| Case report | Peer reviewed |
Cite as: Marruchella G, Mosca F, Di Provvido A, et al. Severe outbreak of adventitious sternal bursitis in a pig herd in Central Italy. J Swine Health Prod. 2017;25(5):256–260.
Also available as a PDF.
SummaryThis case report describes the main features of an outbreak of adventitious sternal bursitis that severely affected a pig herd in Central Italy between April and May 2016. All cases involved pigs aged 4 to 5 months, originating from the same farm and sharing the same genetic background. Concurrent infections with beta-hemolytic streptococci were observed. The intrinsic and extrinsic causative factors, as well as the significance of this disease in pigs, are herein reviewed and discussed. | ResumenEste reporte de caso describe las características principales de un brote inesperado de bursitis esternal que afectó severamente a un hato de cerdos en Italia Central entre abril y mayo de 2016. Todos los casos incluyeron cerdos entre 4 a 5 meses de edad, provenientes de la misma granja y con la misma genética. Se observaron infecciones simultáneas por estreptococo beta hemolítico. Los factores causantes intrínsecos y extrínsecos, así como la importancia de esta enfermedad en los cerdos, se revisan y discuten aquí. | ResuméCe rapport de cas décrit les principales caractéristiques d’une éclosion de cas de bursites sternales accidentelles qui ont affecté sévèrement un troupeau porcin en Italie centrale entre avril et mai 2016. Tous les cas ont impliqué des porcs âgés de 4 à 5 mois qui provenaient de la même ferme et partageaient le même bagage génétique. Des infections concomitantes par des streptocoques β-hémolytiques ont été observées. Les facteurs causals intrinsèques et extrinsèques, ainsi que la signification de cette condition chez les porcs sont revus et discutés. |
Keywords: swine, adventitious bursitis, beta-hemolytic streptococci
Search the AASV web site
for pages with similar keywords.
Received: September 20, 2016
Accepted: February 6, 2017
Under conventional (ie, intensive) breeding conditions, pigs are often exposed to stressful and harmful environments. As a consequence, traumatic lesions are commonly observed in pigs of different ages, from the farrowing crates to the abattoir. Among those are pressure-induced injuries due to inappropriate flooring, which include calluses, focal skin necrosis, claw fissures and erosions, decubitus ulcers, and “bursitis.”1,2
Strictly speaking, bursitis means inflammation of a bursa, which is a small sac-like cavity lined with a synovial membrane and filled with synovial fluid.3 Bursae are physiologically located around joints and serve to decrease friction where muscles and tendons glide over bones.4 Although used improperly, the terms “adventitious bursa and bursitis” indicate an acquired fluid-filled sac which develops within the subcutis – where “normal” bursae do not exist – after persistent trauma to the skin overlying the bony prominences.3,5
Adventitious bursitis can affect piglets as young as 1 to 2 weeks old and becomes more evident in growing pigs (body weight 30 to 70 kg), when the lesions increase in size and large amounts of fluid accumulate.1,6,7
We report herein the main and peculiar features of a severe outbreak of adventitious bursitis that affected a pig herd in Central Italy.
Case description
The present outbreak occurred in a medium-sized farrow-to-finish pig farm. The herd consisted of two barns (A and B), located approximately 5 km apart and under the same management. Barn A housed approximately 500 sows (Landrace × Large White) and their piglets, which were weaned at 28 to 35 days and therein reared up to 2 months of age. Pigs were then moved to Barn B and raised to market weight. As usual for the Italian pork industry, pigs were marketed at 9 to 10 months of age (average body weight 160 kg) to produce typical seasoned hams and salami.
The herd was free from pseudorabies and vesicular disease, and positive for porcine reproductive and respiratory syndrome (PRRS) virus, Mycoplasma hyopneumoniae, and Actinobacillus pleuropneumoniae. Sows were regularly vaccinated against pseudorabies, erysipelas, and porcine parvovirus, while pigs were vaccinated only against pseudorabies. Both barns had slatted floors without bedding. No all in-all out strategy was carried out.
During the previous few months, the farm’s management had undergone major changes. Gilt replacement ceased, greatly reducing the number of sows. At the same time, in April 2016, 2500 growing pigs (commercial hybrids, approximately 25 kg body weight), were purchased and housed in Barn B.
Also in Barn B, between April and May 2016, a relevant number of pigs (approximately 30 of 2800) developed impressive swellings in the sternal region. The prevalence of these lesions is likely to have been higher, since they were often discrete and detectable only after careful inspection. Sternal lesions affected only the newly introduced pigs, becoming evident within the few weeks after their arrival (Figure 1). Herd history showed that sternal swellings had occasionally occurred in Barn A in suckling and weaned piglets, which usually recovered within a few weeks (Figure 2). In two pigs, such lesions were so severe as to require euthanasia (Figure 3). Both euthanized pigs were necropsied and submitted to cytological (MGG quick stain; Bio-Optica, Milano, Italy) and <>bacteriological investigations (culture and antimicrobial sensitivity). In addition, lesions were sampled, fixed in 10% buffered formalin, and routinely processed for histopathology (hematoxylin and eosin stain).
Figure 1: Pigs in a farrow-to-finish farm into which 2500 growing pigs (25 kg body weight) had recently been introduced (Barn B). Both pigs had developed impressive, symmetrical swellings at the cranial end of the thorax within a few weeks after their arrival. Similar lesions affected approximately 30 pigs. The pig on the left had a very large lesion shaped like a soccer ball, which rubbed on the floor and developed traumatic injuries. Skin injuries foster bacterial infections, worsening the outcome of such lesions.

Figure 2: Piglet from a farrowing crate (Barn A). A prominent swelling was evident at the sternal region in a 4-week-old suckling piglet. On palpation, the fluid content could be easily appreciated.
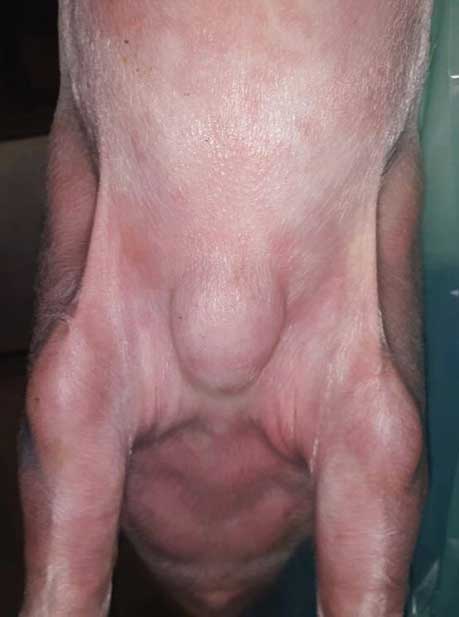
Figure 3: Pig in the growing-finishing unit (Barn B). A very large swelling severely affected the health status of this pig and made euthanasia necessary.

At necropsy, the lesions appeared well circumscribed and surrounded by enlarged lymph nodes. On cut section, the lesions consisted of a thick fibrotic wall and contained many liters of a watery, brownish, fetid exudate (Figure 4). Cytology revealed that such exudates contained a large number of neutrophils and bacterial aggregates (cocci). Histologically, the lesion wall consisted of dense connective tissue embedded with a number of foci of purulent inflammation and bacterial aggregates, and lined with a continuous layer of necrotic tissue on the inner side.
Figure 4: Excised lesion from the pig in Figure 3. After excision, the lesion measured 40 cm in diameter and weighed 15 kg. Cut section showed that it contained many liters of fluid.

A few days later, three more pigs were sampled for diagnostic purposes. Approximately 10 mL of fluid, aseptically collected by <>means of a needle and syringe, was submitted to the diagnostic laboratory of the University of Teramo (Faculty of Veterinary Medicine, Teramo, Italy) for cytological and standard bacteriological investigations.
In all pigs under study, bacteriological culture on sheep blood agar yielded isolation of pure cultures of beta-hemolytic, Gram-positive, catalase-negative and oxidase-negative cocci, which were identified as Streptococcus agalactiae by means of biochemical tests (API 20 STREP<>; bioMèrieux Italia, Bagno a Ripoli, Firenze, Italy). Results were interpreted using the API 20 STREP V8.0 software. The API 20 STREP profile is shown in Figure 5. Antimicrobial susceptibility disk diffusion tests showed that isolates were susceptible to β-lactams (amoxicillin plus clavulanic acid), cephalosporins (cefazolin, cefoperazone), and quinolones (enrofloxacin).
Figure 5: Biochemical profile of the bacterial isolates from the fluid in the lesion shown in figures 3 and 4. Biochemical tests were carried out by means of the API 20 STREP method (bioMèrieux Italia, Bagno a Ripoli, Firenze, Italy) and interpreted by the API 20 STREP V8.0 software. Streptococcus agalactiae was confirmed with a high percent of identity (85.2%). Assuming that S agalactiae contributed to the development of adventitious bursitis in this case, most relevant unexpected results were negative hippuric acid hydrolysis (HIP; 99%) and positive pyrrolidonyl arylamidase (PYRA; 1%). VP = Voges Proskauer (acetoin production); HIP = hippuric acid hydrolysis; ESC = β-glucosidase hydrolysis; PYRA = pyrrolidonyl arylamidase; αGAL = α-galactosidase; βGUR = β-glucuronidase; βGAL = β-galactosidase; PAL = alkaline phosphatase; LAP = leucine aminopeptidase; ADH = arginine dihydrolase; RIB = D-ribose acidification; ARA = L-arabinose acidification; MAN = D-mannitol acidification; SOR = D-sorbitol acidification; LAC = D-lactose acidification; TRE = D-trehalose acidification; INU = inulin acidification; RAF = D-raffinose acidification; AMD = starch acidification; GLYG = glycogen acidification.

On the basis of the obtained results, the diagnosis of adventitious bursitis, complicated by secondary streptococcal infection, was made. Approximately 30 pigs with severe lesions were sent to slaughter before they reached market weight. No additional cases of sternal bursitis were observed. However, in a high percentage of finishing pigs (approximately 50%) large swellings occurred below the hock. In most pigs, the lesions regressed after treatment with ampicillin by intramuscular injection.
Discussion
Adventitious bursae and bursitis are considered to be the result of “pathological responses to an environment that is less than ideal.7” Although adventitious bursitis can develop <>anywhere, it most commonly arises on the boney prominences of the hocks and elbows.1,8,9 The etiology and pathogenesis of adventitious bursitis is considered multi-factorial. A growing body of evidence indicates that a key role is played by the floor type and, more generally, by floor quality and pen conditions that increase the likelihood of injuries. Further extrinsic and interrelated factors include stocking density and the presence of bedding in the resting area.1,3,5,6,10
As a consequence, adventitious bursae or bursitis can represent a relevant issue for pig farms by causing economic losses (low body weight, poor quality of carcasses, rejection of breeding stock), and raising serious animal welfare concerns, especially when secondary infection occurs.1,5,10,11 In fact, the “Welfare Quality Assessment Protocols”12 consider bursitis among the most relevant animal-based measures to evaluate good housing in pig herds.
Genetic effects have also been identified, and white breeds appear to be at higher risk of bursitis, their mean heritability being estimated at 25% and 30%, respectively.13,14 Rapid growth has been suggested as a further intrinsic predisposing factor.1
A kind of sternal bursitis (so-called “breast blister”) is recognized in chickens and turkeys, caused by prolonged pressure from sitting. The morbidity of breast blisters in poultry can exceed 50% and is often exacerbated by Staphylococcus species infection.15 To the best of the authors’ knowledge, no data are currently available concerning the etiology, occurrence, or prevalence of adventitious sternal bursitis in pigs. <>It may be assumed that the described mixture of intrinsic and extrinsic factors caused the present outbreak, which so severely involved a large number of pigs in quite a short time. The concurrent onset of hock bursitis further supports such an assumption.
Improving the quality of flooring surfaces is explicitly considered by the European legislation for the protection of pigs (<>Council Directive 2008/120/EC),16 which lays down the design requirements for the various categories of animals. The farm herein investigated complies with such rules. However, the present report suggests that improving floor quality may not be sufficient to ensure animal welfare and makes adoption of additional measures desirable (eg, the use of straw bedding).
The time course in this outbreak might support an etiological role also for the genetic background of the pigs, although that is difficult to prove, assess, and explain. Moreover, the farm manager reported that the affected pigs spent more time than usual lying on the floor; <>such behavior, probably linked to foot and (or) leg injuries and discomfort, might have further contributed to the onset of bursitis.
<>According to the literature,1 bacterial infections likely exacerbate sternal lesions, which often reached an impressive size as in this case. Streptococcus agalactiae (Lancefield’s group B streptococcus) is known to be a major agent of contagious mastitis in cattle and is considered a human health hazard. Biomolecular and serological typing indicate that human and bovine S agalactiae represent largely distinct populations.17 Occasionally, S agalactiae has been isolated from lesions in pigs.18 However, concerns still remain <>about the true identity of streptococci isolated from such lesions. <>There may be some confusion in distinguishing S agalactiae from Streptococcus porcinus and Streptococcus pseudoporcinus.19,20 The wide zone of hemolysis, along with some biochemical features observed in these isolates (negative reaction to the hippurate hydrolysis test, positive reactions to the pyrolidonylarylamidase and Voges Proskauer tests) might argue against identification of S agalactiae and stimulate further serological and biomolecular investigations.19-22
Implications
- This case report documents that the sternal region can be severely affected by adventitious bursitis.
- Streptococcal infections can be identified in adventitious sternal bursitis lesions, but the routine biochemical characterization of streptococci may be misleading and should be complemented by additional investigations.
Acknowledgement
We gratefully thank Alfreda Tonelli (MS) for kindly revising the English format.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Carr J. Bursitis. In: Carr J, ed. Managing Pig Health. 2nd ed. Sheffield, UK: 5M Publishing; 2013:402–403.
2. Gourreau JM, Drolet R, Martineau GP, Morvan H, Pastoret PP, Pin D, Scott DW. Pressure-induced conditions. In: Gourreau JM, Drolet R, Martineau GP, Morvan H, Pastoret PP, Pin D, Scott DW, eds. Atlas of Porcine Dermatology. 1st ed. Paris, France: OIE World Organization for Animal Health; 2015:391–395.
3. Thompson K. Miscellaneous inflammatory lesions of joint structures. In: Maxie MG, ed. Pathology of Domestic Animals. 5th ed. Philadelphia, Pennsylvania: Saunders Elsevier: 2007;172–173.
4. Barone R. Annessi dei muscoli striati [in Italian]. In: Barone R, ed. Anatomia Comparata dei Mammiferi Domestici. 2nd ed. Vol II. Bologna, Italia: Edagricole; 1981:289–291.
5. Mouttotou N, Hatchell FM, Green LE. Prevalence and risk factors associated with adventitious bursitis in live growing and finishing pigs in south-west England. Prev Vet Med. 1999;39:39–52.
6. Gillman CE, Kilbride AL, Ossent P, Green LE. A cross-sectional study of the prevalence and associated risk factors for bursitis in weaner, grower and finisher pigs from 93 commercial farms in England. Prev Vet Med. 2008;83:308–322.
7. KilBride AL, Gillman CE, Ossent P, Green LE. A cross-sectional study of the prevalence and associated risk factors for capped hock and the associations with bursitis in weaner, grower and finisher pigs from 93 commercial farms in England. Prev Vet Med. 2008;83:272–284.
8. Smith WJ, Taylor S. Adventitious bursitis of the pig hock – a slaughterhouse survey. Veterinary Research Project collection. University of Bristol, UK; 1993.
9. Mouttotou N, Hatchell FM, Lundervold M, Green LE. Prevalence and distribution of foot lesions in finishing pigs in south-west England. Vet Rec. 1997;141:115–120.
10. Mouttotou N, Hatchell FM, Green LE. Adventitious bursitis of the hock in finishing pigs: prevalence, distribution and association with floor type and foot lesions. Vet Rec. 1998;142:109–114.
11. Mouttotou N, Sterry J, Green LE. Cohort study of the association between adventitious bursitis of the hock and the age at slaughter and carcass quality of the pigs on one farm. Vet Rec. 1998;142:52–55.
12. Dalmau A, Velarde K, Scott K, Edwards S, Veissier I, Keeling L, Butterworth A. Welfare Quality Assessment Protocol for pigs. Lelystad, Netherlands: Welfare Quality Assessment Consortium, 2009. Available at <>http://www.welfarequalitynetwork.net/network/45848/7/0/40. Accessed 4 May 2017.
13. Penny RH, Hill FW. Observations of some conditions in pigs at the abattoir with particular reference to tail biting. Vet Rec. 1974;94:174–180.
*14. Smith WJ, Morgan M. The relationship between adventitious bursitis of the hock, breed and heritability. Proc IPVS. Bangkok, Thailand. 1994;447.
15. Riddell C. Developmental, metabolic, and other noninfectious disorders. In: Calnek BW, ed. Diseases of Poultry. 10th ed. London, UK: Mosby-Wolfe; 1997:940–941.
16. Council Directive 2008/120/EC. Available at http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:047:0005:0013:EN:PDF. Accessed 2 May 2017.
17. Zadoks RN, Middleton JR, McDougall S, Katholm J, Schukken YH. Molecular epidemiology of mastitis pathogens of dairy cattle and comparative relevance to humans. J Mammary Gland Biol Neoplasia. 2011;16:357–372.
18. Hommez J, Devriese LA, Castryck F, Miry C. Beta-hemolytic streptococci from pigs: bacteriological diagnosis. Zentralbl Veterinarmed B. 1991;38:441–444.
19. Thompson T, Facklam R. Cross-reactions of reagents from streptococcal grouping kits with Streptococcus porcinus. J Clin Microbiol. 1997;35:1885–1886.
20. Shewmaker PL, Steigerwalt AG, Whitney AM, Morey RE, Graziano JC, Facklam RR, Musser KA, Merquior VL, Teixeira LM. Evaluation of methods for identification and determination of the taxonomic status of strains belonging to the Streptococcus porcinus-Streptococcus pseudoporcinus complex isolated from animal, human, and dairy sources. J Clin Microbiol. 2012;50:3591–3597.
21. Duarte RS, Barros RR, Facklam RR, Teixeira LM. Phenotypic and genotypic characteristics of Streptococcus porcinus isolated from human sources. J Clin Microbiol. 2005;43:4592–4601.
22. Katsumi M, Kataoka Y, Takahashi T, Kikuchi N, Hiramune T. Biochemical and serological examination of beta-hemolytic streptococci isolated from slaughtered pigs. J Vet Med Sci. 1998;60:129–131.
* Non-refereed reference.
