| Original research | Peer reviewed |
Cite as: Pairis-Garcia MD, Johnson AK, Millman ST, et al. Effects of yohimbine, an alpha 2-antagonistic reversal agent, on physiological recovery parameters of anesthetized sows. J Swine Health Prod. 2014;22(1):16-23.
Also available as a PDF.
SummaryObjective: To evaluate the efficacy of yohimbine as an anesthetic reversal agent for sows anesthetized with a combination of xylazine, ketamine, and telazol. Materials and methods: Anesthesia was induced with xylazine, ketamine, and telazol in a single syringe, injected intramuscularly (IM). Following a 20-minute stabilization period, palpebral reflex was evaluated, and if absent, sows were injected IM with sterile saline (Control sows; n = 12) or yohimbine HCl (0.1 mg per kg; Yohimbine sows; n = 12). Data collected included insensibility measures (palpebral reflex, jaw tone, nose prick, alertness to human approach test, body posture) and physiologic measurements (heart rate, rectal temperature, respiratory rate, oxyhemoglobin saturation). Data was collected every 10 minutes until complete sensibility was attained. Results: Yohimbine sows recovered from anesthesia 162 minutes earlier than Control sows (P < .01). For all insensibility measures, Yohimbine sows regained a normal response more quickly than Control sows (P < .001). In addition, Yohimbine sows maintained greater heart rate (P < .05) and rectal temperature (P < .001) between onset of anesthesia (the time anesthetic agents were injected) to completion of the trial (when sow attained complete return to sensibility). Respiratory rate and oxyhemoglobin saturation were maintained within normal physiological ranges throughout anesthesia, confirming that respiratory capability was not compromised under this anesthetic protocol. Implications: Yohimbine is an effective reversal agent in sows anesthetized with xylazine, ketamine, and telazol administered simultaneously. This agent can be used by veterinarians to ensure a quicker recovery from anesthesia with minimal complications. | ResumenObjetivo: Evaluar la eficacia de la yohimbina como un agente de inversión anestésica para hembras anestesiadas con una combinación de xilazina, ketamina, y telazol. Materiales y métodos: La anestesia fue inducida con xilazina, ketamina, y telazol en una sola jeringa inyectada intramuscularmente (IM por sus siglas en inglés). Después de un periodo de estabilización de 20 minutos, se evaluó el reflejo palpebral y si estaba ausente, se inyectó a las hembras con solución IM de solución salina estéril (hembras Control; n = 12) o yohimbina HCl (0.1 mg por kg; hembras Yohimbina; n = 12). Los datos recopilados incluyeron medidas de insensibilidad (reflejo palpebral, respuesta de quijada, punción de nariz, prueba de alerta de cercanía humana, postura corporal) y medidas fisiológicas (ritmo cardiaco, temperatura rectal, ritmo respiratorio, saturación de oxihemoglobina). Los datos se recopilaron cada 10 minutos hasta que se logró sensibilidad completa. Resultados: Las hembras yohimbina se recuperaron de la anestesia 162 minutos antes que las hembras control (P < .01). Para todas las medidas de insensibilidad, las hembras Yohimbina recobraron una respuesta normal más rápido que las hembras Control (P < .001). Además, las hembras Yohimbina mantuvieron un ritmo cardiaco mayor (P < .05) y temperatura rectal (P < .001) entre el inicio de la anestesia (el tiempo en que se inyectaron los agentes anestésicos) y el término de la prueba (cuando la hembra regresó a sensibilidad total). El ritmo respiratorio y la saturación de oxihemoglobina se mantuvieron dentro de los rangos fisiológicos normales durante la anestesia, confirmando que la capacidad respiratoria no se afectó bajo este protocolo anestésico. Implicaciones: La yohimbina es un agente de inversión efectivo en hembras anestesiadas con xilazina, ketamina, y telazol administrados simultáneamente. Este agente puede ser utilizado por veterinarios para asegurar una recuperación más rápida de la anestesia con mínimas complicaciones. | ResuméObjectif: Évaluer l’efficacité de la yohimbine comme agent renversant l’anesthésie chez des truies anesthésiées avec une combinaison de xylazine, kétamine, et telazol. Matériels et méthodes: L’anesthésie a été induite avec un mélange de xylazine, kétamine, et telazol dans une même seringue, et injecté par voie intramusculaire (IM). Suite à une période de stabilisation de 20 minutes, le réflexe palpébral a été évalué, et si absent, les truies étaient injectées IM avec de la saline stérile (truies Témoins; n = 12) ou de la yohimbine HCl (0,1 mg par kg; truies Yohimbine; n = 12). Les données accumulées incluaient des mesures d’insensibilité (réflexe palpébral, tonus de la mâchoire, piquage du groin, attention au test d’approche humaine, posture corporelle) et des mesures physiologiques (rythme cardiaque, température rectale, rythme respiratoire, saturation de l’oxyhémoglobine). Les données étaient prélevées toutes les 10 minutes jusqu’à ce que la sensibilité complète fut atteinte. Résultats: Les truies Yohimbine ont récupéré de l’anesthésie 162 minutes plus tôt que les truies Témoins (P < .01). Pour toutes les mesures d’insensibilité, les truies Yohimbine ont retrouvé une réponse normale plus rapidement que les truies Témoins (P < .001). De plus, les truies Yohimbine ont maintenu un rythme cardiaque plus élevé (P < .05) et une température rectale plus élevée (P < .001) entre le début de l’anesthésie (moment de l’administration des agents anesthésiants) jusqu’à la complétion de l’essai (retour complet de la sensibilité). Le rythme respiratoire et la saturation de l’oxyhémoglobine se maintenaient à l’intérieur des valeurs physiologiques normales durant toute l’anesthésie, confirmant ainsi que la capacité respiratoire n’était pas compromise avec ce protocole d’anesthésie. Implications: La yohimbine est un agent renversant efficace chez les truies anesthésiées avec un mélange de xylazine, kétamine, et telazol administré simultanément. Cet agent peut être utilisé par les vétérinaires afin d’assurer un retour plus rapide de l’anesthésie avec un minimum de complications. |
Keywords: swine, anesthesia, yohimbine, reversal agent
Search the AASV web site
for pages with similar keywords.
Received: February 12, 2013
Accepted: April 9, 2013
Sows represent a unique population in the breeding herd, as physiological compromise (disease) and age can make anesthesia induction risky. According to the American Society of Anesthesiologists,1 age (geriatric), weight,2 disease status, and anatomical variation3 contribute to a heightened anesthetic risk and can lead to prolonged recovery times and increase post-anesthetic complications.4 In addition, studies evaluating natural on-farm sow deaths confirmed cardiovascular failure as one of the top three causes of mortality.5-7 This increases sow anesthetic risk, as the cardiovascular system is a key system altered during anesthesia. Furthermore, direct observations in our laboratory revealed that anesthetized sows (anesthesia induced with xylazine, ketamine, and telazol injected simultaneously in a single syringe) exhibited prolonged recoveries, on average between 5 and 10 hours. Acknowledging inherent sow risk factors, it is critical to design a protocol that minimizes risks associated with anesthesia.
Swine may be anesthetized in order to complete routine production procedures or surgical operations.8 Laboratory and on-farm anesthesia examples include, but are not limited to, coronary angiography, ischemia and reperfusion models for human disease,9,10 tracheal culture and bronchoalveolar lavage for respiratory disease diagnosis,11,12 and assistance with aggressive animals when performing reproductive procedures13 or euthanasia.14 A major disadvantage with anesthesia in swine is the unpredictable recovery time, which results in increased post-anesthetic risks and costs attributed to employee time spent monitoring the animal. Although utilization of on-farm anesthesia on a daily basis is not common, anesthesia combined with an effective reversal agent can provide an additional diagnostic tool for veterinarians. Sows are difficult to restrain due to their large size and can be easily stressed by physical restraint and handling. Anesthetic administration routes are limited in adult swine due to inaccessible superficial veins and thick subcutaneous fat layers.15 In addition, responses and reactions of swine to anesthesia can vary, as noted by resistant responses to certain sedative drug combinations.16,17 Xylazine, ketamine, and telazol are a common combination of drugs used for anesthesia of swine both on farm and under research conditions.18 The choice to use all three drugs in combination in our laboratory was based on a toxicologically wide margin of safety in swine and prolonged analgesic properties attained using all three drugs, as compared to xylazine and ketamine administered together19 and telazol administered alone.20 Yohimbine is an alpha-2 adrenoceptor antagonist that has been reported to be effective in reversing xylazine effects in nursery-age swine and other food-producing animals.21-23 In cats, yohimbine acts as a stimulant, shortening both ketamine-induced anesthesia and the effects of xylazine.24 Providing a quicker recovery may decrease post-anesthetic complications in sows, providing a more efficient, cost-saving method for anesthesia to be applied on farms. Although yohimbine has proven effective in nursery-age swine, inherent anesthetic risk of sows makes it inappropriate to infer that sows will respond in the same manner as younger, healthier swine. The objective of this study was to determine yohimbine efficacy as an anesthetic reversal agent in sows anesthetized with xylazine, ketamine, and telazol injected simultaneously in a single syringe.
Materials and methods
The protocol for this study was approved by the Iowa State University Animal Care and Use Committee.
Animals and housing
Twelve multiparous, non-pregnant, crossbred commercial maternal-line cull sows were used (mean bodyweight ± standard deviation = 233.6 ± 18.7 kg). All sows received a physical examination, which included lung and heart auscultation, rectal temperature, and reproductive tract ultrasonography. These sows were handled daily for research projects and were familiar with their environment and caretakers. The laboratory was located at Iowa State University, College of Veterinary Medicine, Ames, Iowa. To avoid confounding injury due to aggression, each sow was housed in an individual pen; however, sows could see, smell, hear, and have nose-to-nose contact with other cohorts. Sows were provided ad libitum access to water via one nipple drinker per pen (Model 65; Trojan Specialty Products, Dodge City, Kansas). Sows were fed twice daily on a single feed bunk with a diet designed to meet or exceed nutrient requirements for gestating sows.
Treatments
Sows were blocked by body weight and randomly allocated using a random number generator to two treatments. Treatments were as follows: Yohimbine, yohimbine HCl (0.1 mg per kg) administered intramuscularly (IM) into the neck muscle (n = 12) and Control, sterile saline administered IM at an equivalent volume (n = 12).
Experimental design
All sows were acclimated to the laboratory environment for 7 days prior to study commencement. All 12 sows received both treatments in a cross-over design with a 10-day washout period. This experimental design provided robust control of intra- and inter-animal variation and reduced the animal number required to find significant differences. Investigators were blinded to treatments to reduce the possibility of observer bias.
Anesthesia protocol
Sows were fasted overnight (16 hours), but were provided ad libitum access to water until 1 hour prior to anesthesia administration. Sows were restrained by a common pig snare in their home pen and anesthetized. Anesthetic agents were combined and injected at the doses indicated: xylazine (4.4 mg per kg; Anased, Lloyd Laboratories, Shenandoah, Iowa); ketamine HCl (2.2 mg per kg; Ketaset, Wyeth, Madison, New Jersey); and tiletamine HCl and zolazepam HCl in combination (4.4 mg per kg; Telazol, Wyeth).18 Anesthesia onset began once anesthetic agents were injected. Ten minutes after anesthesia onset, sows were placed in lateral recumbency, and postural adjustments were made if involuntary movements resulted in compromised respiratory or circulatory capability. Twenty minutes after anesthesia onset, sows were evaluated for a palpebral reflex. This was determined by placing a finger on the medial canthus of the accessible eye and gently running the finger along the eyelashes. The presence or absence of the palpebral reflex was determined by attempting to elicit a blink response with three successive attempts. If a palpebral reflex was absent, one of the two treatments was administered. If a palpebral reflex was present, the sow was monitored every 10 minutes until the palpebral reflex was absent, and treatment was then administered. To prevent confounding effects of external stimuli such as human traffic and talking within and between the pens during anesthesia induction, these distractions were minimized. During anesthesia and recovery, electrical heating pads and blankets were utilized when the sow’s rectal temperature dropped below 36°C.
Measures
Insensibility measures. Insensibility measures collected included the human approach test,25 body posture, palpebral reflex, jaw tone, and nose prick test. Each measure was scored on a 0 to 2 scale, with score 0 representing a normal alert response, score 1 representing diminished response from normal, and score 2 representing no response (Table 1). Insensibility was measured immediately before anesthesia administration (Baseline), and every 10 minutes after anesthesia onset until sows reached a 0 score (Recovery). A sow was considered to have completed the trial once a 0 score for all measures was attained.
Table 1: Criteria and scoring system* used to assess insensibility throughout anesthesia† and recovery in sows treated with yohimbine (n = 12) or saline (n = 12)
* Adapted from Kim et al21 and Heinonen et al.26 † Sows were anesthetized with xylazine (4.4 mg/kg), ketamine HCl (2.2 mg/kg), and a combination of tiletamine HCl and zolazepam (4.4 mg/kg) administered simultaneously in a single intramuscular injection. Treatments administered following anesthesia onset were yohimbine (alpha-2 adrenoceptor antagonist) administered intramuscularly at 0.1 mg/kg (Yohimbine sows) or an equivalent volume of saline (Control sows). Insensibility measures were assessed every 10 minutes from injection of anesthetic agents until sows reached a 0 score. ‡ Measures are in order of return to sensibility for Yohimbine sows. |
Physiologic measures. Physiologic measures were collected at the same time points as insensibility measures and included heart rate by auscultation (WLS5605-Cl Stethoscope; United Inc, India), rectal temperature (Jumbo Display Digital Thermometer; Graham Field Health Products Inc, Atlanta, Georgia), respiratory rate, and oxyhemoglobin saturation (SpO2) collected from a pulse oximeter probe placed on the sow’s lip or tongue (OxiMax N-65 Quick Guide; NellCor, Boulder, Colorado).
Statistical analysis
Data were analyzed using SAS software version 9.3 (SAS Institute Inc, Cary, North Carolina). Data were analyzed for normality by plotting a predicted residual plot and a quantile-quantile plot. Insensibility and physiologic measures were analyzed using a mixed model procedure utilizing polynomial regression in SAS. The insensibility statistical models included the fixed effect of treatment (Control versus Yohimbine), day (2 days), day-by-treatment interaction, and weight as a linear covariate. Sow was included as a random effect. The physiologic statistical models included the fixed effect of treatment (Control versus Yohimbine), day (2 days), day-by-treatment interaction, weight, and time (minutes) categories. Time categories were created starting at anesthesia onset. Sixty-minute time-point interval blocks were included. All interactions were included in the model. A P value of < .05 was considered significant for the MIXED analysis of variance and when separating means. Fixed effect least squares (LS) means were separated using the PDIFF option in SAS, and data were expressed as LS means (95% confidence intervals) and mean (± SD).
Results
Insensibility measures
No difference was observed between treatments (Control versus Yohimbine) for time to administration, with all sows receiving either Yohimbine or saline on average between 23.5 minutes (95% CI, 17.6-29.4) and 27.0 minutes (95% CI, 21.4-32.6) post anesthesia administration. Yohimbine sows recovered from anesthesia 162 minutes earlier than Control sows (290 minutes, 95% CI, 195.4-384.6 versus 452 minutes, 95% CI, 364.2-559.7; P < .01). Time to return to sensibility for all measures (score 0) was shorter for Yohimbine sows than for Control sows (P < .01; Figure 1).
Figure 1: Latency to regain sensibility least squares means (± standard error) (minutes) for anesthetized sows administered yohimbine or saline to mitigate recovery effects (P < .01). Sows were anesthetized and treatments administered after anesthesia onset as described in Table 1. Statistical analysis was performed using a mixed model. Insensibility measures included palpebral reflex, nose prick test, jaw tone, human approach test (HAT), and body posture. 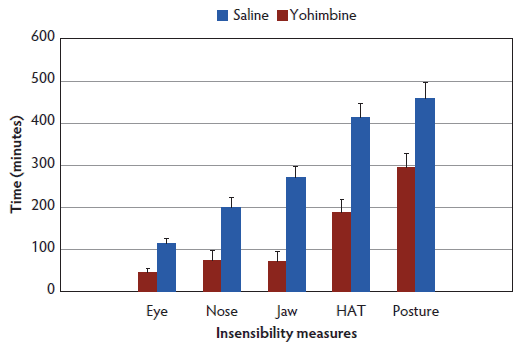 |
Physiologic measures
Heart rate. There was a treatment-by-time interaction, with Yohimbine sows demonstrating a greater heart rate over the anesthesia course than Control sows (P < .05; Figure 2). Within 3 hours post anesthesia administration, heart rate did not differ between treatments. During the following 4 hours, Yohimbine sows maintained greater heart rates (P < .01). When a 0 score was attained, mean heart rate in Yohimbine sows, 69.9 beats per minute (95% CI, 63.0-76.8), was greater than in Control sows, 49.1 beats per minute (95% CI, 42.7-55.5).
Figure 2: Heart rate least squares means by time for anesthetized sows administered yohimbine. Sows were anesthetized and treatments administered after anesthesia onset as described in Table 1. Statistical analysis was performed using a mixed model. Best fit lines for each treatment were fitted using a polynomial function. Black vertical line represents the first time that heart rate differed between Yohimbine and Control sows (P < .05). 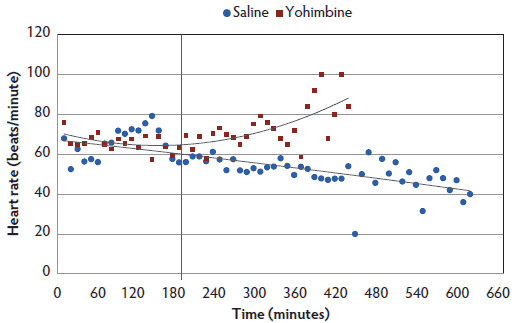 |
Rectal temperature. The interaction between treatment and time (P < .001) demonstrated greater rectal temperatures throughout anesthesia for Yohimbine sows than for Control sows (Figure 3). Within 3 hours post anesthesia administration, rectal temperature did not differ between treatments. During the following 7 hours, Yohimbine sows maintained greater rectal temperatures (P < .001). When a 0 score was attained, mean rectal temperature was greater in Yohimbine sows (34.8°C; 95% CI, 34.2°C-35.3°C) than in Control sows (32.2°C; 95% CI, 31.7°C-32.8°C).
Figure 3: Rectal temperature least squares means by time for anesthetized sows administered yohimbine or saline to mitigate recovery effects (P < .001). Sows were anesthetized and treatments administered after anesthesia onset as described in Table 1. Statistical analysis was performed using a mixed model. Best fit lines for each treatment were fitted using a polynomial function. Black vertical line represents the first time that rectal temperature differed between Yohimbine and Control sows (P < .001). 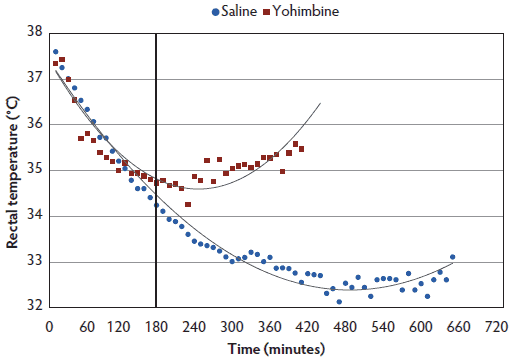 |
Respiratory rate and SpO2. Respiratory rate differed by treatment, time, treatment-by-time interaction, and body weight between Yohimbine sows (21.0 breaths per minute; 95% CI, 18.6-23.3) and Control sows (21.9 breaths per minute; 95% CI, 20.0-23.7): P < .01 (treatment); P < .001 (time); P < .001 (treatment-by-time interaction); P < .001 (weight) (Figure 4). For the first 3 hours following anesthesia induction, Control sows had greater respiratory rates than did Yohimbine sows. For the remaining 5 hours, respiratory rates in Control and Yohimbine sows did not differ. There was no difference in respiratory rate between treatments once sows attained a 0 score.
Figure 4: Respiratory rates least squares means by time for anesthetized sows administered yohimbine or saline to mitigate recovery effects (P < .01). Sows were anesthetized and treatments administered after anesthesia onset as described in Table 1. Statistical analysis was performed using a mixed model. Best fit lines for each treatment were fitted using a polynomial function. 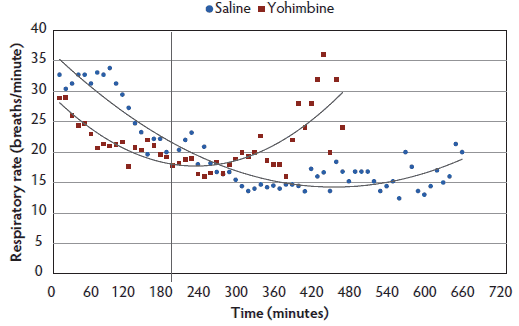 |
There were no treatment differences for SpO2, but for both Yohimbine and Control sows, SpO2 percentage increased with time under anesthesia (Figure 5).
Figure 5: Oxyhemoglobin saturation least squares means by time for anesthetized sows administered yohimbine or saline to mitigate recovery effects (P > .05). Sows were anesthetized and treatments administered after anesthesia onset as described in Table 1. Statistical analysis was performed using a mixed model. Best fit lines for each treatment were fitted using a polynomial function. Data points ended earlier in Yohimbine sows due to difficulty in continuous probe placement during recovery. 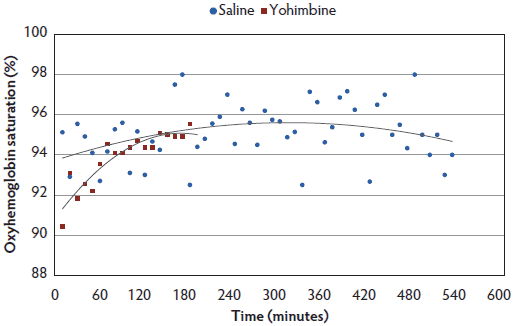 |
Discussion
The objective of this study was to determine yohimbine’s efficacy as an anesthetic reversal agent in sows. On the basis of previous work conducted on nursery pigs,21,27,28 we expected treatment with yohimbine to decrease overall recovery time, decrease latency time to regain sensibility, and maintain physiologic parameters closer to normal homeostatic levels than treatment with saline.
When insensibility measures were evaluated, Yohimbine sows recovered sooner than Control sows, with Control sows taking over 7 hours to regain full sensibility. The results of this study are in agreement with previously published findings that yohimbine reduces overall time under anesthesia, but the anesthesia duration for mature sows was longer than for nursery-age swine. Kim and colleagues21 reported that pigs receiving yohimbine 20 minutes after anesthesia induction regained sternal recumbency (52.2 ± 8.9 versus 76.2 ± 20.6 minutes) and the ability to stand (77.0 ± 9.8 versus 98.7 ± 15.8 minutes) and walk (81.3 ± 11.3 versus 110.8 ± 18.6 minutes) faster than did pigs that did not receive yohimbine. Overall, in the present study, recovery from anesthesia was three to five times longer in sows receiving the same anesthetic protocol at the same dose without administration of yohimbine. This may be explained by differences in body composition and repartitioning of drug in mature animals. However, further studies should be conducted. Regardless of prolonged anesthesia duration, yohimbine effectively reduced the time under anesthesia and in turn decreased the risk of post-anesthetic complications.2,4,29
During anesthesia recovery, Yohimbine sows maintained physiologic parameters more closely resembling a healthy sow at rest; however, Control sows had depressed physiological measures. In all species undergoing anesthesia, it is expected that the animal’s physiologic state (ie, heart rate, temperature, respiratory rate, and oxygen exchange) will be altered from normal homeostatic levels.30 This is due to effects on receptors in the heart, lungs, and peripheral veins by selected anesthetic agents such as xylazine.31 Antagonistic agents like yohimbine may alter the impact that anesthetic agents like xylazine have on the sow’s physiologic status.
Yohimbine sows had faster heart rates than did Control sows, with Yohimbine sows maintaining heart rates within a normal range32 throughout anesthesia (60 to 90 beats per minute). Bradycardia is a common side effect noted in animals anesthetized with xylazine.33 Xylazine is an alpha-2-adrenergic agonist that generates systemic vascular resistance by acting on the alpha-2 receptors located on peripheral veins.31 This in turn produces hypertension and a short transient tachycardia, followed by a “compensatory baroreceptor-mediated reflex” resulting in bradycardia and decreased cardiac output.31 This physiologic event can lead to inadequate oxygen-rich blood perfusion to vital organs and compromise basal metabolic requirements.34 This may cause complications during recovery and may cause permanent organ-system damage. The Yohimbine treatment effectively antagonized xylazine effects on the sow’s cardiovascular system, as was demonstrated in Yohimbine sows that did not become bradycardic during the anesthesia procedure and maintained a normal heart rate once a 0 insensibility score was attained.
During the anesthesia course, Yohimbine and Control sows reached subnormal body temperatures, but Yohimbine sows maintained higher overall temperatures within the last anesthetic hours. Rectal temperature in both treatment groups dropped approximately 1°C per hour within the first 2 hours after anesthesia. Between the third and fourth anesthesia hours, temperature dropped only 0.3°C per hour for Yohimbine sows, whereas the temperature of Control sows dropped 0.7°C per hour. Neither treatment group attained normal rectal temperature (38.0°C to 39.0°C) 30 when the sows reached a 0 insensibility score. However, Kim and colleagues21 reported that nursery pigs had lower rectal temperatures when yohimbine was administered than did control pigs. In their study,21 from the time pigs were anesthetized until recovery, rectal temperature dropped by 1°C. In the present study, rectal temperatures of both Yohimbine and Control sows dropped approximately 1°C within the first hour. However, temperature continued to drop in both treatment groups due to prolonged recovery times, which differs from previous work.21
Hypothermia in anesthetized companion animals is often overlooked, but can have severe consequences.35 To date, to the authors’ knowledge, there are no published studies evaluating hypothermia in swine. In companion animals, hypothermia has been defined as core body temperature dropping below 36°C,36 and this was used in our study as a critical control point for thermal heat supplementation. Electrical heating pads and blankets were utilized when the sow’s rectal temperature dropped below 36°C. Yohimbine sows maintained greater rectal temperatures and exhibited a slower rate of decline in rectal temperature over time. This is an advantage to the animal, as consequences of heat loss under anesthesia increase the risk of impaired immune-system function, impaired blood coagulation, cardiovascular depression, acidemia, and increased morbidity and mortality.34
Baseline respiratory rate was different between treatment groups, with Control sows having greater mean respiratory rate than Yohimbine sows. It is unclear why respiratory rate was greater in Control sows than in Yohimbine sows and why baseline respiratory rates in both groups were greater than reported normal respiratory rates (10 to 20 breaths per minutes)32 in adult swine. Housing conditions were regulated to maintain a constant ambient temperature throughout the study and were unlikely to be the source for elevated respiratory rates. Behavioral excitability is a possible explanation for the increased baseline respiratory rate. Sows were not provided with a morning feed ration in order to minimize risk of aspiration or regurgitation while under anesthesia. It was noted that the sows exhibited increased vocalization and activity on trial day, compared to their typical behavior. This may have resulted from the sows not being fed that morning, which in turn elevated their respiratory rates. In addition, differences in baseline respiratory rate between treatments (Yohimbine versus Control) may have also been influenced by the sows’ respiratory capability, which is influenced by lung development, maturation, and structural status. Previous sow history was not known, and therefore previous respiratory disease or compromised lung function may have influenced respiration under anesthesia.37 It was noted that body weight had an effect on respiratory rate. However, because of the experimental design, weight or behavioral excitability would be unlikely to influence respiratory rate, as the same individual received both treatments.
Although Control sows did have greater mean respiratory rate during the first 2 hours, respiratory rate did not differ between treatment groups during the remaining time under anesthesia. A common side effect observed with xylazine administration includes respiratory depression.31 Kim et al21 reported that Yohimbine did not reverse respiratory depression in younger pigs until 5 minutes after administration. The results of our study and those of Kim et al21 suggest that either yohimbine does not play a substantial role in controlling or regulating swine respiration under anesthesia, or the measurement methods (evaluating abdominal movements for 15 seconds) may not have been sufficiently sensitive to detect respiratory changes.
Treatment had no effect on SpO2 concentrations in the present study, but all sows demonstrated a gradual increase in SpO2 as time under anesthesia increased. No sows were provided with supplemental oxygen, therefore increased SpO2 over time must be attributed to improved oxygen exchange by the sow. Gianotti and colleagues38 determined that normal SpO2 concentration in swine aged 60 to 90 days was 96% (± 2.10%). On the basis of data from this study, SpO2 averages were within normal levels and did not fall below 90%. These data suggest that this anesthetic protocol did not compromise sow respiratory or oxygen exchange capability. However, caution should be taken when evaluating these results, as methods chosen for measurement may not be sensitive enough. In comparing pulse oximetry accuracy to capnography in dogs, cats, horses, and white-tailed deer, it has been demonstrated that the accuracy and consistency of pulse oximetry varies widely and does not provide readings as accurate as arterial blood gas analysis.39,40 Although capnography may be a more accurate method than pulse oximetry, additional expense and technical skills make it difficult to apply on farm, and it was not chosen for this study. In addition, pulse oximetry results were difficult to collect once sows began regaining consciousness, as the probe needed to be clamped onto either a tongue or lip. Difficulty in placing the probe when sows were regaining consciousness resulted in less data collected for the Yohimbine sows.
In conclusion, on the basis of insensibility and physiologic measures, yohimbine was an effective reversal agent in sows anesthetized with xylazine, ketamine, and telazol. Overall anesthetic recovery time was shorter, and sows in an anesthetized state were able to maintain physiological parameters closer to normal homeostatic values. However, the effects of yohimbine on physical and behavioral recovery remain unknown. Video data analysis may provide additional information regarding the degree of post-anesthesia ataxia or thrashing with and without yohimbine. Yohimbine could be used by veterinarians to provide a desired analgesic and anesthetic effect while surgical procedures are performed, with a shorter recovery time that may decrease physiologic complications associated with anesthesia.
Implications
• Yohimbine is an effective reversal agent in sows anesthetized with xylazine, ketamine, and telazol administered simultaneously in a single syringe.
• Sows treated with xylazine, ketamine and telazol recover sooner when yohimbine is administered as a reversal agent, and physiological parameters return to normal homeostatic ranges more quickly.
• Recovery time after administration of xylazine, ketamine and telazol may be longer in sows than in nursery pigs, and anesthesia protocols may need to be adjusted for mature sows.
Conflict of interest
None reported.
References
1. American Society of Anesthesiologists. ASA physical status classification system. 2011. Available at: www.asahq.org/clinical/physicalstatus.htm. Accessed 25 June 2013.
*2. Petry C, Pereira ALB, Lemos JL, Mira A. Retrospective study: assessment of anesthetic risk of obese patients. Proc World Small Anim Vet Assoc Cong. Sao Paulo, Brazil. 2009; unpaginated.
*3. Sinclair M. Managing pediatric and geriatric patients. Proc NAVC. Gainesville, Florida. 2011; 166–169.
4. Trim CM, Braun C. Anesthetic agents and complications of Vietnamese potbellied pigs: 27 cases (1999–2006). JAVMA. 2011;239:114–121.
5. Engblom L, Eliasson-Selling L, Lundeheim N, Belak K, Andersson K, Dalin AM. Post mortem finding in sows and gilts euthanized or found dead in a large Swedish herd. Acta Vet Scand. 2008;50:25–35.
6. D’Allaire S, Drolet R, Brodeur D. Sow mortality associated with high ambient temperature. Can Vet J. 1996;37:237–239.
7. Chagnon M, D’Allaire S, Drolet R. A prospective study of sow mortality in breeding herds. Can J Vet Res. 1991;55:180–184.
8. Lu DZ, Fan HG, Kun M, Song ZL, Ming YS, Sheng J, Wang HB. Antagonistic effect of atipamezole, flumazenil, and naloxone following anaesthesia with xylazine, tramadol, and tiletamine/zolazepam combination in pigs. Vet Anesth Analg. 2011;38:301–309.
9. Williams PD, Malik N, Kingston PA. Coronary angiography and percutaneous coronary intervention in the porcine model: a practical guide to the procedure. Animal. 2012;6:311–320.
10. Dominguez HA, Carrasco MA, Olivera A, Santan I, Reyes Z, Rodriguez M, Murguia G, Alfonso C. Effect of sex on heart frequency in an Ischaemia/reperfusion model in pigs under intravenous total anaesthesia. Revista Computadorizada de Produccion Porcina. 2011;18:115–117.
*11. Solano G, Pijoan C. Diagnostic notes. A simple technique for tracheal culture to detect respiratory pathogens in live pigs. Swine Health Prod. 1997;5:30–31.
12. Jolie R, Backstrom L, Olson L, Chase C. Respiratory and systemic health parameters in pigs raised in a conventional farm or in isolation. Swine Health Prod. 1999;7:269–275.
*13. Shipley CF. Diagnostic notes. Breeding soundness examination of the boar. Swine Health Prod. 1999;7:117–120.
*14. Pelland C. Boar and sow euthanasia. Proc AASV. Dallas, Texas. 2009;333–334.
15. Bîrtoiu IA, Badea R, Sîrbu-Boeti M, Efrimescu C. Different anesthesia protocols used for experimental swine surgery Lucrari Stiintifice. 2008;41:526–529.
16. McClellan RO. Applications of swine in biomedical research. Lab Anim Care. 1968;18:120–126.
17. Swindle MM. Anesthesia, analgesia and perioperative care. In: Swindle MM, ed. Swine in the Laboratory: Surgery, Anesthesia, Imaging and Experimental Techniques. 2nd ed. Boca Raton, Florida: CRC Press; 2007:59–61.
18. St-Jean G, Anderson DE. Anesthesia and surgical procedures in swine. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, eds. Diseases of Swine. 9th ed. Victoria, Australia: Blackwell Publishing; 2006:1107–1131.
19. Green CJ, Knight J, Precious S, Simpkin S. Ketamine alone and combined with diazepam or xylazine in laboratory animals: a 10 year experience. Lab Anim. 1981;15:163–170.
20. Ko JC, Williams BL, Smith VL, McGrath CJ, Jacobson JD. Comparison of telazol, telazol-ketamine, telazol xylazine, and telazol-ketamine-xylazine as chemical restraint and anesthetic induction combination in swine. Lab Anim Sci. 1993;43:476–480.
21. Kim MJ, Park CS, Jun MH, Kim MC. Antagonistic effects of yohimbine in pigs anaesthetized with tiletamine/zolazepam and xylazine. Vet Rec. 2007;161:620–624.
22. Kitzman JV, Booth NH, Hatch RC, Wallner B. Antagonism of xylazine sedation by 4-aminopyridine and yohimbine in cattle. Am J Vet Res. 1982;43:2165–2169.
23. Mohammed FK, Zangana IK, Abdul-Latif AR. Reversal of medetomidine sedation in sheep by atipamezole and yohimbine. Vet Hum Toxicol. 1995;37:97–99.
24. Hsu WH, Lu ZX. Effect of yohimbine on xylazine-ketamine anesthesia in cats. JAVMA. 1984;185:886–888.
25.Marchant JN, Whittaker X, Broom DM. Vocalisations of the adult female domestic pig during a standard human approach test and their relationship with behavioural and heart rate measures. Appl Anim Behav Sci. 2001;72:23–39.
26. Heinonen ML, Raekallio MR, Olivero C, Ahokas S, Peltoniemi OAT. Comparison of azaperone-detomidine-butorphanol-ketamine and azaperone-tiletamine-zolazepam for anaesthesia in piglets. Anesth Analg. 2009;36:151–157.
27. Lee J, Kim M. Antagonistic effects of atipamezole and yohimbine against anesthesia with medetomidine and ketamine combination in pigs. J Vet Clin. 2011;28:291–296.
*28. Kim MC, Kim MJ, Jun MH, Park CS. Effect of yohimbine as a reversing agent of tiletamine/zolazepam and xylazine anesthesia in swine. Proc IPVS. Hamburg, Germany. 2004:569.
29. Dodman NH, Gray L, Williams R, Goldspink G. Intracompartmental muscle pressure, temperature and pH in the horse under general anesthesia. J Equine Vet Sci. 1985;5:11–15.
30. Smith AC, Swindle MM. Anesthesia and analgesia in swine. In: Fish RE, Brown MJ, Dannerman PJ, Karas AZ, eds. Anesthesia and Analgesia in Laboratory Animals. 2nd ed. Oslo, Norway: Academic Press; 2008:413–436.
31. Lamont, L. a2-Agonists. In: Gaynor JS, Muir WW, eds. Handbook of Veterinary Pain Management. 2nd ed. St Louis, Missouri: Mosby; 2009:210–230.
32. Jackson PGG, Cockcroft PD. Investigations of clinical problems on pig farms. In: Jackson PGG, Cockcroft PD, eds. Handbook of Pig Medicine. Philadelphia, Pennsylvania: Elsevier; 2007:1–15.
33. Hsu WH. Xylazine-pentobarbital anesthesia in dogs and its antagonism by yohimbine. Am J Vet Res. 1985;46:852–855.
* 34. Posner L. Complications during anesthesia: evaluation and treatment. Proc NAVC. Orlando, Florida. 2010;131–133.
35. Byers CG. Cold critters: understanding hypothermia. Vet Med. 2012;107:82–87.
36. Knaepal A. Inadvertent perioperative hypothermia: a literature review. J Perioper Pract. 2012;22:86–90.
37. Klein C, Reinhold P. Analysis of respiratory mechanics by impulse oscillometry in non-sedated and diazepam-sedated swine. Res Vet Sci. 2001;70:181–189.
38. Gianotti GC, Beheregaray W, Passos W, Bianchi S, Santos Mombach V, Bonfirm Carregaro A, Contestino E. Swine in biomedical research: normal physiological values. Acta Sci Vet. 2010;38:133–137.
39. Matthews NS, Hartke S, Allen JC Jr. An evaluation of pulse oximeters in dogs, cats and horses. Anesth Analg. 2003;30:3–14.
40. Koenig J, McDonnell W, Valverde A. Accuracy of pulse oximetery and capnography in healthy and compromised horses during spontaneous and controlled ventilation. Can J Vet Res. 2003;67:169–174.
* Non-refereed references.
