| Case study | Peer reviewed |
Cite as: Scherba G, Bromfield CR, Jarrell VL, et al. Evaluation of responses to both oral and parenteral immunization modalities for porcine epidemic diarrhea virus in production units. J Swine Health Prod. 2016;24(1):29–35.
Also available as a PDF.
SummaryThe immune responses (serum anti-porcine epidemic diarrhea virus [PEDV] immunoglobulin G [IgG] and milk antiviral neutralizing antibodies) induced by various combinations of two PEDV immunization modalities (vaccine and oral immunization) were examined in unrelated swine production units in different locations. Anti-PEDV antibodies were undetectable in serum and milk of the control group (non-vaccinated and non-infected). Sows in the unit that received only the PED vaccine (iPED+; Harrisvaccines, Inc, Ames, Iowa) (two doses) remained naive for the wild-type virus and did not develop milk anti-PEDV neutralizing immunoglobulin titers as high as those in the other three production units, which had received oral immunization. Milk anti-PEDV antibody titers in the orally immunized sows appeared to be of longer duration than serum antiviral IgG concentrations. This indicates that oral immunization may be the more efficacious PEDV immunization modality, especially with regard to the production of milk antiviral antibody levels. | ResumenLa respuesta inmune (suero contra el virus de la diarrea epidémica porcina [PEDV por sus siglas en inglés], inmunoglobulina G [IgG por sus siglas en inglés], y anticuerpos neutralizantes antivirales en la leche) inducida por varias combinaciones de dos modalidades de inmunización del PEDV (vacuna e inmunización oral) fueron examinadas en unidades de producción porcina no relacionadas, y en diferentes ubicaciones. Los anticuerpos contra el PEDV no se detectaron en suero y leche en el grupo control (no vacunados y no infectados). Las hembras en la unidad que recibieron únicamente la vacuna contra PED (iPED+; Harrisvaccines, Inc, Ames, Iowa) (dos dosis) y que no tuvieron contacto con el virus de campo no desarrollaron en leche, una carga de Igs neutralizantes contra el PEDV tan alta, al compararlas con las de las hembras, en las otras tres unidades de producción, que recibieron inmunización oral. Las cargas de anticuerpos contra el PEDV de leche en las hembras inmunizadas oralmente parecen ser de más larga duración que las concentraciones de IgG antivirales en suero. Esto indica que la inmunización oral puede ser la modalidad de inmunización PEDV más eficaz, especialmente en lo que se refiere a la producción de los niveles de anticuerpos antivirales de la leche. | ResuméLes réponses immunitaires (immunoglobulines G [IgG] sériques anti-virus de la diarrhée épidémique porcine [VDEP] et anticorps neutralisants antiviraux du lait) induites par diverses combinaisons de deux modalités d’immunisation contre le VDEP (vaccin et immunisation orale) ont été examinées dans des unités de production porcine non-apparentées dans des localisations différentes. Les anticorps anti-VDEP étaient non-détectables dans le sérum et le lait des animaux témoins (non-vaccinés et non-infectés). Les truies dans les unités qui ont reçu uniquement le vaccin DEP (iPED+; Harrisvaccines, Inc, Ames, Iowa) (deux doses) et qui sont demeurées naives pour la souche sauvage du virus, ne développèrent pas de titres d’Ig anti-VDEP neutralisants dans le lait aussi élevés que celles dans les trois autres unités de production qui avaient reçu une immunisation orale. Les titres d’anticorps anti-VDEP dans le lait des truies immunisées par voie orale ont semblé durer plus longtemps que les concentrations d’IgG antivirales sériques. Ceci indique que l’immunisation orale pourrait être la modalité la plus efficace d’immunisation contre le VDEP, surtout en ce qui concerne la production d’anticorps antiviraux dans le lait. |
Keywords: swine, porcine epidemic diarrhea virus, immunofluorescent antibody assay, oral immunization, parenteral vaccine, PEDV
Search the AASV web site
for pages with similar keywords.
Received: December 17, 2014
Accepted: August 10, 2015
Since the incursion of porcine epidemic diarrhea virus (PEDV) into the United States in May 2013,1 its clinical disease presentation and pathology have appeared indistinguishable from those of another coronavirus, transmissible gastroenteritis virus (TGEV).1,2 Both cause significant enteric disease in the young animal, with 30% to 100% mortality in newborn and early-weaned pigs in naive herds.1 Although both viruses are classified in the Alphacoronavirus genus, they are antigenically distinct.1 In addition, empirical observations from swine practitioners and researchers indicate that a protective immune response to PEDV, unlike the response to TGEV, is of short duration. Animals that have recovered from an infection may be re-infected and manifest clinical disease just months later. Nevertheless, viral family characteristics suggest that immune responses and disease prevention approaches similar to those used against TGEV may be successful.
Coronaviruses infect their hosts by the oral route, with direct invasion of enterocytes from the intestinal lumen, and hence do not require viremic systemic spread. Transmission occurs through virus shedding in the feces where these enveloped viruses can remain highly infectious. Such pathogenesis suggests that mucosal immunity (secretory immunoglobulin A [IgA]) would be important, as opposed to serum antibodies (IgG). For example, previous studies have shown that serum antibodies do not provide significant protection against TGEV infections.3,4 Consequently, initial protection of the neonate depends upon receiving colostrum-derived neutralizing anti-viral antibodies. The colostrum and milk of sows orally inoculated or naturally infected with virulent TGEV contains primarily secretory IgA (considered optimal lactogenic immunity due to the resistance of IgA to proteolytic degradation in the neonatal gut), whereas colostrum and milk of sows that receive parenteral TGEV inoculation contains mainly IgG antibodies that do not persist in high levels.3
Traditionally, TGEV outbreaks in production units were successfully controlled by oral immunization of gilts and sows through feeding intestinal tracts from euthanized infected neonates.1 This method stimulated mucosal (gut-associated lymphoid tissues [GALT]) immunity in the dam.5 Such production of colostral anti-TGEV IgA and IgG antibodies would help protect the neonates from clinical disease. Consequently, this strategy has been employed to control PEDV outbreaks. However, advancements in vaccinology have presented a new approach. Specifically, Harrisvaccines, Inc, Ames, Iowa, generated a PEDV vaccine (iPED+) consisting of a transcriptional unit of the viral S (spike) gene encapsulated into particles for parenteral administration to gilts and sows. The PEDV S gene was selected because it encodes the viral attachment protein, a major neutralization target of the immune response. Although the company has received a conditional marketing license for this first-generation vaccine from the US Department of Agriculture, published field data are lacking. The parenteral route of immunization suggests that mucosal immunity may not be sufficiently engaged in the vaccinated animals for their disease protection, yet may produce adequate anti-PEDV antibody titers in colostrum or milk or both for protection of their neonates.
This case study examined the immune responses of sows in four independent production units to the various combinations of two immunization modalities (parenteral vaccine or oral immunization). Anti-PEDV antibody titers were determined in both serum and milk samples to elucidate whether oral immunization with infectious PEDV is required to develop detectable milk neutralizing-antibody titers or if parenteral iPED+ vaccination alone is sufficient.
Case farm systems
This project was reviewed and approved by the Institutional Animal Care and Use Committee of the University of Illinois.
Four swine production units were investigated. During the investigation period, Unit A remained naive (non-infected) to the wild-type virus, whereas the other three units (units B, C, and D) had experienced recent PEDV outbreaks. These four units provided a significant opportunity to evaluate the immune responses of sows following various combinations of the two immunization modalities (parenteral first generation iPED+ vaccine and oral immunization using neonatal intestines or contents). Since sampling was conducted in active production units, as opposed to experimentally designed cohorts, treatment group sizes varied; however, each group included at least six animals. A control group, consisting of gilts from an isolated research facility (n = 3) that were neither infected nor vaccinated, served to provide baseline data. Samples included serum (collected 48 hours and 3 weeks post partum) and milk (collected 48 hours post farrowing) from the sows in this study. We selected 48-hour samples so as not to interfere with colostral intake by the neonates, as colostrum begins to be replaced by milk approximately 24 to 36 hours post partum.6 Fecal swab samples from sows to monitor PEDV fecal shedding were collected only for unit D.
Unit A was a closed-herd, farrow-to-finish production unit of approximately 150 breeding females. The unit maintained three full-time dedicated workers (did not work at other farms). Feed was purchased from a central feed mill. Semen was purchased from boar stud farms free of porcine reproductive and respiratory syndrome virus (PRRSV) and PEDV. Most sows were parity 2 to 4; sows were rarely retained past parity 6. The unit farrowed approximately 25 to 30 sows or gilts every 5 weeks, annually adding approximately 60 replacement females. Gilts were vaccinated with FarrowSure Gold B (Zoetis, Inc, Kalamazoo, Michigan) 5 and 2 weeks prior to breeding to protect against parvovirus, erysipelas, and six Leptospira serovars, including bratislava. Gilts were also vaccinated with Litter Guard LTC (Zoetis) at 5 and 2 weeks pre-farrowing to provide protection for the piglets against Escherichia coli and Clostridium perfringens Type C. Sows received boosters with the same vaccines for each subsequent gestation. Both gilts and sows were boostered with Toxivac AD and E (Boehringer Ingelheim Vetmedica, Ames, Iowa) before each gestation to protect against Bordetella bronchiseptica, Erysipelothrix rhusiopathiae, and Pastueurella multocida. Neonates were vaccinated with CircoFlex (Boehringer Ingelheim Vetmedica) to protect against circovirus and Toxivac AD and E prior to weaning. The sows and gilts, six of which were included in this study, received two doses of first-generation iPED+ vaccine 3 weeks apart (per manufacturer’s recommendation), at 6 and 3 weeks pre-farrowing (Figure 1A). Milk and serum samples were collected 48 hours post partum, and serum samples were again collected 3 weeks later.
Figure 1: Timeline of events for the four production units in a case study to determine whether oral immunization of gilts or sows with infectious porcine epidemic diarrhea virus (PEDV) is required to develop detectable milk neutralizing-antibody titers, or if parenteral iPED+ vaccination (Harrisvaccines, Inc, Ames, Iowa) alone is sufficient. The approximate temporal occurrences of one or more natural PEDV outbreaks, oral immunization by feeding intestinal tracts from euthanized PEDV-infected neonates, parenteral administration of first generation iPED+ vaccine, and farrowing within each production unit (A, B, C, and D) are shown.
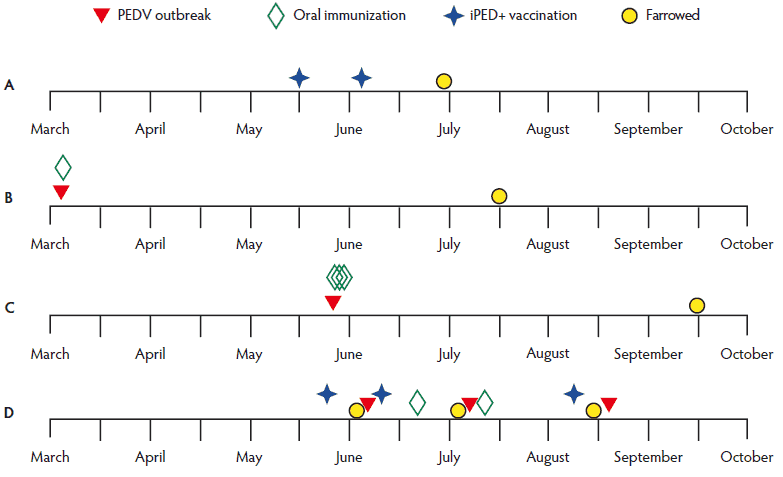
Unit B was a closed-herd, farrow-to-finish production system consisting of animals that were not vaccinated against PEDV prior to experiencing their first PEDV outbreak in March 2014; disease status was determined by diagnostic testing. The unit lacked any connection with the other production units. The farm internally generated their replacement gilts and used artificial insemination (AI) (semen purchased from boar stud farms free of PRRSV and PEDV), with the exception of natural service in an outdoor breeding system. Each month the unit bred approximately 30 gilts and batch-farrowed 150 sows or gilts or both, for an annual target of approximately 2000 liters. Gilts were vaccinated with PRRS MLV (Ingelvac), FarrowSure Gold B, autogenous swine influenza A virus, and CircoFlex at the time of selection and 1 month later. Sows were vaccinated with FarrowSure Gold B prior to breeding. All breeding animals were vaccinated quarterly with PRRS MLV. Both gilts and sows were vaccinated against swine influenza A virus and with ProSystem (Merck, Kenilworth, New Jersey) and Porcine Ecolizer 3 (Novartis, Greenfield, Indiana) at 5 and 2 weeks pre-farrowing. The PEDV outbreak initially involved second parity or greater sows, but eventually affected all parities, and occurred approximately 4 months pre-farrowing for the six sows included in this study. One oral immunization was performed in the herd immediately after the March 2014 outbreak by feeding back the intestines from clinically affected, euthanized neonates (Figure 1B); PEDV real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) cycle threshold (Ct) values were not determined. Decontamination protocol consisted of routine disinfection of all barns after the outbreak. Serum and milk samples were obtained from the sows 48 hours post partum.
Unit C was a 2650-head, breed-to-wean sow farm that was one of two in the system. Standard biosecurity practices consisted of dedicated caregivers, showers, washing trailers, and composting on site. The unit milled feed on-site that fed this farm as well as its off-site wean-to-market barns. The animals ranged from parity 1 (post wean) through 8, with all breeding done by AI (semen purchased from boar stud farms free of PRRSV and PEDV). The herd had no gilts on site, rather, on a weekly basis, receiving 26 first-parity sows from a separate, PEDV-free sow herd (parity segregation). Prior to receipt, gilts were vaccinated at 15 and 18 weeks of age with PRRS MLV (Ingelvac), at 20 weeks of age with FarrowSure Gold and iFluVent (Harrisvaccine, Ames, Iowa), at 22 weeks of age with Circumvent PCVM G2 (Merck), and at 24 weeks of age with FarrowSure Gold and iFluVent. Sows received ProSystem CE (Merck) and iFluVent at 5 and 4 weeks pre-farrowing, respectively. Annually (September), the herd was boostered with PRRS MLV. Even though the farm was PEDV-negative, management also included routine feedback, which included feeding mummies and feces to 22-week old gilts prior to breeding, and feeding scour material from neonates to sows at 6, 5, and 4 weeks pre-farrowing. The animals were not vaccinated against PEDV prior to experiencing their first PEDV outbreak in late May 2014. Disease status was determined by diagnostic testing. Sows were orally immunized on each of the 3 days after the outbreak began, approximately 12 weeks pre-farrowing for the six sows included in this study (Figure 1C); PEDV rRT-PCR Ct values were not determined. This PEDV immunization protocol was then discontinued as the farm worked to eradicate the virus. In addition, all neonates were euthanized for 14 consecutive days after the outbreak. Moreover, farrowing and gestation barns were aggressively washed and disinfected during this 12-week down period. Serum and milk samples were obtained from the sows 48 hours post partum.
Unit D was a closed-herd, farrow-to-finish production system of approximately 40 breeding females. The unit maintained two full-time, dedicated workers. Feed was purchased from a central feed mill. Semen was purchased from boar stud farms free of PRRSV and PEDV. Most sows were parity 2. The unit farrowed approximately 8 to 12 sows or gilts or both every 6 weeks, annually adding approximately 20 replacement females. Gilts were vaccinated with FarrowSure Gold 5 and 2 weeks prior to breeding. For each successive breeding, animals were boostered with Litter Guard LTC at 5 and 2 weeks pre-farrowing. Before each gestation, sows were boostered with the same vaccines, and both gilts and sows were boostered with Toxivac AD and E. Piglets were vaccinated with CircoFLEX prior to weaning and then vaccinated with Parasail (Newport Laboratories, Worthington, Minnesota) to protect against Hemophilus parasuis in the nursery. Each pregnant sow and gilt was vaccinated with the first-generation iPED+ vaccine. Within 2.5 weeks after their first iPED+ immunization, sows in the farrowing unit became PEDV-infected through a natural outbreak (June 2014), during which both adults and neonates manifested severe acute PEDV disease (Figure 1D). Initially the infection remained limited to the farrowing unit, but all pigs on the farm were exposed intentionally at this time (included gestation, growers, and finishing units). All neonates were euthanized and their intestinal contents were harvested. The infection status of the neonates born during this outbreak was verified by histopathology, bacterial culture, and PEDV rRT-PCR testing as routinely performed at the University of Illinois Veterinary Diagnostic Laboratory (UI VDL). Each pregnant sow in the gestation unit received 10 mL of intestinal slurry (Ct value of 18) added to their individual feed. Also, the farrowing unit was decontaminated prior to entry of the next group of sows. Subsequently, a second outbreak (July) occurred 4 weeks after the initial wild-type infection and 2 weeks after oral immunization of all gestating sows, including the next group of sows that farrowed just prior to this outbreak. This time, clinical disease was limited to the neonates in the farrowing unit; however, they manifested much milder clinical disease than that observed in the first outbreak. As before, all neonates were euthanized, then the farrowing unit was decontaminated using the same procedure used after the first outbreak. All sows in the gestation unit received their second oral immunization with intestinal contents, as described, 5 weeks before the next farrowing. In addition, they received a third iPED+ vaccination approximately 1 week before the third group of sows farrowed. Therefore, the pregnant sows in this third group (nine of which were included in this study) had received prepartum three doses of iPED+ and two oral immunizations. Serum and fecal-swab samples were obtained 1 day before oral immunization (week 0) and weekly thereafter for a total of five sampling time points, with the last (4 weeks) just prior to farrowing and the third PEDV outbreak (August; Figure 1D). Porcine epidemic diarrhea virus infections during the outbreaks were identified only by molecular assay; none of the S genes were sequenced. Milk samples were obtained 48 hours post partum. Decontamination efforts after each outbreak included power-spray washing using 1-Stroke Environ (Steris Corp, St Louis, Missouri) to wash walls and floors. Additionally, after the second and third outbreaks, washing was followed by 160˚C heat treatment of each farrowing room. Environmental samples for molecular PEDV testing were collected from farrowing, gestation, nursery, and office, and other traffic areas after the second and third clean-up efforts.
Laboratory assays
The humoral (IgG) immune response to PEDV was quantified by using an immunofluorescent antibody assay (IFA; VMRD, Inc, Pullman, Washington) performed at the UI VDL. Similar to ELISA systems, such assays detect any anti-virus antibodies, therefore neutralizing antibody levels cannot be specifically quantified. Serum samples were tested in duplicate using twofold dilutions from 1:40 to 1:320. Samples that lacked a detectable antibody response at the 1:40 dilution were tested at a 1:20 dilution. Samples were considered negative if anti-PEDV IgG antibodies were undetectable at the 1:20 dilution.
Maternal immunoglobulin (IgG, IgA, IgM) response to PEDV was also evaluated in milk samples obtained from sows 48 hours post parturition. Antibodies to PEDV were measured using a PEDV fluorescent focus neutralizing (FFN) assay performed at the South Dakota State University Animal Disease Research and Diagnostic Laboratory. Such assays detect neutralizing anti-virus antibodies. Samples were considered negative if anti-PEDV neutralizing antibodies were undetectable at a 1:40 dilution.
A PEDV rRT-PCR assay routinely performed at the UI VDL was used to assess virus shedding in fecal samples. Samples were considered positive for detection of the viral genome if Ct values were ≤ 37 and negative if Ct values were > 40. Counts of 38 and 39 were considered suspect, and retesting may be suggested.
Statistical analysis was not performed, given the type of data obtained from this case study in active production units.
Antibody responses
An IFA assay was used to determine anti-PEDV IgG in the serum samples. As anticipated, the control group (non-vaccinated and non-infected) lacked a detectable humoral response to the virus. An FFN assay was used to evaluate neutralizing anti-PEDV immunoglobulin in milk samples obtained 48 hours postpartum. As expected, the control group lacked detectable anti-PEDV antibodies in their milk.
Unit A animals were non-infected and had been iPED+ vaccinated twice at a 3-week interval, with the last dose administered 3 weeks before farrowing (Figure 1A). As shown in Table 1, serum samples obtained 3 weeks post partum (approximately 6 weeks after the last vaccination) had anti-PEDV IgG reciprocal titers that ranged from undetectable (< 20) in four sows to 80 in one of the six animals. Similarly, their 48-hour post-farrowing milk samples had neutralizing antibody titers ranging from undetectable in three to 80 in one sow.
Table 1: Serum and milk anti-PEDV titers in sows in three independent swine facilities, units A, B, and C*
| Sow | Group A | Group B | Group C | |||
|---|---|---|---|---|---|---|
| Serum† | Milk‡ | Serum† | Milk‡ | Serum† | Milk‡ | |
| 1 | 80 | Neg | Neg | 320 | 160 | 2560 |
| 2 | Neg | Neg | Neg | 160 | 80 | 2560 |
| 3 | Neg | 80 | Neg | 1280 | 80 | 640 |
| 4 | 20 | 40 | Neg | 1280 | 20 | 320 |
| 5 | Neg | Neg | Neg | 320 | 40 | 320 |
| 6 | Neg | 20 | Neg | 320 | 80 | 1280 |
* Units B and C had experienced outbreaks of porcine epidemic diarrhea, while Unit A remained naive. Serum and milk samples were collected 48 hours post partum. Group name indicates the farm of origin.
† Serum anti-PEDV IgG titers were determined by an indirect fluorescent antibody (IFA) assay. Samples were tested in duplicate using twofold dilutions from 1:20 to 1:320. Titers are given as the reciprocal of the highest dilution of a sample in which a detectable anti-PEDV IgG result was obtained. Negative (Neg) result indicates that anti-PEDV IgG was not detected at the 1:20 dilution.
‡ Milk anti-PEDV neutralizing Ig titers were measured by a fluorescent focus neutralization (FFN) assay. Titers are given as the reciprocal of the highest dilution of a milk sample in which a detectable result was obtained.
PEDV = porcine epidemic diarrhea virus.
Animals in two separate production units (B and C) were non-vaccinated prior to experiencing a PEDV outbreak. Only oral immunization by feeding back intestines from euthanized moribund neonates was performed in both units following the outbreaks (Figure 1B and 1C). In Unit B, one oral immunization was performed within days after the outbreak, which was approximately 4.5 months prior to farrowing. None of the six sows had detectable anti-PEDV IgG serum antibodies at the time of farrowing (Table 1). In contrast, their milk samples contained antiviral neutralizing antibodies, ranging in titers from 160 to 1280. Unit C sows had received three consecutive oral immunizations starting 24 hours after their outbreak, which was approximately 3.5 months prior to farrowing. In this case, all animals at the time of parturition had serum anti-PEDV IgG titers ranging from 20 to 160 and milk neutralizing-antibody titers ranging from 320 to 2560 (Table 1).
Unit D animals experienced one natural PEDV outbreak that initiated in the farrowing unit within 2.5 months prior to parturition of the third group of sows. This third group of sows had three iPED+ vaccinations approximately 12 and 9 weeks and 1 week prefarrowing, as well as two oral immunizations (intestinal contents of known Ct value) administered 8 and 5 weeks prior to farrowing (Figure 1D). A total of five serum samples were obtained from each study animal, starting 1 day prior to oral immunization, and weekly thereafter, with the last one obtained a few days prior to parturition. The highest serum anti-PEDV IgG titer was ≥ 320 for four sows on the first sampling, and was maintained in only one sow for the next two tests (Table 2). In general, humoral immunity inconsistently fluctuated and tended to decrease over the 5-week period, even following oral immunization. The milk antiviral neutralizing-antibody titers ranged from 80 to 1280 and did not necessarily correlate with the serum IgG titers in the last week serum samples were collected, a few days prior to parturition.
Table 2: Serum and milk anti-PEDV reciprocal antibody titers and results of PEDV rRT-PCR on fecal samples in Unit D*
| Sow | Serum† (fecal swabs‡) | Milk§ | ||||
|---|---|---|---|---|---|---|
| 0 weeks | 1 week | 2 weeks | 3 weeks | 4 weeks | ||
| 1 | ≥ 320 (39.8) | 160 (39.0) | 40 (38.10) | Neg (Neg) | Neg (Neg) | 320 |
| 2 | ≥ 320 (Neg) | ≥ 320 (Neg) | 160 (36.9) | 80 (Neg) | 160 (Neg) | 640 |
| 3 | ≥ 320 (Neg) | ≥ 320 (38.7) | 160 (Neg) | 160 (Neg) | 160 (Neg) | 80 |
| 4 | ≥ 320 (Neg) | ≥ 320 (Neg) | ≥ 320 (Neg) | 160 (Neg) | 160 (Neg) | 1280 |
| 5 | 80 (Neg) | 40 (Neg) | 40 (Neg) | 20 (Neg) | 80 (35.6) | 640 |
| 6 | 80 (Neg) | 20 (Neg) | 40 (34.5) | Neg (Neg) | 20 (Neg) | 160 |
| 7 | 160 (Neg) | 80 (Neg) | 20 (Neg) | Neg (Neg) | Neg (Neg) | 320 |
| 8 | 160 (Neg) | 40 (38.4) | 40 (Neg) | Neg (Neg) | 40 (Neg) | 1280 |
| 9 | 80 (Neg) | 20 (Neg) | 40 (Neg) | 20 (Neg) | Neg (36.8) | 320 |
* Serum and fecal swabs were obtained from sows prior to oral inoculation (0 weeks) and weekly thereafter for four additional time points, with the last samples collected a few days prior to parturition.
† Serum anti-PEDV IgG levels of sows were determined by an indirect fluorescent antibody assay. Samples were tested in duplicate using twofold dilutions from 1:20 to 1:320. Titers are given as the reciprocal of the highest dilution of a sample in which a detectable anti-PEDV IgG result was obtained. Negative (Neg) result indicates that anti-PEDV IgG antibodies were not detected at the 1:20 dilution.
‡ PEDV rRT-PCR Ct values for fecal swab samples are provided: positive (Ct value ≤ 37), suspect (Ct value, 37.01 to 40), or negative (Neg), Ct value > 40).
§ Neutralizing antibodies to PEDV in milk were measured using a fluorescent focus neutralizing assay. Samples were considered negative if antibodies were undetectable at a 1:40 dilution.
PEDV = porcine epidemic diarrhea virus; rRT-PCR = real-time reverse transcriptase polymerase chain reaction; Ig = immunoglobulin; Ct = cycle threshold.
PEDV shedding
The relative amount of PEDV shed in the feces was temporally determined in Unit D, as units B and C were not available for such testing. Natural PEDV infections occurred in Unit D in the two previously pregnant groups of animals at their time of farrowing. Subsequently, the third group of pregnant sows was orally immunized 8 and 5 weeks prior to farrowing, as described. The second and third outbreaks were attributed to the oral immunization procedure as well as likely residual environmental contamination. Overall, virus shedding was not consistent over the 5-week prior (Table 2). Only low levels of virus were sporadically detected in seven of the nine sows during the five time points, with Ct values ranging from 36 (positive) to 39 (suspect). Three of the seven sows were in the suspect range, with one animal yielding three and the other two sows only one such result. The other four sows had one positive Ct value each, but were otherwise negative; for two of these sows, their first detectable fecal shedding was 2 to 3 days prior to parturition. When PEDV is being shed in the feces just prior to farrowing, the neonates are at risk of infection, regardless of the level of colostral protection they may have received. In fact, the neonates did succumb to PEDV infection (as determined by PEDV rRT-PCR), albeit with milder disease than the previous two litters.
Follow-up to case study
Unit A remained PEDV-free. Unit B did not experience further PEDV outbreaks in the batch farrowings subsequent to oral immunization. Similarly, after the oral immunization protocol, Unit C continued on a successful path of PEDV eradication 9 weeks post introduction. Unit D was depopulated due to the rRT-PCR-detectable environmental levels of PEDV that remained despite the rigorous decontamination attempts, and because of the change of plans for the unit (depopulation with repopulation).
Discussion
This case study was undertaken to provide data on whether parenteral iPED+ (first generation) vaccination alone was adequate to illicit detectable milk anti-PEDV antibodies, or if oral immunization with infectious PEDV was required. It is well-documented that porcine neonates rely upon passive immunity obtained from the colostrum and milk of their dam, as there is negligible placental transfer of antibodies during gestation.7 Nearly all the colostral IgG and IgM and only 40% of the IgA originates in the systemic circulation of the dam; the change to mammary tissue origin occurs later in lactation.8 During the first 48 hours of lactation after parturition, the Ig content of colostrum or milk is very high, with IgG in the highest relative concentration followed by IgA then IgM (ratio of 76:17:7).9
Unit A sows received only iPED+ vaccinations at 6 and 3 weeks pre-farrowing. Both their serum IFA IgG and milk anti-PEDV neutralizing antibody levels were low, with titers of 80 or less. Unit B sows were orally immunized once approximately 4 months prior to their subsequent batch farrowings. Although the milk titers in these sows ranged from 160 to 1280, all serum titers were negative. The serum results may reflect an empirical observation that serum anti-PEDV antibodies do not appear to persist post infection. Similarly, in Unit C, sows were orally immunized on 3 successive days after their initial PEDV outbreak. Following this more rigorous immunization approach, the animals that farrowed nearly 3 months later had the highest milk anti-PEDV neutralizing antibody titers of the three units, with all six study sows having detectable serum IgG. These outcomes indicate that oral immunization induced higher levels of milk anti-PEDV-neutralizing antibodies than parenteral vaccination with PEDV antigen alone. However, the protective passive antibody titer for neonates has yet to be determined.
The Unit D sows in the study were provided with parenteral iPED+ vaccination at 9 and 2 weeks prior to farrowing, as well as oral immunization at 8 and 5 weeks pre-farrowing. The temporal serum sampling of the sows revealed that overall anti-PEDV antibody titers wane fairly rapidly (Table 2). As for TGEV, serum antibodies may not provide significant protection against PEDV infections.3,4 Empirical information relates that feedback of neonatal intestines would stop a TGEV outbreak in a herd for the remainder of the “TGEV season.” This has not been the case for PEDV. After feedback, a herd may be re-infected and manifest clinical disease months later in the same “season.” Such empirical information may suggest the possibility that PEDV has immunosuppressive properties not evident in TGEV; such a hypothesis has yet to be examined. It is known that stimulation of GALT by oral inoculation or natural exposure to virulent virus achieves the most effective protective anti-TGEV immunity.3 The active immunity that results from such enteric replication of the virus also involves induction of cell-mediated immunity (CMI), as well as production of intestinal secretory IgA.10 The GALT CMI to TGEV was found to persist for only 14 days after oral inoculation in neonates (7 days of age) and lacked a natural killer cell component (part of innate immunity) in pigs of this age. In comparison, the GALT CMI persisted at least 110 days in pigs 6 months of age.3 This reflects the noted age-dependent resistance to TGEV infections.10,11 Such age resistance does not appear to be associated with PEDV infection, as the virus is known to cause disease in adults.
Although serum immunoglobulins are the initial source of colostrum and milk immunoglobulins, the measured milk anti-PEDV neutralizing-antibody titers did not necessarily correlate with the serum antiviral IgG titers, specifically the last samples collected a few days prior to parturition. This is also evident between units in this study in that while serum anti-PEDV IgG titers for Unit D were highest, their milk antiviral neutralizing antibodies were not. Such lack of correlation may be real or simply reflect a sensitivity disparity in the type of antibodies the two assays are detecting (IFA IgG detecting any anti-viral antibody; FFN detecting only neutralizing antibodies). From the perspective of the neonate, one would consider the milk anti-PEDV neutralizing antibody titers to be critical.
Furthermore, at the time of parturition, sows in the Unit D farrowing unit were still shedding PEDV in their feces (25 days after their last oral immunization). This is not unexpected, as neonates that recovered from a natural PEDV infection under field conditions shed the virus in their feces for up to 56 days post infection.12 Interestingly, neonates of the Unit D sows did not manifest clinical disease until about 5 days of age and their disease was much milder than that during the initial PEDV outbreak in this herd. Therefore, passive immunity appeared to offer a level of protection, but eventually was overwhelmed. Although the duration of PEDV shedding from such infected adult animals may vary, it would be prudent to perform oral inoculations at least 40 days prior to parturition and in a different location than the farrowing unit.
Implications
• Under the conditions of this case study, oral immunization may be the more efficacious PEDV immunization modality
• There appears to be a lack of correlation between milk anti-PEDV neutralizing antibody titer and serum antiviral IgG titer.
Acknowledgements
This project was funded by the National Institute of Food and Agriculture (Hatch) project no. ILLU-888-358. We thank Drs William J. Armbruster (Greenhaven Animal Clinic, PC) and Aaron J. Lower (Carthage Veterinary Services) for submission of clinical samples. D. Cassout and D. Robison of the University of Illinois Veterinary Diagnostic Laboratory provided technical support.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Stevenson, GW, Hoang H, Schwartz KJ, Burrough ER, Sun D, Madson D, Cooper VL, Pillatzki A, Gauger P, Schmitt BJ, Koster LG, Killian ML, Yoon KJ. Emergence of porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J Vet Diag Inv. 2013;25:649–654.
2. Pensaert MB. Porcine epidemic diarrhea. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames, Iowa: Iowa State University Press; 1999:179–185.
3. Saif LJ, Heckert RA. Enteric coronaviruses: Transmissible gastroenteritis: immunity. In: Viral Diarrheas of Man and Animals. Saif LJ, Thiel KW, eds. Boca Raton, Florida: CRC Press, Inc; 1989: 200–203.
4. Shoup DI, Jackwood DJ, Saif LJ. Active and passive immune responses to transmissible gastroenteritis virus (TGEV) in swine inoculated with recombinant baculovirus-expressed TGEV spike glycoprotein vaccines. Am J Vet Res. 1997;58:242–250.
5. Saif LJ, van Cott JL, Brim TA. Immunity to transmissible gastroenteritis virus and porcine respiratory coronavirus infections in swine. Vet Immunol Immunopath. 1994;43:89–97.
6. Rooke JA, Bland IM. The acquisition of passive immunity in the new-born piglet. Livest Prod Sci. 2002;78:13–23.
7. Kim YB, Bradley SG, Watson DW. Ontogeny of the immune response. I. Development of immunoglobulins in germfree and conventional colostrum- deprived piglets. J Immunol. 1966;97:52–63.
8. Lund A. Immunological phenomena in sows and piglets. Norsk Veterinaertidsskrift. 1979;91:751–757.
9. Klobasa F, Werhahn E, Butler JE. Composition of sow milk during lactation. J Anim Sci. 1987;64:1458–1466.
10. Brim TA, Van Cott JL, Lunney JK, Saif LJ. Cellular immune responses of pigs after primary inoculation with porcine respiratory coronavirus or transmissible gastroenteritis virus. Vet Immunol Immunopath. 1995;48:35–54.
11. Moon HW, Norman JO, Lambert G. Age dependent resistance to TGE of swine. I. Clinical signs and some mucosal dimensions in the small intestine. Can J Comp Med. 1973;37:157–166.
12. Sun R, Leng Z, Dekun C, Song C. Multiple factors contribute to persistent porcine epidemic diarrhea infection in the field: An investigation on porcine epidemic diarrhea repeated outbreaks in the same herd. J Anim Vet Adv. 2014;13:410–415.
