| Brief communication | Peer reviewed |
Cite as: Kang I, Kang HS, Jeong J, et al. Comparison of growth performance under field conditions in growing pigs each vaccinated with one of two commercial modified-live porcine reproductive and respiratory syndrome vaccines. J Swine Health Prod. 2017;25(1):24–28.
Also available as a PDF.
SummaryUnder field conditions, in groups of pigs each vaccinated with one of two modified live virus porcine reproductive and respiratory syndrome vaccines, growth performance was better and lung lesions were fewer than in nonvaccinated controls. Growth performance and number of lung lesions did not differ between the two vaccinated groups. | ResumenBajo condiciones de campo, en grupos de cerdos vacunados con una de las dos vacunas vivas modificadas del virus del síndrome reproductivo y respiratorio porcino, el desempeño de crecimiento fue mejor y las lesiones de pulmón menores que en los controles no vacunados. El desempeño del crecimiento y el número de lesiones de pulmón no difirieron entre los dos grupos vacunados. | ResuméDans des groupes de porcs gardés en conditions de champs et chacun vaccinés avec un des deux vaccins vivants modifiés contre le virus du syndrome reproducteur et respiratoire porcin, les performances de croissance étaient meilleures et il y avait moins de lésions pulmonaires que chez les témoins non-vaccinés. Les performances de croissance et la quantité de lésions pulmonaires ne différaient pas entre les deux groupes d’animaux vaccinés. |
Keywords: swine, porcine reproductive and respiratory syndrome, respiratory disease, vaccine, PRRS
Search the AASV web site
for pages with similar keywords.
Received: February 11, 2016
Accepted: June 28, 2016
Porcine reproductive and respiratory syndrome (PRRS) virus is an enveloped positive-stranded RNA virus belonging to the order Nidovirales, family Arteriviridae, and genus Arterivirus.1 PRRS virus (PRRSV) is divided into type 1 (European) and type 2 (North American) genotypes on the basis of the 3ʹ-terminal structural genes or the entire genome.2,3 PRRS is one of the most devastating diseases of swine, causing enormous economic losses for the global pork industry due to reproductive failure in sows and respiratory disease in growing pigs.4 Vaccination is still a major tool for control of PRRSV infection. Currently, two commercial PRRS modified-live vaccines (MLVs) are available in Korea: Fostera PRRS (Zoetis, Florham, New Jersey) and Ingelvac PRRS MLV (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri). Hence, the objective of this study was to compare the efficacy of the two MLV PRRS vaccines, under field conditions, in healthy pigs from a herd infected with type 2 PRRSV.
Materials and methods
All animal protocols were approved by the Seoul National University Institutional Animal Care and Use Committee.
The clinical field trial was conducted on a 1000-sow herd with two-site production: farrowing-nursery and growing-finishing system. The farm had suffered recent losses due to respiratory disease caused by type 2 PRRSV in post-weaning and late-growing pigs at the time of the study. However, reproductive failure had been reported in breeding females from the farm 4 months prior to the study. All pigs were routinely vaccinated with a commercial porcine circovirus type 2 (PCV2) vaccine at 3 weeks of age, but clinical signs indicative of PCV2 had not been observed.
Type 2 PRRSV (SNUVR 150324 strain, lineage 5, GenBank no. KU301048) was isolated from lung samples from weaned pigs at 42 days of age, prior to the beginning of this study. The SNUVR 150324 strain and Fostera PRRS vaccine virus (GenBank no. AF494042) share 91.5% nucleotide identity for open reading frame 5 (ORF5). The SNUVR 150324 strain and Ingelvac MLV vaccine virus (GenBank no. AF066183) share 99.1% nucleotide identity for ORF5. Fostera PRRS vaccine and Ingelvac PRRS MLV vaccine virus share 91.3% nucleotide identity for ORF5.
This study used a randomized, blinded, weight-matched, controlled clinical trial design (Table 1). Sample size was calculated assuming a 90% power (1 - β = .90) of detecting a difference at the 5% level of significance (α = .05), which was based on expected results of average daily gain (ADG).5 To minimize sow variation, six piglets at 7 days of age were selected from each sow using the random number generator function in Excel (Microsoft Corporation, Redmond, Washington). Pigs were assigned evenly to three groups (30 pigs per group) using the Excel random number generator. Pigs in Group 1 were injected intramuscularly with 2.0 mL of the Fostera PRRS vaccine (Zoetis, lot no. A405013B) in the right side of the neck at 21 days of age according to the manufacturer’s instructions. Pigs in Group 2 were injected intramuscularly with 2.0 mL of the Ingelvac PRRS MLV (Boehringer Ingelheim Vetmedica Inc, lot no. 245-659A) in the right side of the neck at 21 days of age according to the manufacturer’s instructions. Pigs in Group 3 were injected in the same anatomic location with 2.0 mL of phosphate buffered saline (0.01M, pH 7.4).
Table 1: Means (with standard deviation) of average daily gain (ADG) in pigs vaccinated for PRRS (Group 1 and Group 2) or injected with phosphate buffered saline (Group 3) at 21 days of age*
| Period between study days | Age (days) | ADG (g/day) | ||
|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | ||
| 0 to 49 | 21 to 70 | 400.51 (56.81) | 405.77 (41.02) | 396.57 (36.86) |
| 49 to 91 | 70 to 112 | 631.57 (86.12) | 639.13 (80.26) | 607.14 (78.75) |
| 91 to 147 | 112 to 168 | 786.80a (59.16) | 783.07a (71.85) | 738.61b (41.41) |
| 0 to 147 | 21 to 168 | 615.82a (34.43) | 615.85a (29.15) | 586.59b (30.38) |
* To minimize sow variation, six piglets at 7 days of age were selected from each sow using the random number generator function in Excel (Microsoft Corporation, Redmond, Washington) and assigned evenly to three groups (30 pigs per group) using the random number generator. At study day 0 (21 days of age), Group 1 pigs were vaccinated with a one-dose PRRS vaccine (Fostera PRRS; Zoetis, Florham Park, New Jersey); Group 2 pigs were vaccinated with a one-dose PRRS vaccine (Ingelvac PRRS MLV; Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri); and Group 3 pigs were injected with phosphate buffered saline. The live weight of each pig in each group was measured at study days 0 (21days of age), 49, 91, and 147 (168 days of age); ADG was compared among the three groups using a Tukey’s multiple comparisons test.
ab Within a row, values with different superscript letters are significantly different (P < .05).
PRRS = porcine reproductive and respiratory syndrome.
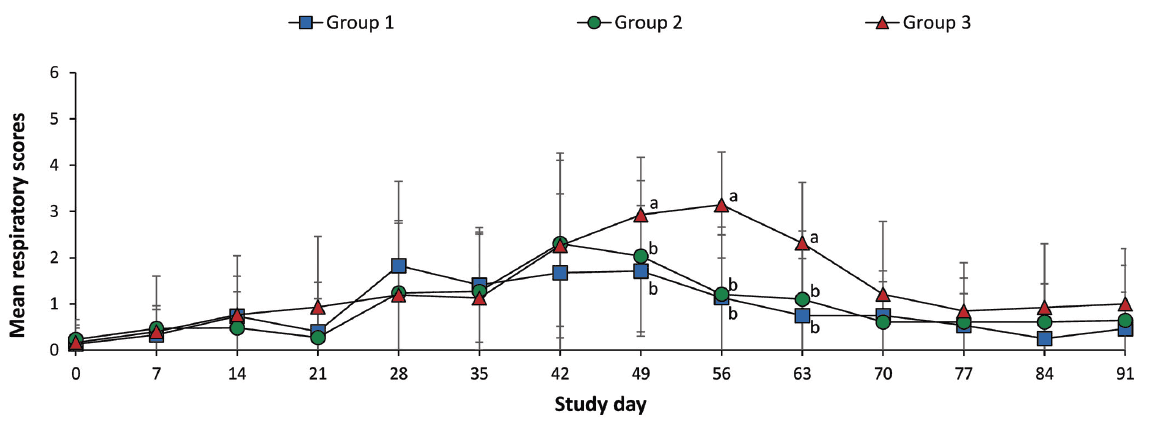
Pigs in each group were randomly assigned into three pens (10 pigs per pen) using the Excel random number generator and were housed in the same barn. Pigs were monitored daily for physical condition, and mean respiratory scores were recorded once weekly. Scores ranged from 0 (normal) to 6 (severe dyspnea, abdominal breathing, and death) at study days 0 to 91 (Figure 1).6 Observers were blinded to vaccination status. Mortality rate was calculated as the number of pigs that died divided by the number of pigs initially assigned to that group within batch.
Figure 1: Mean respiratory scores (with standard deviation) of pigs in the study described in Table 1. Mean respiratory signs were scored on a scale from 0 to 6: 0 = normal; 1 = mild dyspnea or tachypnea or both when stressed; 2 = mild dyspnea or tachypnea or both when at rest; 3 = moderate dyspnea or tachypnea or both when stressed; 4 = moderate dyspnea or tachypnea or both when at rest; 5 = severe dyspnea or tachypnea or both when stressed; and 6 = severe dyspnea or tachypnea or both when at rest at study days 0, 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, 77, 84, and 91. Different letters (a, b) at a study day indicate significant differences among groups (P < .05; Kruskal-Wallis and Mann-Whitney tests used sequentially).
The live weight of each pig in groups 1, 2, and 3 was measured at study days 0 (21 days of age), 49, 91, and 147 (168 days of age). The ADG (grams per pig per day) was analyzed over three time periods: between study days 0 and 49; 49 and 91; 91 and 147, respectively (Table 1). The ADG during these various production stages was calculated as the difference between the starting and final weights divided by the duration of the stage. Data from dead pigs were included in the calculation.
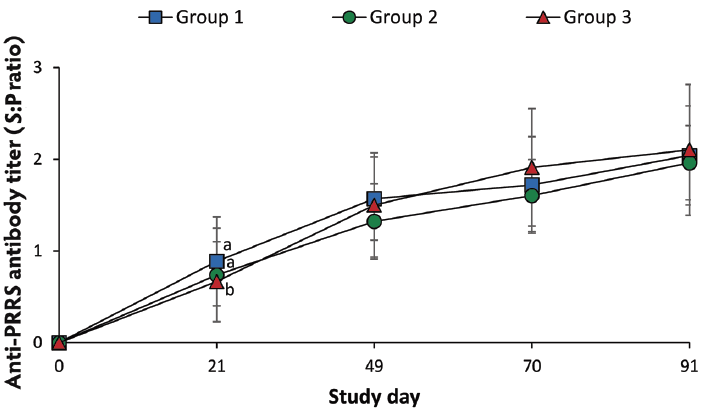
Blood samples from pigs were collected at study days 0, 21, 49, 70, 91, and 147. Blood samples were also collected from sows at study days 0, 21, 49, 70, and 91. Serum samples from sows at study days 0, 21, 49, 70, and 91 were tested using a commercial PRRSV ELISA (Idexx Laboratories Inc, Westbrook, Maine). Serum samples were considered positive for anti-PRRSV antibody if the sample-to-positive ratio (S:P) was ≥ 0.4, according to the manufacturer’s instructions.
QIAamp RNA Mini Kit (Qiagen Inc, Valencia, California) was used to extract RNA from the pigs’ serum samples at study days 0, 21, 49, 70, 91, and 147. The RNA extracts were used to quantify the number of PRRSV genomic RNA copies by real-time PCR as previously described.7,8 Real-time PCR for the vaccine virus was also performed to quantify the number of PRRSV genomic RNA copies.8,9 Numbers of copies of PRRSV genomic RNA per mL of serum were converted to base 10 logarithms for analysis.
Five serum samples from pigs PCR-positive for field or vaccine virus, randomly selected using the Excel random number generator at study days 21, 49, 70, 91, and 147, were used to analyze the sequence of ORF5 by PCR as previously described.10 The PCR products were purified using a commercial kit (Wizard PCR Preps DNA Purification and PCR Clean-Up System, Promega, Madison, Wisconsin), cloned with the TOPcloner Blunt kit (Enzynomics, Daejeon, Korea), and propagated in DH5α competent cells (Enzynomics) according to the manufacturer’s instructions. Plasmid DNA was purified with a plasmid purification kit (iNtRON Biotechnology, Sungnam, Kyeonggido, Korea) and sequenced by a commercial service (Sol Gent Co Ltd, Daejeon, Korea). Three clones of each PCR product were independently sequenced at least three times.
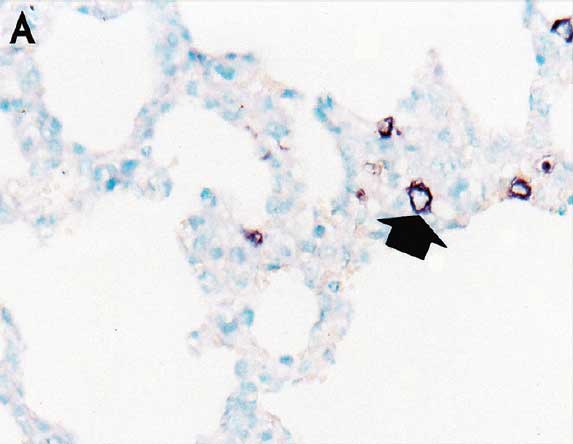
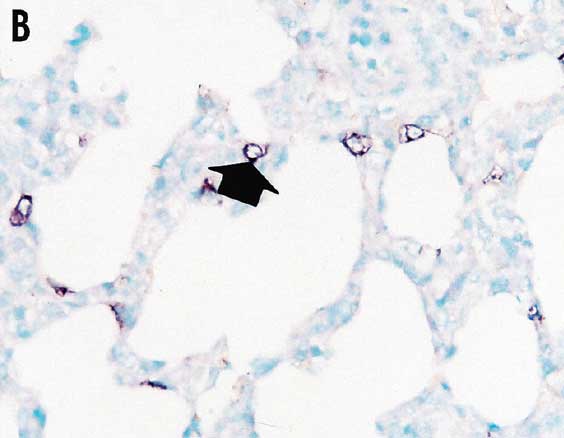
Lung samples were collected from all pigs in each group at study day 147 (the time of slaughter). For morphometric analysis of histopathological lesion scores in lungs, eight pieces of lung tissue (two pieces from the right cranial lobe, two from the right middle lobe, one from the ventromedial part of the right caudal lobe, one from the dorsomedial part of the right caudal lobe, one from the mid-lateral part of the right caudal lobe, and one from the accessory lobe) were collected from each pig. Three tissue sections from the eight lung pieces were prepared and examined blindly by two veterinary pathologists (authors JJ and CC) at Seoul National University (Seoul, Republic of Korea) as previously described.6 Lung lesions were scored on a scale from 0 to 4: 0 = no microscopic lesions; 1 = mild interstitial pneumonia; 2 = moderate multifocal interstitial pneumonia; 3 = moderate diffuse interstitial pneumonia; and 4 = severe interstitial pneumonia.6 In situ hybridization for detection and differentiation of type 1 and type 2 PRRSV nucleic acids in lung tissues was performed and analyzed morphometrically as previously described.9,11
Statistical analyses were performed using SPSS software (version 21; IBM, Armonk, New York). Continuous data included ADG determined by the difference between the starting and final weights divided by the duration of the stage; PPRSV RNA (numbers of log10 PRRSV genomic copies per mL) determined by real-time PCR; PRRSV antibody titer; and numbers of lung sections positive for PRRSV nucleic acid per unit area (0.25 mm2) determined by in situ hybridization. Continuous data were analyzed using Tukey’s multiple comparisons test for comparison between groups in order to estimate the difference at each time point. Discrete data (clinical signs and lung lesion scores) were analyzed with the Kruskal-Wallis test. When the Kruskal-Wallis test was significant, the Mann-Whitney test was performed to determine the significant differences between groups. Fisher’s exact test was applied to evaluate mortality rate. A value of P < .05 was considered significant.
Results
The mean respiratory scores were significantly lower (P < .05) in vaccinated pigs (Group 1 and Group 2) than in nonvaccinated pigs (Group 3) from day 49 to 63 (Figure 1). The overall mortality rates were 6.6% (two of 30 pigs) both in Group 1 and in Group 2, and 13.3% (four of 30 pigs) in Group 3. Diagnostic test results indicated the cause of death was primarily streptococcal meningitis in Group 1, primarily pneumonic pasteurellosis in Group 2, and primarily related to Glasser’s disease (Hemophilus parasuis) in Group 3.
The ADGs were significantly higher (P < .05) in vaccinated pigs (Group 1 and Group 2) than in nonvaccinated pigs (Group 3) between day 91 and 147, and between day 0 and 147 (Table 1).
On day 21, anti-PRRSV antibody titers were significantly higher (P < .05) in vaccinated pigs (Group 1 and Group 2) than in nonvaccinated pigs (Group 3) (Figure 2). Anti-PRRSV antibody titers were detected in 15 sows, with S:P ratios ranging from 0.4 to 0.7.
Figure 2: Mean anti-PRRSV antibody serum titers (with standard deviation) of pigs in the study described in Table 1. Blood samples were collected from pigs for serological testing at study days 0, 21, 49, 70, and 91 (PRRS ELISA; Idexx Laboratories, Inc, Westbrook, Maine; sample-to-positive [S:P] ratios reported). Different letters (a, b) at a study day indicate significant differences among groups (P < .05; Tukey’s multiple comparisons test).
Numbers of genomic copies of type 2 PRRS field virus in serum did not differ between vaccinated pigs (Group 1 and Group 2) and nonvaccinated pigs (Group 3) throughout the experiment. ORF5 sequences from five randomly selected serum samples in all three groups were highly homologous (99.1% to 100%) with field PRRS virus (SNUVR150324 strain). Vaccine virus was detected in the blood of Group 1 (vaccinated pigs) at study days 21 (four pigs) and 49 (one pig). ORF5 sequences from the serum samples of Group 1 (vaccinated pigs) at study days 21 and 49 identified the Fostera PRRS vaccine virus. Vaccine virus was detected in the blood of Group 2 (vaccinated pigs) at study days 21 (five pigs) and 49 (two pigs). ORF5 sequences from the serum samples of Group 2 (vaccinated pigs) at study days 21 and 49 identified the Ingelvac PRRS vaccine virus. Determined by PRRSV ORF5 sequencing after vaccination, cross-contamination of vaccine virus was not observed between Group 1 and Group 2 vaccinated pigs. Vaccine virus was not detected in the blood of nonvaccinated pigs (Group 3). Type 1 PRRSV was not detected in any of the three groups throughout the experiment.
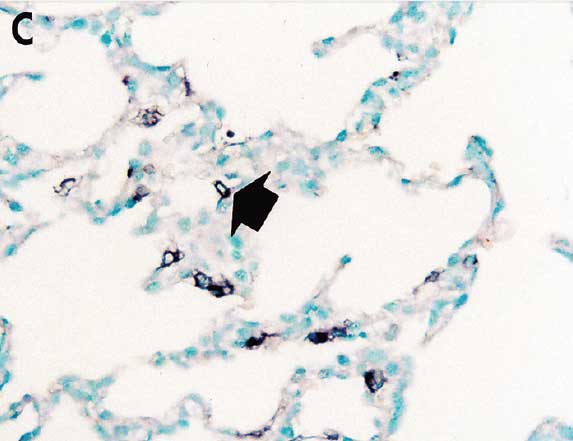
Pulmonary lesion scores were significantly lower (P < .05) in vaccinated pigs (Group 1 and Group 2) than in nonvaccinated pigs (Group 3) (Table 2). The number of lung cells positive for type 2 PRRSV nucleic acid was not significantly different between vaccinated pigs (Group 1 and Group 2) (Figure 3) and nonvaccinated pigs (Group 3) (Table 2).
Table 2: Means (standard deviation) of pulmonary lesion score and numbers of pulmonary cells positive for type 2 porcine reproductive and respiratory syndrome virus (PRRSV) nucleic acid*
| Group (n) | Vaccination† (21 days) | Lung | |
|---|---|---|---|
| Lesion score‡ | No. of type 2 PRRSV-positive cells§ | ||
| 1 (30) | Fostera PRRS | 0.69 (0.51)a | 3.33 (1.35) |
| 2 (30) | Ingelvac PRRS MLV | 0.81 (0.53)a | 3.94 (1.85) |
| 3 (30) | None | 1.64 (0.44)b | 4.17 (2.16) |
* Study described in Table 1.
† Vaccines: Fostera PRRS: Zoetis, Florham, New Jersey and Ingelvac PRRS MLV; Boehringer Ingelheim Inc, St Joseph, Missouri.
‡ Lung samples were collected from pigs in each group at study day 147 (168 days of age). Eight pieces of lung tissue (two from the right cranial lobe, two from the right middle lobe, one from the ventromedial part of the right caudal lobe, one from the dorsomedial part of the right caudal lobe, one from the mid-lateral part of the right caudal lobe, and one from the accessory lobe) were collected from each pig, and three tissue sections from each of the eight lung pieces were examined blindly. Lung lesions were scored on a scale from 0 to 4: 0 = no microscopic lesions; 1 = mild interstitial pneumonia; 2 = moderate multifocal interstitial pneumonia; 3 = moderate diffuse interstitial pneumonia; and 4 = severe interstitial pneumonia. Scores were compared between groups using the Kruskal-Wallis and the Mann-Whitney tests sequentially.
§ Numbers of lung cells positive for type 2 PRRSV nucleic acid per unit area (0.25 mm2) of lung were counted using an NIH Image J 1.45s program (http://imagej.nih.gov/ij/download.html). Numbers of positive cells were compared between groups using a Tukey’s multiple comparisons test.
ab Within a column, values with different superscript letters are significantly different (P < .05).
Figure 3: In situ hybridization testing was performed using a type 2 PRRSV-specific probe to detect type 2 PRRSV nucleic acid in lungs of pigs in the study described in Table 1. Few type 2 PRRSV nucleic acid-positive cells (arrows) were detected in macrophages in pigs from Group 1 (Panel A), Group 2 (Panel B), or Group 3 (Panel C) (magnification ×200).
Discussion
The results of this study demonstrate that under field conditions, in pigs vaccinated with MLV vaccines for PRRS, growth performance was better and lung lesions were fewer than in nonvaccinated controls. In addition, no significant differences between two commercial MLV PRRSV vaccines were found in this study, as determined by four types of outcomes: clinical (ADG and clinical signs), immunologic (antibodies), virologic (PCR testing), and pathologic (lesions and viral antigen). Measurement of PRRSV viremia was one of the parameters in assessing the efficacy of PRRS vaccines under an experimental challenge study.12-14 However, in contrast to previous studies,15-17 in the current study, under field conditions, the number of genomic copies of type 2 PRRS field virus RNA did not differ between vaccinated and nonvaccinated pigs. This difference may be due to varying conditions, such as ventilation and feeding systems, in experimental and field studies. In this field study, vaccinated and nonvaccinated pigs were housed in separate pens within the same barn. Therefore, vaccinated pigs could have been exposed to the circulating PRRS field virus. This might explain why the number of genomic copies of type 2 PRRS field virus RNA did not differ significantly between vaccinated and nonvaccinated pigs.
Although reproductive failure had occurred within 4 months of this study on the sow farms, maternally derived anti-PRRSV antibodies were not detected in any pigs from the three groups. In the 15 sows used in this study, PRRSV ELISA S:P ratios were low (0.4 to 0.7), suggesting that the majority of newborn piglets might have received small quantities of colostral anti-PRRSV antibodies from their dams. These passively acquired antibodies might decay in pigs by 21 days of age, which could explain why the 21-day-old pigs in this study had no detectable maternally derived anti-PRRSV antibodies at the time of vaccination.
Comparison of two commercial MLV PRRS vaccines provides swine practitioners and producers with clinical information concerning control of PRRSV infection. Regardless of the commercial MLV PRRS vaccine, growth performance was better and lung lesions were fewer in vaccinated pigs than in nonvaccinated pigs. However, there were no significant differences in growth performance or lung lesions between pigs vaccinated with either commercial MLV PRRS vaccine.
Implications
- Under the conditions of this study in a PRRS-positive herd, growth performance and lung lesions do not differ between pigs vaccinated with either of two commercial PRRS vaccines.
- Efficacies of MLV PRRS vaccines are independent of the genetic similarity between the MLV PRRS and wild-type virus.
Acknowledgements
This research was supported by contract research funds of the Research Institute for Veterinary Science from the College of Veterinary Medicine and by BK 21 PLUS Program for Creative Veterinary Science Research in the Republic of Korea. The first two authors contributed equally to this work.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Snijder EJ, Meulenberg JJM. The molecular biology of arteriviruses. J Gen Virol. 1998;79:961–979.
2. Murtaugh MP, Elam MR, Kakach LT. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch Virol. 1995;140:1451–1460.
3. Nelsen CJ, Murtaugh MP, Faaberg KS. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J Virol. 1999;73:270–280.
4. Zimmerman JJ, Benfield DA, Dee SA, Murtaugh MP, Stadejek T, Stevenson GW, Torremorell M. Porcine reproductive and respiratory syndrome virus (porcine arterivirus). In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, eds. Diseases of Swine. 10th ed. Ames, Iowa: Wiley-Blackwell; 2012;461–486.
5. Chow SC, Wang H, Shao J. Comparing means. In: Chow SC, Wang H, Shao J, eds. Sample Size Calculations in Clinical Research. 2nd ed. New York: Chapman & Hall/CRC; 2008:60–92.
6. Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng X-J, Lum MA, Andrews JJ, Rathje JA. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32:648–660.
7. Wasilk A, Callahan JD, Christopher-Hennings J, Gay TA, Fang Y, Dammen M, Reos ME, Torremorell M, Polson D, Mellencamp M, Nelson E, Nelson WM. Detection of U.S., Lelystad, and European-like porcine reproductive and respiratory syndrome viruses and relative quantitation in boar semen and serum samples by real-time PCR. J Clin Microbiol. 2004;42:4453–4461.
8. Park C, Seo HW, Han K, Kang I, Chae C. Evaluation of the efficacy of a new modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine (Fostera PRRS) against heterologous PRRSV challenge. Vet Microbiol. 2014;172:432–442.
9. Han K, Seo HW, Park C, Chae C. Vaccination of sows against type 2 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) before artificial insemination protects against type 2 PRRSV challenge but does not protect against type 1 PRRSV challenge in late gestation. Vet Res. 2014;45:12. doi: 10.1186/1297-9716-45-12.
10. Do DT, Park C, Choi K, Jeong J, Nguyen TT, Le DTH, Vo KM, Chae C. Nucleotide sequence analysis of Vietnamese highly pathogenic porcine reproductive and respiratory syndrome virus from 2013 to 2014 based on the NSP2 and ORF5 coding regions. Arch Virol. 2016;161:669–675.
11. Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng X-J, Andrews JJ, Lum MA, Rathje JA. Comparison of the antigen distribution of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1996;33:159–170.
12. van Woensel PAM, Liefkens K, Demaret S. Effect on viraemia of an American and a European serotype PRRSV vaccine after challenge with European wild-type strains of the virus. Vet Rec. 1998;142:510–512.
13. Roca M, Gimeno M, Bruguera S, Segalés J, Díaz I, Galindo-Cardiel IJ, Martínez E, Darwich L, Fang Y, Maldonado J, March R, Mateu E. Effect of challenge with a virulent genotype II strain of porcine reproductive and respiratory syndrome virus on piglets vaccinated with an attenuated genotype I strain vaccine. Vet J. 2003;193:92–96.
14. Díaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Different European-type vaccines against porcine reproductive and respiratory syndrome virus have different immunological properties and confer different protection to pigs. Virology. 2006;351:249–259.
15. Martelli P, Gozio S, Ferrari L, Rosina S, De Angelis E, Quintavalla C, Bottarelli E, Borghetti P. Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: Clinical protection and cell-mediated immunity. Vaccine. 2009;27:3788–3799.
16. Mavromatis I, Kritas SK, Alexopoulos C, Tsinas A, Kyriakis SC. Field evaluation of a live vaccine against porcine reproductive and respiratory syndrome in fattening pigs. J Vet Med B. 1999;46:603–612.
17. Park C, Seo HW, Kang I, Jeong J, Choi K, Chae C. A new modified live porcine reproductive and respiratory syndrome vaccine improves growth performance in pigs under field conditions. Clin Vaccine Immunol. 2014;21:1350–1356.
