Original research
Peer-Reviewed
Monitoring of Lawsonia intracellularis by indirect serum immunofluorescence assay in a commerical swine production system
Steven D. Just, DVM, MS; Charles O. Thoen, DVM, PhD; Brad J. Thacker, DVM, PhD; John U. Thompson, DVM, MS
SDJ: Morris Veterinary Center, 605 W. 5th St. Morris, Minnesota 56267; COT, BJT: Iowa State University College of Veterinary Medicine, Ames, Iowa 50011; JUT: Mississippi State University College of Veterinary Medicine, Starkville, Mississippi 39762
Just SD, Thoen CO, Thacker BJ, et al. Monitoring of Lawsonia intracellularis by indirect serum immunofluorescence assay in a commerical swine production system. J Swine Health Prod. 2001;9(2):57-61. Also available in PDF format (124k).
Summary
Objectives: To determine the serological prevalence of Lawsonia intracellularis and the time of seroconversion in a commercial swine production system.
Methods: In the first phase of the study, prevalence of Lawsonia intracellularis (LI) was determined at six of approximately 150 finishing sites. Pigs were randomly selected with stratification by the source of pigs within or from outside the production system. Blood was collected from 33 to 35 pigs at each site, and serum was tested for LI antibodies by indirect immunofluorescence assay (IFA). In phase two, timing of exposure to LI was determined by IFA serum assay of 64 tagged pigs from four sow farms. Blood was first collected from suckling pigs, then at 5-weekintervals until they were sent to market (six samples per pig).
Results: Seroprevalence at the six finishing sites ranged from 0 to 54% (mean 20%). Seroprevalence was 0%, 11.4%, and 31.4% at the three Source A finishing sites; 8.5% and 14.3% at the two Source B finishing sites; and 54.3% at the outside source finishing site. In phase two, pigs seroconverted at the finishing site. Only nine of the 64 pigs tested seroconverted, and all were seronegative 5 weeks later.
Implications: Seroconversion occurred afterthe pigs entered the finishing site, suggesting exposure takes place in the nursery. As IFA titers to LI are short-lived, current and (or) previous infections may be missed when a single, cross-sectional sampling is tested.
Keywords: swine, PPE, ileitis,
Lawsonia intracellularis, indirect immunofluorescent antibody
assay
swine, PPE, ileitis,
Lawsonia intracellularis, indirect immunofluorescent antibody
assay
Received: June 6, 2000
Accepted: November 24, 2000
Lawsonia intracellularis
(LI) is a microaerophilic, curved, gram-negative obligate intracellular
bacillus that infects the enterocytes of the porcine small intestine.1,2
This bacterium was thought to be a "campylobacter-like"
organism until 1993, when Koch's postulates were fulfilled. The
organism was named Lawsonia intracellularis in 1995.1,3
Infection may cause proliferation of immature crypt cells, with
diarrhea and decreased growth performance in young pigs, and sudden
death in more mature pigs.4,5,6 The clinical disease
is called porcine proliferative enteropathy (PPE) or, more commonly,
ileitis. The 1995 National Animal Health Monitoring System (NAHMS)
surveyreported that on the basis of serological testing, the organism
occurred in 6.8% of all swine operations, and in 18.5% of those
marketing over 10,000 animals per year.7 In a study
conducted at the Veterinary Diagnostic Laboratory at Iowa State
University, 5% of all samples of intestinal mucosa submitted from
swine, regardless of age or clinical history, were positive for
LI by polymerase chain reaction (PCR).8 Korean investigators
reported that 20% of herds were infected, and within herd prevalencewas
3.3%.9 In England, the annualcost of PPE to the British
swine industry was estimated to be 2 to 4 million pounds (approximately
1.6 to 3.2 million $US).10 The annual cost to the American
swine industry was estimated to be $98 million, based on an assumed
prevalence of 40%.11
Two clinical forms of PPE occur. Porcine hemorrhagic enteropathy (PHE) is the acute hemorrhagic form that occurs in pigs approximately 4 to 12 months of age and older, resulting in sudden death.6 It should be noted that PHE is not synonymous with hemorrhagic bowel syndrome (HBS), a disease clinically similar to PHE, but with different gross and histological lesions. The cause of HBS is unknown. Porcine intestinal andenomatosis (PIA) is the chronic proliferative form of PPE that occursin pigs approximately 6 to 20 weeks of age or younger, causing diarrhea and poor growth performance.6 Chronic lesions from which the organism has been cleared may regress, and an increase in apoptotic events returns the tissue to normal structure and function.12
As treatment is often unrewarding, control measures are directed towards disease prevention. Tylosin, the only feed grade antibiotic labeled for prevention and control of PPE, is effective at a dosage of 110 grams per ton (110 ppm) for 21 days.13 Chlortetracycline is effective in preventing PPE when administered as a continuous in-feed medication at doses of 272 and 545 g per ton (300 and 600 ppm, respectively).14 A combination of bacitracin methylene disalicylate (BMD, Alpharma Inc., Fort Lee, New Jersey 07024), 30 g per ton, and chlortetracycline (Chlormax(TM), Alpharma Inc., Fort Lee, New Jersey 07024), 22 mg per kg (10 mg per lb), has recently been approved for control of ileitis. Tiamulin is effective for prevention and control of PPE when used as a feed medication at 35 or 50 grams per ton for 35 days.15 Lincomycin decreases shedding of the organism, a significant finding, as severity of PPE is dose dependent.16 When included in the feed at 200 g per ton for 21 days, lincomycin decreases fecal shedding of LI and improves pig performance.17 Lincomycin-spectinomycin, administered as a water soluble powder for 7 days at 10 mg per kg, is also an effective method for the treatment and control of ileitis, indicated by improved ADG and fecal consistency.18 The most effective means of control is to prevent development of PPE lesions by administering feed grade antibiotics. Identifying the stage at which LI infection occursin a production system allows producers to more effectively target their antimicrobial prevention and treatment regimes.
The objectives of this study were to determine the serological prevalence of Lawsonia intracellularis in a commercial swine herd, and identify the time at which animals seroconverted.
Materials and methods
This study was conducted in a large commercialswine production system that had previously experienced clinical PPE, primarily PIA. Sow units, and the nurseries and finishers they supplied, were divided into two pig flows (the movement of pigs from birth to market) designated Source A and Source B on the basis of their historical and disease status. There were approximately 90,000 sows in 20 sow units, each housing approximately 5,000 sows. Each nursery facility housed 16,000 animals. The 120 finishing units each housed 5,000 to 15,000 animals. The animals used for the study were housed in finishing units of varying size. On each site there were eight to ten barns, each housing 1,200 to 1,500 animals. The farm site was the experimental unit.
An indirect immunofluorescent antibody (IFA) serum assay was used to detect anti-Lawsonia intracellularis IgG antibodies, in the procedure described by Knittel in 1997.19 The coating antigen was a pure culture of LI strain N343. Both pig serum and the anti-swine immunoglobulin were diluted 1:30 in PBS before testing. Samples that showed fluorescence were reported as positive. Tests were performed at two different laboratories, Boehringer Ingelheim Vetmedica, Ames, Iowa 50010, and the University of Minnesota - Twin Cities, College of Veterinary Medicine, St. Paul, Minnesota 55108.
Prevalence Determination: Phase 1
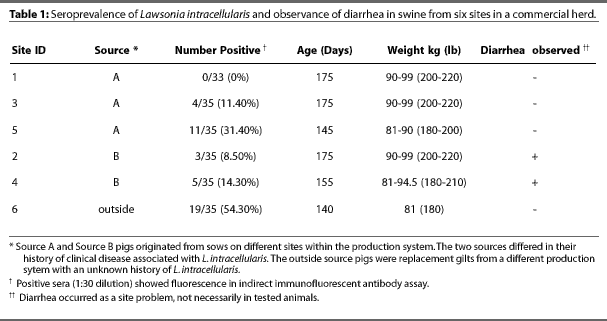
Six finishing sites stocked with pigs of similar age (approximately 175 days old) and weighing 82 to 100 kg (180 to 220 lb) were selected at random. Three sites were stocked with pigs from Source A sow units, two with pigs from Source B sow units, and one with replacement gilts from an outside source with an unknown LI history. Finishing buildings were double curtain-sided,with total slat flooring and deep manure pits.
In order to obtain a 95% confidence level for detecting a 30% prevalence, 33 to 35 blood samples were collected from pigs in different barns at each of the six finishing sites, on different days for each site. Table 1 shows age of pigs at each site. Blood was collected into Vacutainers(TM) by jugular venipuncture. Samples were centrifuged and serum was separated within 24 hours, delivered on ice to Boerhinger Ingleheim Vetmedica Laboratories in Ames, Iowa the day they were collected, and assayed as they were obtained. The number of seropositive animals within the test population was used to determine the seroprevalance of LI in the experimental herd.
Time of Exposure Determination: Phase 2
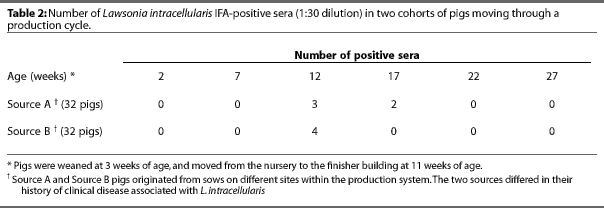
After the prevalence of seropositive animals had been determined, Phase 2 of the study was initiated. Two sow units from Source A and two from source B were used. Pigs were weaned at 3 weeks of age, and spent 8 weeks in the nursery and 16 weeks in the finishing unit, with separate nurseries and finishers for pigs from each source. Littermates were housed in the same nursery room and finishing barn but not necessarily in the same pen.
Eight litters approximately 15 days old were randomly selected in each unit. Two pigs from each litter were tagged (64 pigs total), and blood samples were collected from these animals at 5-week intervals untilthe pigs were marketed. Six samples were collected from each animal: one before weaning (2 weeks of age), one in the nursery (7 weeks of age), and four in the finishing unit (12, 17, 22, and 27 weeks of age). Feed-grade antimicrobials were administered only in the nursery. Pigs from Source A received 181 g per ton of tilmicosin (Pulmotil(R), Elanco Animal Health, Indianapolis, Indiana 46285) for 21 days and pigs from Source B received 399 g per ton of chlortetracycline (Aureomycin(R), Roche Vitamins, Inc., Paramus, New Jersey ) for 14 days.
Blood samples were centrifuged and serum was separated at the farm's laboratory and stored at -80C until testing. Sera were packed on ice and delivered overnight to Dr. Connie Gebhart at the University of Minnesota for IFA testing. Representative feed samples were obtained at the time of each bleeding to verify feed medication inclusion levels in the nursery diets or the absence of feed medication in the finisher diets. Feed samples were sent to Woodson-Tenet Laboratories (Des Moines, Iowa 50305) and to Elanco Animal Health AnalyticalLaboratories (Greenfield, Indiana 46401). Feed and water medication histories were obtained from the nurseries and finishers. Fecal samples were collected from tagged animals that developed clinical signs of ileitis, i.e., diarrhea, and sent to Dr. Gebhart's laboratory for PCR to detect LI.
Results
Prevalence Determination: Phase 1
The results of IFA tests are shown in Table 1. Seroprevalence of LI in the six sites ranged from 0% to 54%, with a mean of 20%. Diarrhea occurred in the two Source B finishing sites, but no observable enteric disease occurred in Source A or outside source sites.
Time of Exposure Determination: Phase 2
Five of the 32 pigs from Source A and four of the 32 pigs from Source B tested positive by IFA. The IFA results by week sampled are presented in Table 2. All pigs were seronegative at 2 and 7 weeks (farrowing and nursery phases, respectively). Three pigs from Source A were seropositive 1 week after arriving at the finishing site but were seronegative 5 weeks later. Two different pigs subsequently became seropositive but were also seronegative 5 weeks after the positive test. One pig from Source B developed diarrhea at approximately 26 weeks of age. A fecal sample from this pig was negative for LI by PCR testing.
Discussion
An IFA assay, rather than PCR, was used to determine the prevalence and timing of exposure to LI because it was likely that PCR would detect only clinically infected pigs or those shedding the organism. AlthoughPCR is a highly sensitive test, only animals with active lesions excrete sufficient organisms for detection by this method.20, 21 Furthermore, shedding of LI appears to be cyclical, even in animals where the ileum is colonized.22 Tests that detect an immune response to LI in either serum or tissue are more accurate for diagnosing ileitis than tests that attempt to detectthe organism in either feces or tissue.24 The IFA has been shown to be a sensitive and specific test.19 However, as with all serological tests, it identifies previous exposure, not active infection.
The seroprevalence of ileitis on this farm is comparable with figures reported for other farms of similar size.7 Little or no recent information on the seroprevalence of LI within infected herds is available. This lack of data is in part due to the recent identification of the causative agent and, until recently, the lack of an accurate ante-mortem diagnostic test.2,23
The results of Phase 2 were unexpected. None of the samples obtained prior to weaning were positive, which casts doubt on the role of passively acquired antibodies in the absence of disease in young pigs, or the ability of the IFA test to detect passively acquired antibodies. There is a possibility that antibodies other than IgG play a role in immunity. In one study, IgM was the major antibody class formed in response to infection with LI, and titers were detected for 8 weeks.25
There is some difference in results between Phase 1 and Phase 2. In Phase 1, animals tested positive at 25 weeks of age; however, in Phase 2, none of the animals tested positive after 17 weeks of age. Although the same protocol was used for the IFA tests in each phase of the study, different laboratories conducted the tests, and this may have contributed to the variation seen between the two phases.
However, although some pigs in Phase 1 tested positive after 22 weeks of age, seroprevalence decreased with age, consistent with Phase 2 results. Seroprevalence in Phase 1 was lowest on the three sites tested when the pigs were more than 22 weeks of age, and was 0% on one of the these sites.
The finding that animals became seronegative within 5 weeks of testing positive was interesting. It appears that the time from exposure to seroconversion and back to seronegative status may be as short as 4 weeks, based upon the IFA test results. More animals may have been infected, but we were unable to detect them because of the short duration of seropositive status. Our data suggest that the single, cross-sectional sampling strategy in combination with IFA testing may not be appropriate for prevalence studies. This test would be more appropriate for timing exposure within a system. A more appropriate testing method for prevalence studies would be to use two or more parallel tests; however, financial constraints did not allow for this in our study. A combination of IFA on serum and PCR on feces would have been a better method. In this method, any animal that tests positive to either test at any time is considered positive. The odds of detecting a true positive increase, but the risk of finding a false positive also increases. Parallel testing has greater sensitivity and negative predictive value than single testing.
In Phase 2, none of the seropositive animals were from the same sow and only two were penmates in the finishing units. This brings into question when and how the organism is transmitted. Infection studies suggest that LI is transmitted via the fecal-oral route.3, 15, 23 Seroconversion and clinical signs occur 2 to 3 weeks after experimental challenge.23 Since none of the animals tested positive until the first week in the finisher, it appears that exposure occurred during the last 2 weeks in the nursery, and exposure from sows shedding the organism either did not occur or was undetected. However, some pigs may have seroconverted earlier but were not detected because of the 5-week intervals between bleedings, or because they had anti-LI antibodies not detected by this test, such as IgM or IgA.
It is possible that in-feed medication delays seroconversion until the medication is withdrawn, or that an infectious dose of LI does not build up in the environment for several weeks. Unfortunately, the location of tagged pigs in the nursery was not recorded, which could have provided information on transmission. Environmental exposure in these facilities is unlikely, because all in-all out management ensured that the nurseries and finishers were thoroughly cleaned and sanitized between groups of pigs.
These results provide veterinarians and producers with additional information for interpretation of PPE serological testing. If the time of exposure to LI is known, the source of exposure may be identified, and control strategies may be improved.
Implications
- Single, cross-sectional sampling to test for exposure to L. intracelluaris by IFA is of limited value, as titers are short-lived, and current and (or) earlier infections may be missed.
- Seroconversion to L. intracelluaris, which occurs 2 to 3 weeks after exposure under research conditions, was detected after the pigs entered the finishing site, suggesting that exposure had taken place in the nursery.
References -- refereed
1. McOrist S, Gebhart C, Boid R, Barns S. Characterization of Lawsonia intracellularis gen. Nov., sp. Nov., the obligately intracellular bacterium of porcine proliferative enteropathy. Inter J Syst Bact. 1995;45:820-825.
2. Gebhart C, McOrist, Lawson G, Collins J, Ward G. Specific in situ hybridization of the intracellular organism of porcine proliferative enteropathy. Vet Pathol. 1994;31:462-467.
3. McOrist S, Jasni S, Mackie R, MacIntyre N, Neef N, Lawson G. Production of porcine proliferative enteropathy with pure culture of ileal symbiont intracellularis. Infect Immun. 1993;61:4286-4292.
4. McOrist S, Mackie R, Neef N, Aitken I, Lawson G. Synergism of ileal symbiont intracellularis and gut bacteria in the reproduction of porcine proliferative enteropathy. Vet Rec. 1994;134:331-332.
5. Gogolewski R, Cook R, Batterham E. Suboptimal growth associated with porcine intestinal adenomatosis in pigs in nutritional studies. Australian Vet J. 1991;68:406-408.
6. McOrist S, Gebhart C. Porcine proliferative enteropathies. In: Straw B, D'Allaire S, Mengeling W, Taylor D, eds. Diseases of Swine. 8th ed. Ames Iowa: Iowa State University Press; 1999:521-534.
7. USDA-NAHMS. Swine'95: Grower/finisher, Part II: Reference of 1995 U.S. Grower/finisher Health and Management of Practices; June 1996.
10. McOrist S, Smith S, Green L. Estimate of direct financial losses due to porcine proliferative enteropathy. Vet Rec. 1997;140:579-581.
12. McOrist S, Roberts L, Jasni S, Rowland A, Lawson G, Gebhart C, Bosworth B. Developed and resolving lesions in procine proliferative enteropathy: possible pathogenic mechanisms. J Comp Pathol. 1996;115:35-45.
14. McOrist S, Shearn M, Morgan J. Control of porcine proliferative enteropathy by oral administration of chlortetracycline. Vet Rec. 1999;144:48-49.
20. Jensen T, Moller K, Leser T, Jorsal S. Comparison of histology, immunohistochemistry and polymerase chain reaction for detection of Lawsonia intracellularis in natural porcine proliferative enteropathy. Euro J Vet Pathol. 1997;3:115-118.
21. McOrist S, Gebhart C, Lawson G. Polymerase chain reaction for diagnosis of porcine proliferative enteropathy. Vet Micro. 1994;41:205-212.
22. Knittel J, Schwartz K, McOrist S, Roof M, Jordan D, Harris D. Diagnosis of porcine proliferative enteritis. Comp Cont Ed Food An. 1997;19:S26-29.
23. Knittel J, Jordan D, Schwartz K, Janke B, Roof M, McOrist S, Harris D. Evaluation of ante-mortem PCR and serological methods for the detection of Lawsonia intracellularis exposed pigs. Am J Vet Res. 1998;59:722-726
25. Lawson S, McOrist S, Rowland A. Serological diagnosis of the porcine proliferative enteropathies: Implications for aetiology and epidemiology. Vet Rec. 1998;122:554-557.
26. Mapother M, Joens L, Glock R. Experimental reproduction of porcine proliferative enteritis. Vet Rec. 1987;121:533-536.
References -- non-refereed
8. Murphy-Jordan DM. Detection and transmission of Lawsonia intracellularis in swine [MS thesis]. Iowa State University; 1998.
11. Veenhuizen MF, Elan TE, Sokensen N. The potential impact of porcine proliferative enteropathy of the US swine industry. Proc IPVS. Bangkok, Thailand. 1994;155
16. McOrist S, Gebhart C, Lawson G. The etiology of porcine proliferative enteropathy. Proc IPVS. Bangkok, Thailand. 1994;155.
17. Winkelman N, Cornell P, Bradford J. Lincomycin feed medication and two water medications against ileitis caused by Lawsonia intracellularis. Proc AASP. Des Moines, Iowa. 199;195.
18. Sjosen CG, Kratzer D, Wager A, Crane J. Treatment of proliferative enteropathy of pigs. Proc AASP. St. Louis, Missouri. 1999;233.
19. Knittel JP. Diagnosis of infection with Lawsonia intracellularis in swine [MS thesis]. Iowa State University; 1997.
24. Guedes R, Gebhart C, Winkelman N, Marsteller T. Comparision of different methods for diagnosis of porcine proliferative enteropathy. Proc Conf of Res Workers in An Dis. 1999;99.
