What's your interpretation?
Detecting the onset of a PRRS outbreak in a PRRSV-negative breeding herd using a PRRS ELISA and process behavior charting methods.
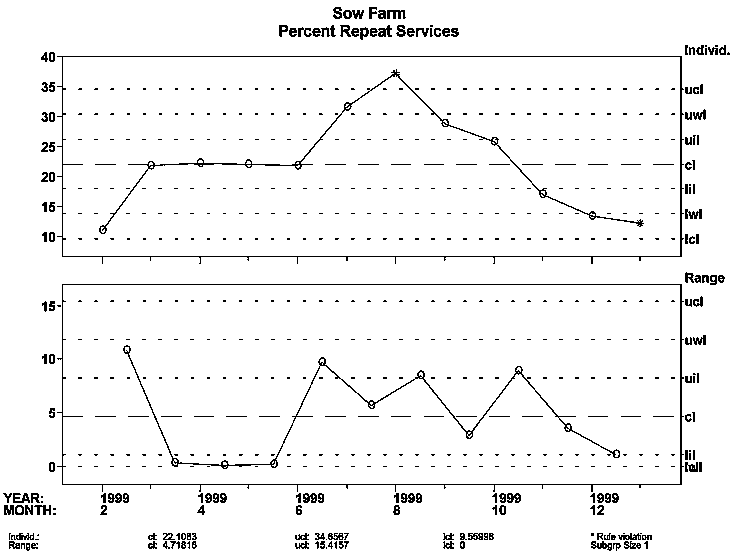
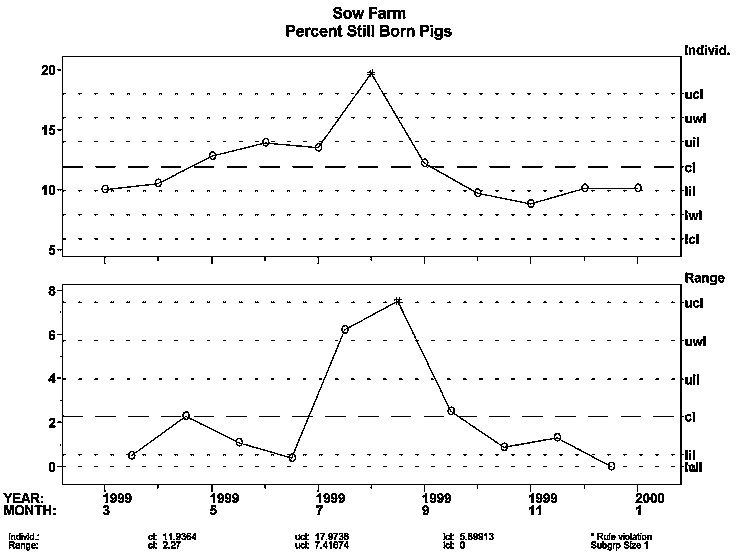
The farm manager of a new breeding herd stocked with PRRSV-negative animals began observing increased stillbirths and repeat services, as indicated by the "3-sigma signals" on the XmR (individual and moving range) charts shown. What could be the possible causes, and how might you rule each in or out?
Continuous process measurement and improvement methods were introduced for tracking a swine breeding herd's health status and performance in February of 1999. Process behavior measurement methods and IDEXX PRRS ELISA serology information were used to measure PRRSV status in a population of breeding females on a monthly basis. At the outset of the process measurement project, the farm was known to be PRRSV-negative.
Health and production data were analyzed using methods for statistical process measurement developed by WA Shewhart (1939)1 and more recently described by Wheeler and Chambers.2 The calculated S:P ratios from the IDEXX PRRS ELISA were charted and analyzed using the X-barR (average and range) charting method. Herd performance data were charted and analyzed using the XmR (individuals and moving range) charting method. Chart "signals", indicating a significant deviation from the range of values expected for a production process, are identified using the decision rules described by Wheeler and Chambers.2
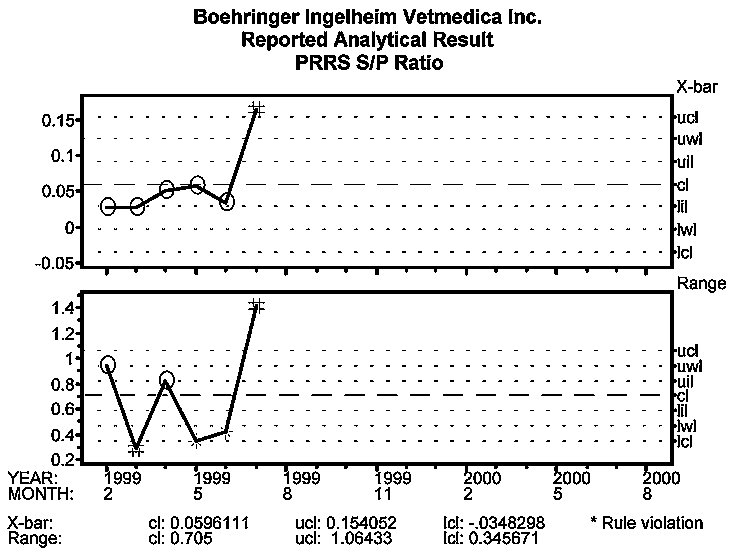
One IDEXX PRRS ELISA sample was positive (S:P=0.74) out of the 30 tested in the first routine monthly sampling in February 1999. There was no clinical evidence of PRRS. In March, all samples were negative. In April, two samples were positive (S:P ratios of 0.51 and 0.42). In May and June, PRRS ELISA results were all negative.
In July, three samples from the routine monthly testing were positive with S:P of 1.41, 1.06, and 0.84. These samples were tested a second time, with S:P of 1.36, 1.17, and 0.74, respectively. These three samples were also pooled and tested using a PRRSV nested polymerase chain reaction (nPCR), which generated a positive result.
In mid-to-late June and early July of 1999, the sow herd manager began observing what he believed to be an abnormal increase in abortions, stillbirths, increased wean-to-service intervals, decreased farrowing rates, and anorexia of breeding animals (Figures 1 and 2). Nursing piglets in the farrowing rooms were healthy during June and through mid-July; however, increased mortality and morbidity became evident in August and continued to late November 1999.
The initial introduction of PRRSV into the breeding herd was detected on PRRS ELISA process behavior charts beginning with the July 1999 sampling. Figure 3 (S:P ratio) indicates that the first signal in the PRRS negative farm was detected in July on both the Average and Range charts.
In early August 1999, 10 piglets were submitted for diagnostic evaluation. Testing by PRRSV PCR yielded 3 of 3 positive sera pools and 2 of 3 positive tissue pools. Virus was isolated from five of the 11 pigs. Restriction fragment length polymorphism (RFLP) results were consistent with a field strain of PRRS virus.
The clinical episode in the breeding herd continued through September. Late in September, approximately 8 to 10 weeks after the onset of the clinical outbreak, all of the females in the breeding herd were vaccinated with a commercially available modified live virus (MLV) PRRS vaccine. Clinical signs in the breeding herd subsided by the end of October 1999.
The breeding herd was vaccinated a second time in November. No new animals were introduced into the herd from June until mid-November. Gilts introduced to the herd starting in mid-November were vaccinated twice, 30 days apart, with the MLV PRRS vaccine.
Gilts known to be actively seroconverting to the PRRS virus were introduced into the herd late in December 1999. In late January 2000, there was an increase in the rate of abortions and anorexia in breeding animals. In February 2000, an aborting animal had a positive PRRSV PCR result. The breeding herd was again vaccinated with the MLV PRRS vaccine in February 2000. Thereafter, the farm has continued to vaccinate all breeding herd animals 'en masse' every 3 months with the modified live virus PRRS vaccine.
As of November 2000, the sow herd, nursery, and grow-to-finish area were showing no clinical signs of PRRS. New replacement gilts have again been introduced with a longer acclimatization periods (4 to 6 weeks). Pigs sent to an off-site nursery are vaccinated upon placement and show evidence of seroconversion at 8 to 10 weeks of age. These pigs are then reared at an off-site finisher, and gilts are selected, revaccinated, and transferred to an isolation-acclimation facility at the breeding herd site, to be integrated into the breeding herd as replacement animals.
Because the change in PRRSV status of this farm from "PRRSV-negative" to "PRRSV-positive" represents a clear process change, process behavior limits were recalculated as of August 1999. The mean S:P ratio chart, Figure 4, depicts a negative population from February through July 1999. In August 1999, the S:P ratio approaches 1.0 and continues to rise, plateaus in November, and increases again in February 2000. The October to December increase may reflect the pigs immune system response. This type of response occurs typically in a vaccinated animal after a heterologous challenge. In this case, the animals first received a natural challenge through exposure to field virus, then were subsequently inoculated with the vaccine virus. The last signal in February may be related to introduction of replacement gilts that were known to be actively seroconverting to PRRS virus upon introduction in December.
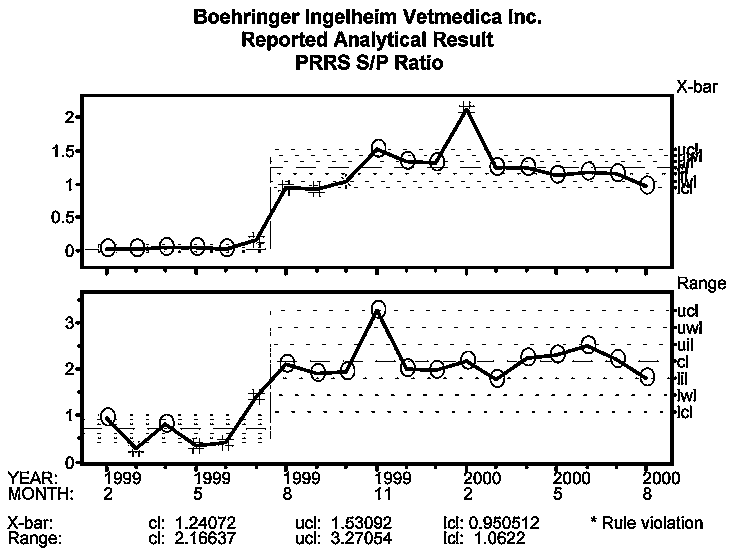
It is expected that vaccination intervention in the breeding herd, in conjunction with the increased acclimatization period, will enable both continued clinical and serological stability (predictable process behavior) in the breeding herd. Pigs are now routinely vaccinated at weaning. This vaccination timing is being changed to entry into the off-site finisher, so that non-vaccinated pigs can be tested late in the off-site nursery. This change is intended to provide for assessment of the PRRSV status of pigs originating from the sow farm on a continual basis. It is expected that as a result of this testing and vaccination process change, PRRSV-negative pigs will be produced from this PRRSV-positive, routinely vaccinating sow farm, and PRRSV-stable internal replacement animals will be introduced back into the sow farm.
Once PRRSV-negative pigs are consistently leaving the off-site nursery, continued breeding herd stabilization will have been achieved. If this is maintained, the next possible phase could be a test-and-removal procedure to attempt to eliminate PRRSV from the breeding herd without depopulation.
References--non-referred
1. Shewhart, WA. Statistical Method from the Viewpoint of Quality Control. New York, New York: Dover Publications, Inc.; 1939.
2. Wheeler DJ, Chambers D. Understanding Statistical Process Control. 2nd ed. Knoxville, Tennessee: SPC Press; 1992.
-- Dale Polson, MS, PHD, DVM;
Dianna Jordan, MS, DVM;
Greg Hartsook;
Larry Graham;
and Reid Philips, DVM
Boehringer Ingelheim Vetmedica, Inc.,
2501 North Loop Drive, Suite 700,
Ames, Iowa 50010, USA
