally dominated by Musca domestica (house fly; n = 13 farms), but on one farm each, Ophyra species (garbage fly) or Drosophila species (fruit fly) were dominant. Also noted were Muscina stabulans (false stable fly), Stomoxys calcitrans (stable fly), and Eristalis species (hover flies); Blatta orientalis cockroaches were noted on two farms. Lawsonia infections were routinely detected in nursery pigs on 14 farms. On five of 12 Lawsonia-positive farms with Musca-dominant insects, Lawsonia DNA was detected within numerous flies (22% to 75% of fly samples from nursery pens). On two farms, larval forms of Eristalis from pen floors were also Lawsonia-positive. Subtyping indicated that the same Lawsonia isolate occurred within pigs and the pig-associated fly stages (Musca adults and Eristalis larvae). The DNA extracted from cockroach samples, and from other flies, was negative. Implication: Musca and Eristalis flies have the greatest potential to carry and transmit Lawsonia intracellularis due to their pig-associated life cycle stages. | ResumenObjetivo: Caracterizar el potencial de las moscas Díptera asociada a cerdos a ser portadora de la Lawsonia intracellularis. Materiales y métodos: En 15 granjas Británicas, se atraparon comunidades de invertebrados (14 por año), se contaron, y se clasificaron por grupo de especie. Se condujeron pruebas serológicas de Lawsonia y se extrajo el DNA total de las heces de los cerdos; también se extrajo DNA de las moscas adultas, pupas, y larvas y vísceras de la especie Blatta. Se probó cada muestra de DNA en busca de L intracellularis a través de la reacción en cadena de la polimerasa; las muestras positivas se subtipificaron a través del análisis de repetición de variables específicas. Resultados: La comunidad de moscas asociada a cerdos generalmente fue dominada por la Musca domestica (mosca de casa; n = 13 granjas), pero en una granja, la especie Ophyra (mosca de basura) ó la especie Drosophila (mosca de fruta) fueron dominantes. También se encontraron la Muscina stabulans (mosca falsa de establo), Stomoxys calcitrans (mosca de establo), y la especie Eristalis (mosca helicóptero); las cucarachas Blatta orientalis se encontraron en dos granjas. La infección por Lawsonia se detectó rutinariamente en cerdos de destete en 14 granjas. En cinco de 12 granjas positivas a Lawsonia con insectos Musca dominantes, se detectó el DNA de la Lawsonia en numerosas moscas (22% a 75% de muestras de mosca de los corrales de destete). En dos granjas, las larvas de Eristalis de los pisos de los corrales también fueron positivas a Lawsonia. La subtipificación indicó que el mismo aislado de Lawsonia ocurrió en los cerdos y en las etapas de la mosca asociadas a cerdos (Musca adultas y larvas de Eristalis). El DNA extraído de las muestras de cucaracha, y de otras moscas, fue negativo. Implicacione: Las moscas Musca y Eristalis presentan el potencial más grande para ser portadoras y transmitir la Lawsonia intracellularis debido a sus etapas de ciclo de vida asociados a cerdos. | ResuméObjectif: Caractériser le potentiel des mouches du genre Diptera associées au porc au portage de Lawsonia intracellularis. Matériels et méthodes: Sur 15 fermes britanniques, les communautés d’invertébrés ont été amassées au moyen de trappes (14 fois par année), comptées, et triées par groupes d’espèces. Des tests sérologiques pour détecter des anticorps envers Lawsonia ont été effectués et l’ADN total a été extrait des fèces de porc; de l’ADN a également été extrait d’échantillons de mouches adultes, de pupes, et de larves ainsi que de viscères des espèces Blatta. Chaque échantillon d’ADN a été testé pour L intracellularis par réaction d’amplification en chaîne par la polymérase; les échantillons positifs furent sous-typés par analyse du nombre variable des séquences spécifiques répétées en tandem. Résultats: La communauté des mouches associées au porc était généralement dominée par Musca domestica (mouche domestique; n = 13 fermes), mais sur une ferme chaque, les espèces Ophyra (mouche à déchets) ou Drosophila (mouche à fruit) étaient dominantes. On nota également la présence de Muscina stabulans (fausse mouche d’étable), Stomoxys calcitrans (mouche d’étable), et Eristalis (syrphes); des blattes (Blatta orientalis) ont également été observées sur deux fermes. Des infections par Lawsonia ont été détectées de routine chez les porcs en pouponnière sur 14 fermes. Sur cinq des 12 fermes positives pour Lawsonia où les insectes dominants appartenaient au genre Musca, l’ADN de Lawsonia a été détecté chez de nombreuses mouches (22% à 75% des échantillons de mouche provenant des enclos de pouponnière). Sur deux fermes, les formes larvaires d’Eristalis provenant du plancher des parcs étaient également positives pour Lawsonia. Le sous-typage a démontré que le même isolat de Lawsonia était présent parmi les porcs et les différents stades associés au porc (les adultes de Musca et les larves d’Eristalis). L’ADN extrait des échantillons de blattes, et des autres mouches, était négatif. Implication: Les mouches de type Musca et Eristalis possèdent le plus grand potentiel à transporter et transmettre Lawsonia intracellularis étant donné leur stade de développement associé au porc. |
Keywords: swine, proliferative enteropathy, Lawsonia intracellularis, Musca, Eristalis
Search the AASV web site
for pages with similar keywords.
Received: August 24, 2010
Accepted: December 3, 2010
Swine farming sites offer a mixture of concrete pens and other habitats, such as feed storage areas, which can each support invertebrate populations differing from those found in adjacent arable or human-activity locations. The importance of invertebrates in the economics of the pig industry is due to their ability to cause direct damage to animal skin (mainly by biting flies or midges), by acting as possible vectors in the transmission of viral and bacterial diseases, and also by acting as agents of feed spoilage. There are few pig-associated zoonotic arboviruses, except for the Japanese encephalitis flavivirus carried by mosquitoes. Studies of the potential for specific invertebrates (usually Musca flies) to carry and transmit other specific pig-associated microbes, such as Salmonella or porcine reproductive and respiratory syndrome (PRRS) virus, have shown such carriage to be likely in on-farm settings.1,2 However, the role of vectors such as invertebrates in on-farm transmission of Lawsonia intracellularis in new cases of proliferative enteropathy (ileitis) is unknown. Our objective was to characterize the potential of pig-associated Diptera flies and other common farm invertebrates to carry Lawsonia intracellularis in their intestinal tracts.
Only one peer-reviewed publication has documented on-farm carriage of L intracellularis in rodent vectors,3 and to our knowledge, there has been no previous work on invertebrates. Lawsonia intracellularis is an obligately intracellular bacterium infecting a certain range of mammals, particularly pigs.4,5 It normally resides and multiplies free in the cytoplasm of infected intestinal epithelial cells.5,6 Previous studies indicated that L intracellularis isolated from swine consists solely of one “pig” strain,7 but that isolates from different farms could be distinguished by analysis of variable tandem repeat elements in intergenic sequences.8 We therefore specifically aimed to establish which (if any) on-farm invertebrates were the most active carriers of L intracellularis and whether isolates carried by these potential vectors matched those carried in adjacent pigs.
Materials and methods
Study locations, invertebrate collections, and population analysis
There were 1250 to 1300 separate swine farming sites in England in 2007-2008; a set of 15 study sites (1.2% of possible farms, labeled Farm 1 to Farm 15) was constructed to represent a uniform geographical spread and examples of each of single-site breeder-to-finisher farms, breeder-to-weaner farms, and weaner-to-finisher units. These English farm study sites were considered typical, with a small-medium size (50 to 1000 sows), buildings > 20 years old, weaning at 24 days of age, infrequent antimicrobial usage, and frequent use of straw bedding materials. Invertebrate trapping was conducted within each study site throughout the period April to November in 2007 and 2008. Within-farm trap locations at each site included feed storage areas, weaner and finisher pig areas, and breeder pig areas (where present). The most productive collection methods were aerial sticky traps and walk-in floor traps. Trap transects were placed in numbers (eight per farm location), periods (2 weeks), and locations (farrowing, nursery, and finisher) that remained constant on each farm throughout the study. The number and identity of all invertebrates on these entire trap devices was annotated via standard taxonomic methods. In brief, textbook and other source keys were followed for genus and species identification of Diptera and other invertebrates by gross and microscopic anatomical features. Because the traps remained constant, a farm population comparison was calculated over time and between study sites.
Pig and invertebrate sample processing
Fresh blood and fecal samples were collected from cross-sectional samples of 10 pigs from each age group (breeder, weaner, finisher) on each farm at monthly intervals during the study. Serum from each blood sample was incorporated into an indirect immunofluorescence assay for Lawsonia-specific IgG antibodies, as described previously.9 The DNA was extracted from each fecal sample by routine commercial kit methods (QIAamp DNA Stool kit; Qiagen, Valencia, California). Each DNA sample was then analyzed by polymerase chain reaction (PCR) incorporating oligo-primers specific for Lawsonia intracellularis DNA, as described previously.10 Known positive and negative control samples were incorporated into each serological assay, DNA extraction, and PCR reaction.
Invertebrates were counted and sorted into genus and species groups and stored at -20°C. The DNA was extracted from samples of each dipterous group per study site by routine commercial kit methods (DNeasy Tissue kit; Qiagen). To achieve adequate DNA amounts, each sample extraction incorporated the whole body of 20 flies, or the internal contents of each pupa or larva, or the dissected viscera of five cockroaches. Each cockroach, pupa, or larva was surface-washed and sterilized for 5 minutes with 100% ethanol prior to removal of its internal contents for DNA extraction. It was therefore aimed to analyze the digestive tracts of these latter intact insect samples for carriage, rather than the outer surfaces. It was not feasible to surface-sterilize the bodies of adult dipterous flies, because they were usually partially crushed by the trapping procedure. Each extracted invertebrate DNA sample was then analyzed by PCR incorporating oligo-primers specific for L intracellularis DNA as described previously.10 Known positive and negative control samples were incorporated into each DNA extraction and PCR. All PCR assays (feces-origin and insect-origin DNA) were carried out in duplicate in coded format in both study-author locations (Nottingham, UK, and Minnesota) to ensure consistency of results.
The entire DNA sequence of one US isolate of L intracellularis was previously established at the University of Minnesota.11 Four loci of variable-number tandem repeat (VNTR) sequences of DNA were identified within the overall sequence using described methods.12 Two of the loci are ATAn nucleic acid repeats and two are CAn nucleic acid repeats. Specific oligo-primer sequences were designed upstream and downstream of these four loci and validated for their ability to amplify and annotate the repeats in L intracellularis isolates from a variety of locations, as described previously.8 The DNA from a range of L intracellularis PCR products derived from our study pig feces, dipterous adults, pupae, and larvae (Table 1) was incorporated as template DNA into PCR assays for each of the four repeat loci. Amplicon PCR products from triplicate assays were then sequenced with an ABI 3100 automated fluorescent DNA sequencer (Perkin-Elmer Applied Biosystems, Foster City, California). This triplicate sequence data was then analyzed to calculate the number of repeats at each locus, creating a four-part profile for each test DNA sample. Previous DNA samples of known profile were incorporated into each PCR reaction and related sequencing steps as controls.
Results
Invertebrate survey summary
Cockroaches (Blatta orientialis) were detected on only two of 15 sampled farms, and were numerous year-round (usually > 10 per trap-point). The fly community on pig farms rose to a peak during July and August and was dominated (> 20 flies per trap-point) by Musca domestica (house fly) on 13 of the 15 farms. On the two other farms, either Ophyra species (black garbage fly) or Drosophila species (fruit fly) was the dominant fly. Other flies noted in nondominant numbers (0 to 10 per trap point) were Muscina stabulans (false stable fly; nine farms), Stomoxys calcitrans (stable fly; two farms), and Syrphidae family (hover flies; two farms), particularly Eristalis species. On the latter two farms, Eristalis larvae (known as rat-tailed maggots) were noted in high numbers in floor traps in pig pens and also within pig feeders (Figure 1). A wide range of other invertebrates was occasionally found across the pig-farm study sites, eg, beetles, weevils, lice, and spiders, to be described elsewhere. Midges (such as Culicoides species) were not evaluated in this study due to lack of the necessary specialized traps.
Figure 1: Rat-tailed maggots (Eristalis species) in a floor trap located in a British pig farm. Farms described in Table 1. Invertebrates were trapped during April to November in 2007 and 2008 in feed storage areas, weaner and finisher pig areas, and breeder pig areas (where present) using aerial sticky traps and walk-in floor traps in constant numbers and locations on each farm.  |
Pig and invertebrate carriage of Lawsonia intracellularis
The 15 farm types and further results are summarized in Tables 1 and 2. No differences were identified in PCR results performed in separate laboratories.
Table 1: Number and subtyping of Lawsonia isolates derived from pig feces on 15 British farms*
* The set of 15 study sites represented 1.2% of possible British pig farms and included examples of single-site breeder-to-finisher farms, breeder-to-weaner farms, and weaner-to-finisher units of small-medium size (50 to 1000 sows). Fecal samples were collected from 10 pigs from each age group (breeder, weaner, finisher) on each farm at monthly intervals during 2007 to 2008. The DNA extracted from each sample was analyzed by PCR. Lawsonia DNA was subtyped by VNTR analysis. The VNTR results are shown as actual band counts per position: 12 or 13 lines at position 1, with other positions identical, were considered to be the same isolate. PCR = polymerase chain reaction; VNTR = variable number tandem repeat; NT = not tested; NA = not applicable. |
Table 2: Diptera samples and subtyping of Lawsonia isolates on 15 British pig farms*
* Farms described in Table 1 and trapping method described in Figure 1. Results for insect Lawsonia DNA carriage summarized as positive (Pos) or negative (Neg). The VNTR results are shown as actual band counts per position. † Dominant fly species on each farm indicated. PCR = polymerase chain reaction; VNTR = variable number tandem repeat; NT = not tested; NA = not applicable.
|
The Lawsonia-specific IgG serological profiles of the 15 farms are shown in Figure 2, indicating typical postweaning exposure patterns. Lawsonia-specific fecal PCR products were routinely detected in 10- to 14-week-old weaner pigs on 14 farms (9% to 88% positive on fecal PCR). Fecal samples from pigs in other farm areas were occasionally positive (finisher areas 0% to 55%; breeders 0% to 23%). On the one farm negative for Lawsonia (Farm 14), Musca domestica was the dominant fly – all trap samples of this fly on this farm were negative on Lawsonia PCR.
Figure 2: Chart of study herds showing five groups of herds: the bars represent the percentages of pigs of each age that were positive when tested for Lawsonia via serological assay (immunofluorescence assay) in 2007-2008. In each of the five groups of herds, percentages of Lawsonia-positive pigs at these different ages were similar (< 3% difference). The five different patterns of Lawsonia seroconversion indicated are consistent with previous studies relating onset of seroconversion with farm management system.13 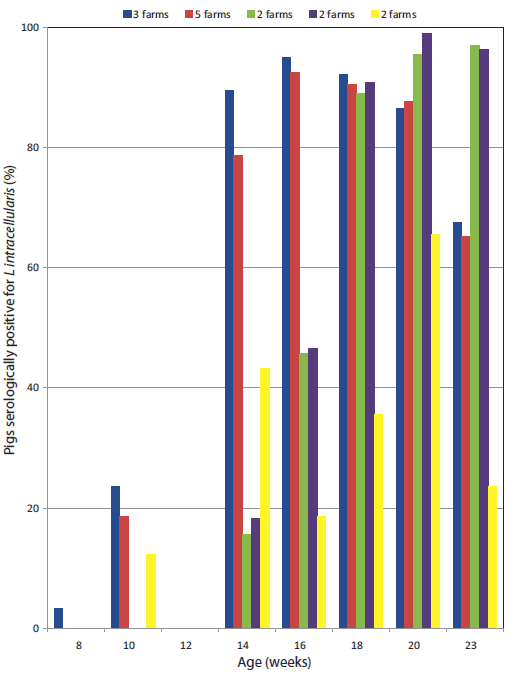 |
Among the 14 Lawsonia-positive farms, the dominant fly was M domestica on 12 farms, Orphyra on one farm, and Drosophila on one farm. On the two latter farms, all trap samples of these dominant flies were negative by Lawsonia PCR. The DNA extracted from all cockroach intestinal samples was also negative. These results are summarized in Tables 1 and 2.
Repeated sampling of adult M domestica collected by aerial traps from within pig pens of the 12 Lawsonia-positive farms where this Diptera fly was dominant consistently showed positive Lawsonia PCR assays in only five of these farms. Flies collected in the weaner-nursery area were most likely to be positive (22% to 75% of fly-trap samples tested), but many positive flies were also noted in the finisher areas of some farms. More details of trap counts and percentages of these samples positive for Lawsonia PCR assays are shown in Table 3. Repeated sampling of larval forms of Eristalis species collected from pig-pen floors and feeders of the two farms (Farms 1 and 2) where this Diptera fly was found consistently showed positive Lawsonia PCR assays. On these two farms, Musca was the dominant adult fly – samples of Musca adult flies from these farms were also positive for Lawsonia DNA reactions (Table 2). Occasional collections of the pupa and adult forms of the Eristalis species hover flies on these two farms were also analyzed and were PCR-positive for Lawsonia.
Table 3: Test results for insect traps with Lawsonia-positive Musca domestica adults on British pig farms in 2007-2008*
* Farms described in Table 1; trapping method described in Figure 1. Each range derived from 12 aerial traps per farm (four per farm area) per collection point every 2 weeks from May to September. Extracted invertebrate DNA from each insect was then analyzed by PCR for Lawsonia intracellularis. † No. of Lawsonia-positive PCR results/no. of traps tested from each farm area for positive farms. NA = not applicable (contract finisher farms). |
||||||||||||||||||||||||||||||||||||||
Identity of Lawsonia within pig and fly-stage samples
Subtyping of Lawsonia DNA by VNTR analysis indicated that farms generally each had different isolates, but two farms had more than one isolate (Tables 1 and 2). The VNTR isolates differed between farms except for Farms 2 and 3. These farm sites were located within 2 km of each other and shared equipment and pigs. On the five farms with Lawsonia-positive Musca flies and pigs, matching VNTR-typed Lawsonia isolate(s) consistently occurred within positive farm pigs and the pig-associated adult house fly stages. On the two farms with Lawsonia-positive Eristalis larvae, Musca adult flies, and pigs, the same individual-farm VNTR-typed isolate occurred within positive farm pigs and these two fly stages.
Discussion
The study results indicate that on many farms, one dominant farm isolate of Lawsonia intracellularis occurs within the pigs and the farm environment, including the insects most closely associated with the pigs. This study also indicates that, of the numerous and varied insects found on pig farms, M domestica and Eristalis species flies have the most likely on-farm potential to carry and transmit L intracellularis. This is most likely due to their numbers and life cycle incorporating stages in regular and close association with pigs and their habitat. These insects probably provide a measure of the environmental contamination of the pig-farm sites by L intracellularis rather than a specific relationship with the bacterium per se. This is suggested by the data indicating that the weaner areas contained both the highest number of infected flies and of infected pigs, whereas other farm areas had lower infection rates of both.
There was a wide diversity in the invertebrates found on the study sites, indicating that pig-farm sites can have a large and possibly distinct invertebrate fauna. European pig farms are perhaps more likely than American ones to incorporate feed preparation areas and bedding in pens. While this may have increased the levels of feed spoilage insects,14 the effect of different farm management systems on pig-associated fly populations, if any, was not clear. Diptera fly stages can have varying food sources, and while most larvae consume rotting fecal or waste matter likely to be found on pig farms,15 fruit flies usually consume rotting vegetable matter. The one farm with dominant fruit flies (Drosophila) was located within a fruit- and vegetable-growing district. These flies do not often interact with animals; therefore, the lack of Lawsonia DNA was not unexpected. All other pig-farm pens contained dominant house flies (Musca) except one farm where the black garbage fly (Orphyra species) was dominant. This is probably due to the larval competition between these two fly species, whereby presence of larvae of one species would capture food sources and exclude the other.2,16
This study is the first to explore subtyping of L intracellularis in European farms. Our results are similar to those of similar studies in North America, whereby one isolate predominates on each single pig-farm site and is distinct from other isolates.8 Lawsonia intracellularis isolates derived from pigs are difficult to distinguish from each other by means other than VNTR, with detailed assays such as 16S rDNA sequencing, outer membrane protein analysis, and others all indicating that L intracellularis forms a single pig strain.7 The VNTR analysis is based on intergenic sequences of no pathogenic consequence; therefore, these results are not considered to dispute the notion of L intracellularis forming a single pig strain. Analysis of key respiratory genes indicates that L intracellularis is a recently evolved bacterium; therefore, it may not have had evolutionary time to develop substrains.17
We suggest the potential of certain Diptera flies to act as vectors for bacterial carriage. Considerable work has been performed in this area for agents of human disease, such as coliforms carried by house flies;18,19 however, studies of agents and invertebrates more relevant to the livestock industries are often lacking. Previous tracking studies of the flight paths of adult M domestica flies suggest that they could move easily between farm sites up to 7 km apart over a 3-day period.20 With the many Lawsonia-positive flies evident on certain of our farm sites, we therefore suggest that there is a possibility of farm-to-farm mechanical transmission of Lawsonia via adult Musca flies, particularly as they often occur in close contact with pigs.
Some previous studies of farm insects18,21 may have ignored adult and larval stages of hover flies or other insect species. We note here that the mobile larval feeding stages of hover flies can occur and be active in pig floor and feed habitats, perhaps enabling greater oral ingestion of environmental-fecal agents and therefore also having significance in disease transmission. Our preliminary data here also suggest that these larvae can retain Lawsonia internally into the static pupa stage, thus suggesting the concept of internal infection of dipterous fly stages and possible biological transmission for Lawsonia infections. A symbiosis or other close relationship has often been indicated between obligately intracellular bacteria and insect cells,22 so this biological transmission concept for Lawsonia is not considered unusual. We did not find Lawsonia in cockroaches (Blatta species) on the two farms where this invertebrate formed large indestructible populations, often in close association with pigs. It is therefore possible that the internal organs of cockroaches are not suitable for carriage of this bacterium.
Our results suggest some variation in the strength of association of particular Diptera as possible vectors of Lawsonia transmission within and perhaps between farms. We found Lawsonia-positive Musca fly stages on only five of 12 potential farms (fly presence with Lawsonia-positive pig feces presence). We speculate that this could reflect the sensitivity of our testing procedures, which we had no way to establish easily, or of perhaps lower levels of pig-fly interaction or of lower levels of actual Lawsonia numbers on some farms, or some combination of these factors. In contrast, we found Lawsonia-positive Eristalis fly stages on two of two potential farms (fly larval presence with Lawsonia-positive pig feces presence). It is likely that the larval stages of this fly form an effective means of ingesting and retaining Lawsonia on the floor of pig pens, as suggested previously for coliform bacteria and Musca fly stages.16,19 It is therefore important that, when ileitis control programs are being formulated, fly control efforts are instituted, as well as ileitis vaccine and medication efforts.
Implications
• Musca (house flies) and Eristalis (hover flies) have the greatest on-farm potential among farm insects to carry and transmit porcine L intracellularis due to their pig-associated life cycle stages.
• As adult M domestica flies can move between farm sites up to 7 km apart, there is a possibility of farm-to-farm mechanical transmission of Lawsonia via these insects.
• Farm sanitation should include insect reduction programs as well as microbial disinfection efforts.
• Intergenic VNTR analysis is a suitable technique for differentiation of L intracellularis isolates globally.
Acknowledgements
We thank Geng Tian, Matthew Chesworth, and Alisdair Gallie for assisting with collections on some farms.
References
1. Dee SA, Schurrer JA, Moon RD, Fano E, Trincado C, Pijoan C. Transmission of porcine reproductive and respiratory syndrome virus under field conditions during a putative increase in the fly population. J Swine Health Prod. 2004;12:242–245.
2. Forster M, Klimpel S, Mehlhorn H, Sievert K, Messler S, Pfeffer K. Pilot study on synanthropic flies (e.g. Musca, Sarcophaga, Calliphora, Fannia, Lucilia, Stomoxys) as vectors of pathogenic microorganisms. Parasitol Res. 2007;101:243–246.
3. Friedman M, Bednar V, Klimes J, Smola J, Mrlik V, Literak I. Lawsonia intracellularis in rodents from pig farms with the occurrence of porcine proliferative enteropathy. Lett Appl Microbiol. 2008;47:117–121.
4. Gebhart CJ, Barns SM, McOrist S, Lin G, Lawson GHK. Ileal symbiont intracellularis, an obligate intracellular bacterium of porcine intestines showing a relationship to Desulfovibrio species. Int J Syst Bacteriol. 2003;43:533–538.
5. McOrist S, Gebhart CJ, Boid R, Barns SM. Characterization of Lawsonia intracellularis gen. nov., sp. nov., the obligately intracellular bacterium of porcine proliferative enteropathy. Int J Syst Bacteriol. 1995;45:820–825.
6. Rowland AC, Lawson GHK. Intestinal adenomatosis in the pig: immunofluorescent and electron microscopic studies. Res Vet Sci. 1974;17:323–330.
7. McOrist S, Gebhart CJ. Genus Lawsonia McOrist, Gebhart, Boid and Barns 1995aVP. In: Garrity GM, ed. Bergey’s Manual of Systematic Bacteriology. 2nd ed. Baltimore Maryland: Williams and Wilkins Press; 2004;148, 161.
*8. Beckler D, Amonsin A, Kapur V, Gebhart C. Multiple-locus variable number tandem repeat analysis for the differentiation of Lawsonia intracellularis isolates. Proc Am Assoc Swine Vet. 2004;41–44.
9. Knittel JP, Jordan DM, Schwartz KJ, Janke BH, Roof MB, McOrist S, Harris DL. Evaluation of antemortem polymerase chain reaction and serologic methods for detection of Lawsonia intracellularis-infected pigs. Am J Vet Res. 1998;59:722–726.
10. Jones GF, Ward GE, Murtaugh MP, Lin G, Gebhart CJ. Enhanced detection of intracellular organism of swine proliferative enteritis, ileal symbiont intracellularis, in feces by polymerase chain reaction. J Clin Microbiol. 1993;31:2611–2615.
11. Gebhart CJ, Kapur V, University of Minnesota; Lawsonia intracellularis genome. Available at: http://www.ncbi.nlm.nih.gov/sites/entrez?Db=genomeprj&cmd=ShowDetailView&TermToSearch=35241. Accessed 29 March 2011.
12. Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acid Res. 1999;27:573–580.
13. Hands I, McOrist S, Blunt R, Lawrence K. Current infection patterns of porcine proliferative enteropathy in Great Britain and the Republic of Ireland. Vet Rec. 2010;167:343–344.
14. Sinclair E. Population estimates of insect pests of stored products on farms on the Darling Downs, Queensland. Aust J Exp Agric An Husb. 1982;22:127–132.
15. Nayduch D, Noblet GP, Stutzenberger FJ. Vector potential of houseflies for the bacterium Aeromonas caviae. Med Vet Entomol. 2002;16:193–198.
16. Rochon K, Lysyk T, Selinger L. Persistence of Escherichia coli in immature house fly and stable fly (Diptera: Muscidae) in relation to larval growth and survival. J Med Entomol. 2004;41:1082–1089.
17. Schmitz-Esser S, Haferkamp I, Knab S, Penz T, Ast M, Kohl C, Wagner M, Horn M. Lawsonia intracellularis contains a gene encoding a functional Rickettsia-like ATP/ADP translocase for host exploitation. J Bacteriol. 2008;190:5746–5752.
18. Davies R, Breslin M. Persistence of Salmonella enteritidis phage type 4 in the environment and arthropod vectors on an empty free-range chicken farm. Environ Microbiol. 2003;5:79–84.
19. Kobayashi M, Sasaki T, Saito N, Tamura K, Suzuki K, Watanabe H, Agui N. Houseflies: not simple mechanical vectors of enterohemorrhagic Escherichia coli O157:H7. Am J Trop Med Hyg. 1999;61:625–629.
20. Nazni WA, Luke H, Rozita WM, Abdullah AG, Sadiyah I, Azahari AH, Zamree I, Tan SB, Lee HL, Sofian MA. Determination of the flight range and dispersal of the house fly, Musca domestica (L.) using mark release recapture technique. Trop Biomed. 2005;22:53–61.
21. Schurrer JA, Dee SA, Moon RD, Deen J, Pijoan C. Evaluation of three strategies for insect control on a commercial swine farm. J Swine Health Prod. 2006;14:76–81.
22. Zientz E, Dandekar T, Gross R. Metabolic interdependence of obligate intracellular bacteria and their insect hosts. Microbiol Mol Biol Rev. 2004;68:745–770.
* Non-refereed reference.
