| Original research | Peer reviewed |
Cite as: Corzo CA, Morrison RB, Fitzpatrick AM, et al. Risk factors for detecting influenza A virus in growing pigs. J Swine Health Prod. 2014;22(4):176–184.
Also available as a PDF.
SummaryObjective: To investigate the association between certain farm-level risk factors and the presence of influenza A virus (IAV) in growing-pig farms. Materials and methods: Twenty-six pig farms participated in the study. Thirty nasal swabs from growing pigs were collected per month from each farm for 12 or 24 consecutive months between 2009 and 2011. Nasal swabs were tested for IAV by real-time reverse transcriptase polymerase chain reaction. Weather stations located at every participating farm monitored temperature, relative humidity, light intensity, and wind speed and gusts. Farm-level data was obtained through a questionnaire to assess the relationship between the presence of IAV and farm-level characteristics. Results: Of the 15,630 nasal swabs collected from growing pigs, 730 (4.6%) tested positive for IAV. Of the 522 groups of growing pigs from which nasal swabs were collected, 110 groups (20.8%) had at least one positive nasal swab. Positive nasal swabs originated from 23 of the 26 participating farms. Farm-level characteristics associated with the presence of IAV included farm type (farrow-to-finish odds ratio [OR] 3.05; nursery OR 16.69), pig flow (all-in, all out OR 0.31 by barn; OR 0.35 by site), gilt source (born at breeding site, raised off-site, and later returned OR 0.17; off-site multiplier OR 0.25), environmental temperature, and wind speed. Implications: Population dynamics, eg, nursery and farrow-to-finish farms and continuous-flow management, play important roles in the epidemiology of IAV. Possible modifications to farm type and pig flow should be considered when constructing IAV control and prevention strategies. | ResumenObjetivo: Investigar la asociación entre ciertos factores de riesgo a nivel granja y la presencia del virus A de la influenza (IAV por sus siglas en inglés) en granjas de crecimiento. Materiales y métodos: Veintiséis granjas de cerdos participaron en este estudio. Mensualmente, se recolectaron 30 muestras de hisopos nasales de cerdos de crecimiento de cada granja durante 12 ó 24 meses consecutivos entre 2009 y 2011. Las muestras nasales se analizaron en busca del IAV por medio de la reacción en cadena de la polimerasa de transcripción reversa en tiempo real. Se colocaron estaciones meteorológicas en cada una de las granjas participantes para monitorear la temperatura, humedad relativa, intensidad de luz, y velocidad de viento y ráfagas. La información a nivel de granja se obtuvo a través de un cuestionario para valorar la relación entre la presencia del IAV y las características a nivel de granja. Resultados: De las 15,630 muestras nasales recolectadas de los cerdos en crecimiento, 730 (4.6%) resultaron positivas al IAV. De los 522 grupos de cerdos de crecimiento de los cuales se recolectaron las muestras, 110 grupos (20.8%) presentaron por lo menos una muestra nasal positiva. Las muestras nasales positivas procedían de 23 de las 26 granjas participantes. Las características a nivel de granja asociadas con la presencia de IAV incluyeron (ciclo completo; índice de probabilidad [OR, por sus siglas en inglés] 3.05; destete OR 16.69), flujo de cerdos (todo dentro-todo fuera OR 0.31 por edificio; OR 0.35 por sitio), origen de primerizas (granjas que crían sus propias primerizas, criadas fuera de sitio y que regresan OR 0.17; multiplicadora fuera de sitio OR 0.25), temperatura medio ambiental, y la velocidad del viento. Implicaciones: La dinámica de la población, por ejemplo, las granjas de ciclo completo y manejo continuo, juegan un papel muy importante en la epidemiología del IAV. Se deberían considerar posibles modificaciones al tipo de granja y flujo de cerdos cuando se planean estrategias de prevención y control del IAV. | ResuméObjectif: Étudier l’association entre certains facteurs de risque à la ferme et la présence du virus de l’influenza A (VIA) sur des fermes de porcs en croissance. Matériels et méthodes: Vingt-six fermes ont participé à cette étude. Trente écouvillons nasaux par mois provenant de porc en croissance furent collectés sur chaque ferme pour 12 ou 24 mois consécutifs entre 2009 et 2011. Les écouvillons nasaux furent testés pour VIA par réaction d’amplification en chaîne en temps réel avec la transcriptase réverse. Des stations météorologiques étaient installées à chaque ferme participante afin de suivre la température, l’humidité relative, l’intensité de la lumière, et la vitesse des vents et des bourrasques. Des données sur la ferme furent obtenues à l’aide d’un questionnaire pour évaluer la relation entre la présence de VIA et les caractéristiques de la ferme. Résultats: Sur les 15,630 écouvillons nasaux prélevés des porcs en croissance, 730 (4,6%) se sont avérés positifs pour VIA. Sur les 522 groupes de porcs en croissance à partir desquels les écouvillons nasaux furent prélevés, 110 groupes (20,8%) avaient au moins un écouvillon nasal positif. Les écouvillons nasaux positifs provenaient de 23 des 26 fermes participantes. Les caractéristiques des fermes associées à la présence de VIA incluaient le type de ferme (ratio de cote [OR] naisseur-finisseur 3,05; OR pouponnière 16,69), le flot des animaux (OR tout plein-tout vide 0,31 par bâtiment; OR 0,35 par site), la source des cochettes (nées sur le site de reproduction, élevées hors-site, et retournées sur la ferme OR 0,17; multiplicateur hors-site OR 0,25), la température environnante, et la vitesse des vents. Implications: Les dynamiques de populations (pouponnières et les fermes naisseur-finisseur et un flot continu d’animaux) jouent des rôles importants dans l’épidémiologie du VIA. Des modifications possibles au type de ferme et au flot des animaux devraient être pris en considération lors de l’élaboration de stratégies de prévention et de limitation du VIA. |
Keywords: swine, influenza A virus, risk factors, meteorological conditions, weather
Search the AASV web site
for pages with similar keywords.
Received: August 26, 2013
Accepted: December 3, 2013
Influenza A virus (IAV) is an RNA, single-stranded, negative-sense virus belonging to the Orthomyxoviridae family. The virus can infect humans and certain domestic and wild animal species, including avian, porcine, equine, canine, feline, and marine mammals. In swine, the virus is considered to play a primary role in polymicrobial respiratory-disease events.1 Swine have been recognized as an important host species for IAV, since they may be potential sources for both zoonotic infections and novel viruses through reassortment.2-4
Among the different IAV subtypes, three (H1N1, H1N2, H3N2) have been circulating in the swine population during recent decades.5-7 Infections in swine are characterized by high morbidity and low mortality. Infected pigs may exhibit sneezing, coughing, lethargy, fever, anorexia, and rhinorrhea.8 Infected pigs start shedding infectious viral particles through their nasal secretions 1 day after infection, and individual pigs can continue to shed for 7 days. Introduction of new viruses into pig farms may accompany infected replacement animals.5,9 Pigs infected with influenza A virus can generate infectious aerosols that may play a role in regional dissemination.10 However, there are gaps in our knowledge of airborne and regional transmission routes and also many unanswered questions regarding the overall epidemiology and possible modes of transmission of influenza in swine.
Risk-factor studies based on serologic data have generated valuable information regarding the epidemiology of influenza in swine. The presence of anti-influenza antibodies has been associated with high farm density, farm type, herd size, female replacement rates, pen density, uncontrolled access of people, and indoor housing.11-16 However, to the authors’ knowledge, there are no risk factor studies associating the presence of the virus itself with farm-level characteristics. Therefore, the objective of this study was to collect virologic and epidemiologic data to investigate whether certain farm-level risk factors are associated with the presence of IAV in growing pigs.
Materials and methods
Procedures performed in this study were approved by the University of Minnesota Institutional Care and Use Committee.
Study population
This study was part of a larger IAV active surveillance study conducted in the midwestern United States that started in June 2009 and ended in December 2011.17 A total of 32 conveniently selected farms were enrolled, for which the primary objective was to actively monitor IAV in growing pigs over time. Six farms withdrew from the surveillance study, leaving 26 farms located in Illinois (n = 4), Indiana (n = 8), Iowa (n = 10), and Minnesota (n = 4) remaining enrolled.
Sample collection and testing
Sample collection began once producers agreed to participate and lasted for 12 or 24 months. On each farm, nasal swabs (Starswab II; Starplex Scientific Inc, Etobicoke, Ontario, Canada) were collected monthly from a convenience sample of 30 growing pigs (95% confident of detecting at least one positive swab when virus prevalence is at least 10%) at approximately 10 weeks old. On pig farms where there were multiple age groups, the investigator selected the age group of pigs that was closest to 10 weeks old. Nasal-swab samples collected during the monthly visit belonged to pigs from the same age-group cohort. Swabs were individually labeled with the farm identification number, state, month, and sample number. Samples were placed in a Styrofoam container with ice packs and shipped overnight to the virology department of St Jude Children’s Research Hospital (Memphis, Tennessee) for IAV testing by real-time reverse transcriptase polymerase chain reaction (RRT-PCR).17-19
Data collection
A questionnaire (Figure 1) containing close-ended questions was designed to capture data on farm characteristics. The survey was modeled after biosecurity questionnaires accepted by the swine industry and available through the American Association of Swine Veterinarians Production Animal Disease Risk Assessment Program (AASV PADRAP at www.padrap.org).
Figure 1: Farm characteristics questionnaire designed to capture data from 26 pig farms in the midwestern United States for an influenza A virus (IAV) risk-factor study. The questionnaire was based on biosecurity questionnaires accepted by the swine industry, such as those available from the American Association of Swine Veterinarians Production Animal Disease Risk Assessment Program (www.padrap.org). The questionnaire was administered to producers from commercial farms participating in a surveillance program wherein monthly nasal swabs were collected from growing pigs for 12 to 24 months to test for IAV in an investigation of the association between certain farm-level risk factors and the presence of IAV.
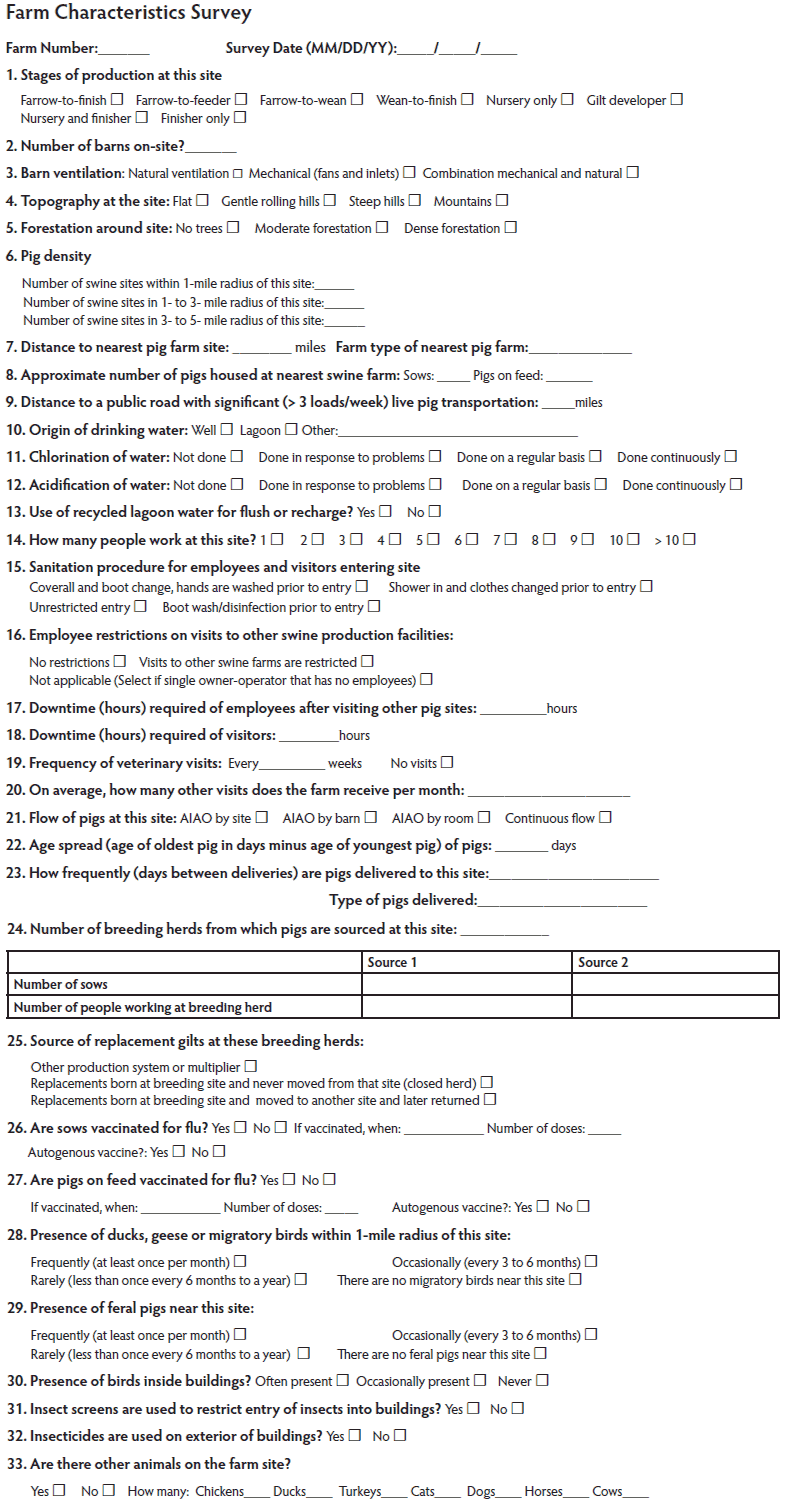
Specifically, the survey assessed farm type, regional farm density, topography surrounding the site, entrance biosecurity measures for employees and visitors, pig flow, origin of pigs, source of gilts, vaccination history, water-treatment protocols, and number of people working at the farm. The person collecting the monthly nasal swabs administered the survey to the owner or farm manager near the time of completion of the study.
Meteorological data collection
A weather station (HOBO; Onset Computer Corporation, Bourne, Massachusetts), set up to log data every hour, was placed 20 to 30 m away from the pig barns on each of the participating farms. Meteorological data recorded included temperature (°C), relative humidity (%), sunlight intensity (watts per m2), wind direction (degrees), wind speed (km per hour), and wind-gust speed (km per hour). Data were downloaded into a portable computer from the weather station monthly on the day nasal swabs were collected.
Data analysis
For the purpose of this study, a group of pigs was defined as the sample set of 30 growing pigs selected for monthly monitoring. A group was considered positive if at least one nasal swab tested RRT-PCR-positive for IAV.
Repeated measures logistic regression with an autoregressive correlation matrix was used to assess the relationship between farm IAV status and farm-level risk factors. Farm was included in the model as a random effect. One logistic model examined the relationship between IAV status of the group and farm-level characteristics, and a second assessed the weather data.
Univariate analysis was performed to screen variables, and those with a P value < .25 were considered for further analysis. A multivariable model was built by forcing all variables that met the screening criteria, and a stepwise backward elimination procedure was employed for model simplification by removing variables with P ≥ .05. Farm was included in the model as a random effect to account for clustering of the groups of pigs per farm. Month was included in the model as the repeated measure under an autoregressive correlation structure matrix. P < .05 was considered statistically significant in all analyses. SAS 9.2 (SAS Institute Inc, Cary, North Carolina) was used for all statistical procedures.
Results
A total of 522 groups with a mean and median age of 13.5 and 13.0 weeks, respectively, were screened for IAV. Age ranged from 3.5 to 31.0 weeks of age. Eight farms were finishing farms, eight were wean-to-finish farms, four were farrow-to-finish farms, four were nursery-to-finisher farms, one was a nursery, and one was a gilt developer unit.
The number of visits per farm was not constant due to absence of pigs, time constraints, or farms withdrawing from the study. Of the 26 farms enrolled in the study, one was visited 25 times, 14 were visited 24 times, one was visited 23 times, one was visited 21 times, two were visited 17 times, six were visited 12 times, and one was visited 11 times between June 2009 and December 2011. Thus, 32.1%, 24.7%, 18.3%, 15.5%, 4.6%, and 4.6% of the samples originated from groups of pigs in finisher, wean-to-finish, farrow-to-finish, nursery-finisher, nursery, and gilt developer unit farms, respectively.
At the individual level, 730 of 15,630 nasal swabs (4.7%) tested positive for IAV, whereas at the group level, 110 of 522 groups of pigs (21.1%) had at least one RRT-PCR-positive nasal swab. All but three farms had at least one IAV-positive group. The three farms with no IAV-positive groups were monitored for 12 months or less.
Farm-level factors
For the univariate analysis, the odds of testing positive for IAV were 3.05 and 16.69 times higher for farrow-to-finish and nursery farms, respectively, than for finishing farms (Table 1). Pig farms that managed their pigs all-in, all-out (AIAO) by barn or by site, compared to farms that managed pigs in a continuous flow, had lower odds of testing positive for IAV (0.31 for AIAO by barn, 0.35 for AIAO by site). Growing pigs born in sow farms in which gilts originated from an off-site facility had lower odds (0.17 if gilts were born at a breeding site, moved to another site and later returned; 0.25 if gilts originated from a multiplier) than did pigs born in sow farms in which replacement gilts were born at the breeding site and never moved from that site. The presence at the site of other animals, such as dogs and cats, was not identified as a significant risk factor for detection of IAV in pigs. Of the four variables included in the multivariable model (ie, farm type, pig flow, gilt source, and presence of other animals), only farm type remained significant.
Table 1: Farm-level factors univariably associated with the presence of influenza A virus in 26 commercial farms in the midwestern United States*
| Risk factor | Odds ratio | 95% CI | P† |
|---|---|---|---|
| Farm type | |||
| Finisher (referent) | 1 | NA | NA |
| Farrow-to-finish | 3.05 | 1.56-5.95 | < .001 |
| Wean-to-finish | 0.89 | 0.44-1.80 | .76 |
| Nursery | 16.69 | 5.34-52.18 | < .001 |
| Gilt developer unit | 1.11 | 0.31-3.94 | .86 |
| Nursery-finisher | 0.78 | 0.33-1.82 | .58 |
| Sow vaccination for influenza | 1.09 | 0.31-3.75 | .89 |
| No. of barns on site | 1.03 | 0.89-1.20 | .64 |
| Barn ventilation | |||
| Natural and mechanical (referent) | 1 | NA | NA |
| Natural ventilation | 0.55 | 0.17-1.77 | .32 |
| Mechanical ventilation | 1.33 | 0.52-3.40 | .55 |
| Topography – gentle rolling hills | 0.76 | 0.30-1.94 | .56 |
| Absence of trees surrounding the site | 0.72 | 0.24-2.13 | .56 |
| No. of pig farms within 1 mile | 1.01 | 0.70-1.46 | .94 |
| Distance to closest pig farm | 0.86 | 0.62-1.19 | .37 |
| Distance to closest road | 0.89 | 0.71-1.10 | .30 |
| Drinking water chlorinated | 1.56 | 0.52-4.70 | .42 |
| Drinking water acidified | 1.56 | 0.52-4.70 | .42 |
| Recycled lagoon water for flush or recharge | 1.08 | 0.11-9.88 | .95 |
| No. of employees at the site | 1.04 | 0.88-1.22 | .62 |
| Entrance sanitation procedure | |||
| Shower in and clothes changed (referent) | 1 | NA | NA |
| No measures | 0.39 | 0.10-1.56 | .19 |
| Boot wash and disinfection | 0.54 | 0.11-2.67 | .45 |
| Coverall and boot change, hands washed | 0.44 | 0.16-1.19 | .11 |
| Employee restrictions on visits to other pig farms | 0.87 | 0.37-2.03 | .76 |
| Downtime required for employees after visiting other pig farms | 1.19 | 0.74-1.93 | .46 |
| Downtime required for visitors | 1.12 | 0.67-1.86 | .64 |
| Frequency of veterinary visits | 0.97 | 0.93-1.02 | .34 |
| Pig flow | |||
| Continuous flow (referent) | 1 | NA | NA |
| All-in, all-out by barn | 0.31 | 0.14-0.66 | < .01 |
| All-in, all-out by site | 0.35 | 0.14-0.88 | .03 |
| No. of sow herds supplying pigs | 1.51 | 0.59-3.82 | .40 |
| Sow herd size | 1 | 1.0-1.0 | .31 |
| No. of workers at the sow herd | 0.97 | 0.90-1.04 | .46 |
| Growing pigs vaccinated for influenza | 1.14 | 0.27-4.78 | .85 |
| Gilt source | |||
| Born at breeding site and never moved from that site (referent) | 1 | NA | NA |
| Born at breeding site; moved to another site, later returned | 0.17 | 0.04-0.63 | < .01 |
| Multiplier | 0.25 | 0.09-0.68 | < .01 |
| Presence of migratory birds within 1-mile radius of the site | |||
| Frequently (once per month) (referent) | 1 | NA | NA |
| Never | 1.48 | 0.38-5.81 | .57 |
| Rarely (< once every 6 months) | 2.12 | 0.70-6.37 | .18 |
| Occasionally (every 3 to 6 months) | 1.71 | 0.53-5.50 | .37 |
| Presence of feral pigs near the site | 1.59 | 0.20-12.14 | .65 |
| Presence of birds inside buildings | 0.76 | 0.33-1.74 | .53 |
| Use of insecticides on building exterior | 1.74 | 0.55-5.49 | .34 |
| Presence of other animals in the farm‡ | 1.58 | 0.72-3.45 | .24 |
* Farms participated in a surveillance program wherein monthly nasal swabs were collected from growing pigs for 12 to 24 months to test for influenza A virus in an investigation of the association between certain farm-level risk factors and the presence of IAV. Farm-specific characteristics obtained through the questionnaire are described in Figure 1.
† Repeated measures logistic regression with an autoregressive correlation matrix; P < .05 considered statistically significant.
‡ Animals such as dogs and cats that are allowed entrance into the farm; not wildlife.
NA = not applicable.
Meteorological factors
All measures met the multivariable model inclusion criteria (Table 2). Temperature and wind speed remained in the final multivariable model after backwards stepwise elimination. Each degree increase in temperature increased the likelihood of a group of pigs testing positive for IAV by 1.04 (95% CI, 1.01-1.07). Similarly, the likelihood of testing positive for IAV increased 1.24 times with every km per hour increase in wind speed (95% CI, 1.08-1.43).
Table 2: Meteorological factors univariably associated with the presence of influenza A virus in growing pigs in the midwestern United States*
| Variable | Odds ratio | 95% CI | P† |
|---|---|---|---|
| Temperature (°C) | 1.02 | 0.99-1.05 | .06 |
| Relative humidity (%) | 0.96 | 0.93-0.99 | .02 |
| Light intensity (watts/m2) | 1.01 | 1.00-1.01 | .02 |
| Wind direction (Ø degrees) | 0.99 | 0.98-1.00 | .11 |
| Wind speed (km/hour) | 1.17 | 1.02-1.34 | .02 |
| Wind gusts (km/hour) | 1.13 | 1.02-1.25 | .02 |
* Farm-specific characteristics obtained through the questionnaire described in Figure 1. Meteorological data recorded by weather stations (Hobo; Onset Computer Corporation, Bourne, Massachusetts) 20-30 m from the barns.
† Repeated measures logistic regression with an autoregressive correlation matrix; P < .05 considered statistically significant
Discussion
Fully understanding the ecology, evolution, and transmission of influenza A viruses requires both virological detection techniques and epidemiological investigation methods such as we have described in the present study. Although previous studies have associated farm-level characteristics with increased risk of seropositivity for IAV, to the authors’ knowledge, this is the first study in which the presence of the virus in pig farms has been associated with farm-level and meteorological risk factors in swine.
The infection dynamics of the IAV are farm-type dependent. Farm type has been found as a significant risk factor according to our study reported here and in a previously published serological risk factor study.14 Both studies concluded that finisher pigs were more likely to be IAV-positive when sows were on site than were finisher pigs raised on farms separated from the sow herd. It has been reported that pigs become infected at an earlier age in farrow-to-finish farms than in finisher-only herds.20 In farrow-to-finish farms, which contain all the different age groups of pigs on the same site, the virus is allowed to perpetuate due to the constant presence of susceptible individuals with waning maternal antibodies (ie, suckling piglets approaching weaning age). The existence of susceptible individuals of varying ages is absent in finishing farms that contain pigs only in the later stages of growth. Nursery farms, like farrow-to-finish farms, also have a constant influx of recently weaned pigs which provides the necessary conditions for IAV to maintain transmission between older pigs and the incoming pigs. Additionally, it has been reported that recently weaned pigs themselves can be a source of virus by introducing new viruses into recipient barns.21,22 Furthermore, personnel and equipment shared between these different age groups may play a role in IAV transmission, since it is known that the virus can survive outside the swine host.23-27 Since introduction of infected animals is one of the most important risk factors for new pathogen introduction into a farm,28 it is not surprising that pig movement within a farm is an important factor to consider when assessing risk of IAV in pigs. There are two main types of animal flows: AIAO and continuous flow. In an AIAO management system, the entire building is emptied out at one time, and then one age group of pigs enters the building, moving through the production system together. Conversely, continuous flow means that pigs are constantly entering and exiting the building, and pigs of different age groups are housed in the same building. It has been reported that AIAO pig flow has a significant positive impact on growth rate, since pigs are not being challenged with infectious agents from older pigs.28-30 Our data show that pigs were less likely to test positive for IAV if they were raised under an AIAO system as analyzed by barn (OR = 0.31; 95% CI, 0.14-0.66) or by site (OR = 0.35; 95% CI, 0.15-0.88) than if they were raised under continuous-flow management. Pigs of different age groups are not exposed to one another when utilizing AIAO flow, which precludes horizontal transmission of infectious agents from older to younger pigs, a known mechanism for IAV maintenance in pig populations.5
In addition to pig flow, gilt replacement source was also associated with IAV positivity. Groups of pigs born in farms where the gilt source was an off-site facility had a lower likelihood of testing IAV- positive than did groups of pigs born in sow farms where gilts were born and raised on-site. This finding contrasts with what has been previously published, in that farms introducing replacement animals were reported to be at higher risk of being IAV-seropositive.5,14-16 Furthermore, introduction of replacement animals should also maintain the circulation of IAV in the population due to the influx of susceptible animals and new virues.5 Yet this appeared not to be the case in our study: detection of IAV in growing pigs was less frequent on farms that introduced gilts raised in off-site facilities. In today’s swine farms, veterinarians are aware of the importance of controlling gilt introduction to reduce disease transmission. It will be important in future studies to determine if incoming gilts from high-health multiplier systems with no detectable IAV, or even from AIAO gilt developer units with infrequent detection of IAV, affect the number of viral introductions into the recipient farms.
Even in today’s swine farms, where pigs are raised entirely indoors, weather conditions still influence the environment of the pig inside the barn (for example, low outside temperatures trigger the need to provide a heat source to increase barn temperature). Data on the relationship between environmental conditions and the presence of IAV in pigs is scarce. However, there is data regarding environment and disease transmission in other species or involving different microbiological agents. Specifically, meteorological conditions have been associated with regional dissemination of equine IAV in Australia31 and with airborne detection of porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae in pigs.32,33 Our study detected an association, albeit with a measurably small effect with OR near 1.0, between two meteorological variables and the presence of IAV in groups of growing pigs. As outside temperature and wind speed increased, the likelihood of a group of pigs being infected with IAV increased. This may seem counter-intuitive, as some experimental work with guinea pigs has shown that at high temperatures (30°C) influenza aerosol transmission ceased.34 However, follow-up studies on the effects of temperature and influenza transmission showed that while aerosol transmission decreased at 30°C, direct-contact transmission was still maintained in the experimentally infected guinea pigs.34,35 A reasonable interpretation for the relationship between temperature increase and presence of IAV in swine farms may be that as environmental temperature increases, the temperature of the barn increases as well, creating the need for increased air movement to reduce ambient temperature. A higher rate of air exchange in the building can be accomplished either by increasing exhaust fan speed or by lowering the curtains, increasing the entry of external air particles that may include airborne pathogens. However, it has been reported36 that higher rates of air exchange in pig units have a protective effect on pneumonia lesions at slaughter, suggesting that as the concentration of housing-unit air particles decreases, respiratory lesions decrease. On the other hand, increasing wind speed may reduce external temperatures, but it is not clear what impact this has on pig-barn environmental conditions. Also unclear is the effect of wind direction on the odds of being IAV-positive. In particular, it would be interesting, and perhaps enlightening, to correlate not only wind direction but direction of the nearest farm in relationship to wind direction (eg, downwind or upwind). In this study, we recorded only the distance to the nearest farm and not the location of the nearest farm. Even though nearest farm location was not recorded, neither wind direction nor nearest neighbor nor number of pigs farms nearby were significant. Thus, directionality or predominant wind direction may be a moot point. Finally, one could speculate that at higher wind speeds, virus particles would become disrupted, and a so-called “viral cloud” could not stay intact. These associations and speculations require further investigation to deepen our understanding of the impact of meteorological conditions on disease within the barn. Until more studies are performed examining larger numbers of farms, complete risk factor analyses that include not only the farm characteristics presented here but also meteorological data, efforts at controlling IAV may best be focused on the more impactful variables of pig flow, gilt source, and farm type rather than environmental conditions and weather. The small number of farms in this study was likely a limitation.
Farm-level risk factors identified in this study provide insights into understanding the epidemiology of influenza in swine. Virological detection coupled with risk-factor identification through epidemiological investigations such as this are encouraged as part of a global surveillance effort not only to more fully understand IAV in pigs, but also to assess the impact swine IAV may have on human health.37 In addition, determining virus genetic characteristics is also encouraged in an attempt to further elucidate possible virus-level characteristics important for IAV control in swine populations. This study emphasizes the importance of population dynamics, in that certain farm-type facilities (nursery and farrow-to-finish) and pig flow (continuous flows) play a role in the epidemiology of the disease due to the constant entry of animals into a population, providing the necessary susceptible hosts for virus maintenance and increased likelihood of IAV detection. Therefore, efforts should be made to decrease IAV transmission and endemic IAV infections by managing closed populations and pig movements AIAO, which will benefit not only pigs but also possibly humans.
Implications
• Growing pigs on farms where sows are present or where replacement gilts are raised on site are at higher risk of testing positive for IAV than pigs in finishing farms or farms that introduce replacement gilts from outside sources.
• Management practices such as AIAO may reduce the likelihood that pigs test positive for IAV.
• More research is needed to fully understand the relationships between weather and IAV in pig populations.
Acknowledgements
This research was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services under Contract No. HHSN266200700005C and by the American Lebanese Syrian Associated Charities.
Conflict of interest
None reported.
References
1. Opriessnig T, Gimenez-Lirola LG, Halbur PG. Polymicrobial respiratory disease in pigs. Anim Health Res Rev. 2011;12:133–148.
2. Ma W, Lager KM, Vincent AL, Janke BH, Gramer MR, Richt JA. The role of swine in the generation of novel influenza viruses. Zoonoses Public Health. 2009;56:326–337.
3. Van Reeth K. Avian and swine influenza viruses: our current understanding of the zoonotic risk. Vet Res. 2007;38:243–260.
4. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179.
5. Brown IH. The epidemiology and evolution of influenza viruses in pigs. Vet Microbiol. 2000;74:29–46.
6. Vincent AL, Ma W, Lager KM, Janke BH, Richt JA. Swine influenza viruses: a North American perspective. Adv Virus Res. 2008;72:127–154.
7. Webby RJ, Rossow K, Erickson G, Sims Y, Webster R. Multiple lineages of antigenically and genetically diverse influenza A virus co-circulate in the United States swine population. Virus Res. 2004;103:67–73.
8. Olsen CW, Brown IH, Easterday BC, Van Reeth K. Swine influenza. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, eds. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Publishing; 2006:469–482.
9. Mohan R, Saif YM, Erickson GA, Gustafson GA, Easterday BC. Serologic and epidemiologic evidence of infection in turkeys with an agent related to the swine influenza virus. Avian Dis. 1981;25:11–16.
10. Corzo CA, Romagosa A, Dee SA, Gramer MR, Morrison RB, Torremorell M. Relationship between airborne detection of influenza A virus and the number of infected pigs. Vet J. 2013;196:171–175.
11. Ewald C, Heer A, Havenith U. Factors associated with the occurrence of influenza A virus infections in fattening swine. Berliner und Munchener Tierarztliche Wochenschrift (Berlin). 1994;107:256–262.
12. Maes D, Deluyker H, Verdonck M, Castryck F, Miry C, Vrijens B, de Kruif A. Herd factors associated with the seroprevalences of four major respiratory pathogens in slaughter pigs from farrow-to-finish pig herds. Vet Res. 2000;31:313–327.
13. Mastin A, Alarcon P, Pfeiffer D, Wood J, Williamson S, Brown I, COSI Consortium, Wieland B. Prevalence and risk factors for swine influenza virus infection in the English pig population. PLoS Curr. 2011;3:RRN1209. doi: 10.1371/currents.RRN1209.
14. Poljak Z, Friendship RM, Carman S, McNab WB, Dewey CE. Investigation of exposure to swine influenza viruses in Ontario (Canada) finisher herds in 2004 and 2005. Prev Vet Med. 2008;83:24–40.
15. Simon-Grife M, Martin-Valls GE, Vilar MJ, Garcia-Bocanegra I, Mora M, Martin M, Mateu E, Casal J. Seroprevalence and risk factors of swine influenza in Spain. Vet Microbiol. 2011;149:56–63.
16. Suriya R, Hassan L, Omar AR, Aini I, Tan CG, Lim YS, Kamaruddin MI. Seroprevalence and risk factors for influenza A viruses in pigs in Peninsular Malaysia. Zoonoses Public Health. 2008;55:342–351.
17. Corzo CA, Culhane M, Juleen K, Stigger- Rosser E, Ducatez MF, Webby RJ. Active surveillance for influenza A virus among swine, midwestern United States, 2009–2011. Emerg Infect Dis. 2013;19:954–960. doi: 10.3201/eid1906.121637.
18. Ducatez MF, Hause B, Stigger-Rosser E, Darnell D, Corzo C, Juleen K, Simonson R, Brockwell-Staats C, Rubrum A, Wang D, Webb A, Crumpton JC, Lowe J, Gramer M, Webby RJ. Multiple reassortment between pandemic (H1N1) 2009 and endemic influenza viruses in pigs, United States. Emerg Infect Dis. 2011;17:1624–1629.
19. Richt JA, Lager KM, Clouser DF, Spackman E, Suarez DL, Yoon KJ. Real-time reverse transcription-polymerase chain reaction assays for the detection and differentiation of North American swine influenza viruses. J Vet Diagn Invest. 2004;16:367–373.
20. Loeffen WL, Hunneman WA, Quak J, Verheijden JH, Stegeman JA. Population dynamics of swine influenza virus in farrow-to-finish and specialized finishing herds in the Netherlands. Vet Microbiol. 2009;137:45–50.
21.Allerson MW, Davies PR, Gramer MR, Torremorell M. Infection dynamics of pandemic 2009 H1N1 influenza virus in a two-site swine herd. Transbound Emerg Dis. 2013. doi: 10.1111/tbed.12053.
*22. Brown WS, Zimmerman J, Karriker L, Schwartz K, Rademacher C, Hoogland M, Main R, Swalla R. Detection of PRRSV and SIV in oral fluids from pigs one week postweaning. Proc AASV. Denver, Colorado. 2012;301.
23. Bean B, Moore BM, Sterner B, Peterson LR, Gerding DN, Balfour HH Jr. Survival of influenza viruses on environmental surfaces. J Infect Dis. 1982:146:47–51.
24. Haas B, Ahl R, Bohm R, Strauch D. Inactivation of viruses in liquid manure. Rev Sci Tech. 1995;14:435–445.
25. Stallknecht DE, Shane SM, Kearney MT, Zwank J. Persistence of avian influenza viruses in water. Avian Dis. 1990;34:406–411.
26. Thomas Y, Vogel G, Wunderli W, Suter P, Witschi M, Koch D, Tapparel C, Kaiser L. Survival of influenza virus on banknotes. Appl Environ Microbiol. 2008;74:3002–3007.
27. Tiwari A, Patnayak DP, Chander Y, Parsad M, Goyal SM. Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Dis. 2006;50:284–287.
28. Scheidt AB, Cline TR, Clark LK, Mayrose VB, Van Alstine WG, Diekman MA, Singleton WL. The effect of all-in all-out growing-finishing on the health of pigs. Swine Health Prod. 1995;3:202–205.
29. Diekman MA, Scheidt AB, Grant AL, Kelly DT, Sutton AL, Martin TG, Cline TR. Effect of vaccination against Mycoplasma hyopneumoniae on health, growth, and pubertal status of gilts exposed to moderate ammonia concentrations in all-in-all-out versus continuous-flow systems. Swine Health Prod. 1999;7:55–61.
30. Ice AD, Grant AL, Clark LK, Cline TR, Einstein ME, Martin TG, Diekman MA. Health and growth performance of barrows reared in all-in/all-out or continuous flow facilities with or without a chlortetracycline feed additive. Am J Vet Res. 1999;60:603–608.
31. Firestone SM, Cogger N, Ward MP, Toribio J, Moloney BJ, Dhand NK. The influence of meteorology on the spread of influenza: Survival analysis of an equine influenza (A/H3N8) outbreak. PLoS One. 2012;7:e35284. doi: 10.1371/journal.pone.0035284.
32. Dee S, Otake S, Oliveira S, Deen J. Evidence of long distance airborne transport of porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae. Vet Res. 2009;40:39. doi: 10.1051/vetres/2009022.
33. Otake S, Dee S, Corzo C, Oliveira S, Deen J. Long-distance airborne transport of infectious PRRSV and Mycoplasma hyopneumoniae from a swine population infected with multiple viral variants. Vet Microbiol. 2010;145:198–208.
34. Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathogens. 2007;3(10):e151. doi: 10.1371/journal.ppat.0030151.
35. Lowen AC, Steel J, Mubareka S, Palese P. High temperature (30°C) blocks aerosol but not contact transmission of influenza virus. J Virol. 2008;82:5650–5652.
36. Flesja KI, Forus IB, Solberg I. Pathological lesions in swine at slaughter. V. Pathological lesions in relation to some environmental factors in the herds. Acta Vet Scand. 1982;23:169–183.
37. Vincent A, Awada L, Brown I, Chen H, Claes F, Dauphin G, Donis R, Culhane M, Hamilton K, Lewis N, Mumford E, Nguyen T, Parchariyanon S, Pasick J, Pavade G, Pereda A, Peiris M, Saito T, Swenson S, Van Reeth K, Webby R, Wong F, Ciacci-Zanella J. Review of influenza A virus in swine worldwide: A call for increased surveillance and research. Zoonoses Public Health. 2014;61:4–17. doi: 10.1111/zph.12049.
* Non-refereed reference.
