Original research
Peer reviewed
Transmission of porcine reproductive and respiratory syndrome virus (PRRSV) to age-matched sentinel pigs
Robert W. Wills, DVM, PhD; Alan R. Doster, DVM, PhD; Fernando A. Osorio, PhD
Department of Veterinary and Biomedical Science, University of Nebraska-Lincoln, Lincoln, NE 68583-0907. Corresponding Author: Dr Robert W. Wills, PO Box 6100, College of Veterinary Medicine, Mississippi State University, Mississippi State, MS 39762. Tel: 662-325-9718; Fax: 662-325-1031; E-mail: wills@cvm.msstate.edu.
Wills RW, Doster AR, Osorio FA. Transmission of porcine reproductive and respiratory syndrome virus (PRRSV) to age-matched sentinel pigs. J Swine Health Prod. 2002;10(4):161-165. Also available as a PDF.
Summary
Objectives: To determine how long PRRSV-infected pigs remain contagious to age-matched sentinel pigs and whether the infected pigs are intermittently or continuously contagious.
Methods: In each of two trials, five 4- to 6-week-old pigs (Principals) housed in one isolation room were inoculated with PRRSV. Pairs of age-matched sentinel pigs (Sentinels) were sequentially placed in direct contact with the Principals for 2-week periods, with 1-week intervals between pairs. Serum samples collected from Sentinels were tested by virus isolation and serology (ELISA) to determine whether transmission had occurred. Eight pairs of Sentinels were rotated through the Principals' rooms over a 165- or 167-day period.
Results: The Principals were contagious through Day 62 but not after Day 69 in the first trial and through Day 60 but not after Day 67 in the second trial.
Implications: This study demonstrated that seropositive, non-viremic, grower-aged pigs may be the source of spread of PRRSV in grow-finish units. The results also suggest that infected grower-aged pigs used as a source of PRRSV for natural infection of gilts of similar age in young-gilt acclimation programs may only be relied upon to transmit PRRSV for a maximum of 60 days.
Keywords: swine, porcine respiratory and reproductive syndrome, transmission, epidemiology.
swine, porcine respiratory and reproductive syndrome, transmission, epidemiology.
Received: October 1, 2001
Accepted: January 14, 2002
Porcine reproductive and respiratory syndrome virus (PRRSV) may cause a potentially devastating disease in swine herds. Understanding the ecology of PRRSV, particularly its transmission, is imperative in the development of successful prevention programs. Research has documented transmission between pigs in direct contact and allowed some estimation of how long pigs remain infectious. In one such study, a sow infected 99 days earlier transmitted virus to naive finisher pigs.1 The use of a previously infected but asymptomatic sow illustrated the potential for "recovered" animals to be the source of infection to naive herds and demonstrated for the first time that PRRSV may persist in vivo. In another study, sentinel pigs seroconverted after they were placed in contact with pigs experimentally infected with PRRSV 56 days earlier.2 However, in this case, transmission no longer occurred when sentinels were placed in contact with pigs infected 140 days earlier. Pigs that had been infected 182 days earlier and then treated with prednisolone acetate for 5 consecutive days in an effort to cause immunosuppression also did not transmit the virus to sentinels. The length of time pigs remained contagious was also investigated in a study in which principal pigs were infected by their experimentally inoculated dams. 3 Sentinel pigs became infected with PRRSV after direct contact with prednisolone-treated, 154-day-old principal pigs. Researchers at South Dakota State University have reported transmission of PRRSV from pigs infected in utero up to 112 days of age.4
Yoon et al5 observed that PRRSV viremia tended to be shorter and took longer to develop as the interval between initial inoculation of principals and introduction of sentinel pigs increased. In their study, only two of four pigs in a sentinel group became viremic when exposed 24 days after the principals were inoculated, indicating that the transmission rate declined with time even though new sentinel groups were exposed to previous sentinels as well as principals.
Although it is evident from previous studies that PRRSV-infected pigs remain contagious for extended periods of time, important questions remain. The goal of this study was to further characterize the transmission of PRRSV to answer some of these questions. The specific objectives of the study were threefold: to determine how long a group of PRRSV-infected pigs remains contagious to age-matched sentinel pigs; to determine whether PRRSV-infected pigs are intermittently or continuously contagious; and to determine whether pigs serologically negative by PRRS ELISA are contagious to age-matched sentinel pigs.
Materials and Methods
Animals
In each of two trials, 35 pigs were obtained, after segregated early-weaning, from a herd known to be free of PRRSV through frequent serological testing. A different herd was used as the source of pigs for each trial. Female pigs were used in Trial One and castrated males in Trial Two. After
arrival from the farm of origin, the pigs were placed in isolation rooms for 24 days (Trial One) or 15 days (Trial Two), until the beginning of the experiment. On the first day of the experiment (Day 0), pigs in Trial One were 36 to 38 days old and pigs in Trial Two were 29 to 33 days old.
On Day 0, pigs were randomly assigned to one of two groups: Sentinels (n = 30) or Principals (n = 5). Sentinels were divided into four groups of seven or eight pigs housed in separate isolation rooms, and Principals were housed together in a fifth isolation room.
All pigs were seronegative by PRRS ELISA (HerdChek PRRS; IDEXX Laboratories, Westbrook, Maine) on Day 0.
Virus inoculation
On Day 0 of Trial One, the Principals were inoculated intranasally on inspiration (0.5 mL per naris) with PRRSV inoculum containing 105.2 median tissue culture infective doses (TCID50) per mL. The virus, PRRSV 16244B, was originally isolated during an acute outbreak of PRRS on a Nebraska farm and had a restricted fragment length polymorphism (RFLP) pattern of 1-5-2.
On Day 0 of Trial Two, the Principals were inoculated intranasally on inspiration (0.5 mL per naris) with PRRSV inoculum containing 105.7 TCID50 per mL. The virus originated from southeast Iowa (obtained from Dr W. Mengeling, USDA, ARS, NADC, Ames, Iowa) and had an RFLP pattern of 1-4-2.
Experimental Design
A pair of randomly selected Sentinels was placed in direct contact with the Principals on post-inoculation (PI) Day 6 (Trial One) or PI Day 4 (Trial Two) for an exposure period of 2 weeks. After these Sentinels were removed from the Principals' room, they were held in another isolation room for an additional 2 weeks (isolation period). One week after the first pair of Sentinels was removed from the Principals' room, two more Sentinels were placed in direct contact with the Principals for a 2-week exposure period and were also moved to another isolation room for a 2-week isolation period. This rotation was continued through eight pairs of Sentinels.
On Day 0 of each trial, blood samples were collected from all pigs. Blood was collected from the Sentinels on the first and last days of their exposure period, and on the last day of the 2-week isolation period. Blood was collected from the Principals on the first and last days of each exposure period.
Sample collection and testing
Blood samples were collected from the jugular or anterior vena cava using Vacutainers (Becton Dickinson, Franklin Lakes, New Jersey). Serum samples were stored at -80°C until tested for PRRSV by virus isolation and for PRRSV antibodies by ELISA (HerdChek PRRS; IDEXX Laboratories). Isolation of virus from serum or positive ELISA results in Sentinels after exposure to the Principals was considered evidence that transmission had occurred. An ELISA sample:positive (S:P) ratio >=0.4 was considered positive.
Virus isolation
Monolayers of 2- to 3-day-old MARC 145 cells in 12- or 24-well plates were inoculated with 300 ml of serum per well and incubated for 1 hour at 37°C with 5% CO2. Medium was removed by pipette and then replaced with 1 mL per well of Minimum Essential Medium with 0.2% gentamicin. Cells were incubated for 7 days at 37°C with 5% CO2 and observed daily for signs of cytopathic effects (CPE). If CPE were observed, or at the end of 7 days, plates were frozen and thawed. After thawing, 200 mL of the medium containing cellular debris from each well of these plates was used to inoculate wells in new 12- or 24-well plates containing monolayers of 2- to 3-day old MARC 145 cells. The procedure for the first passage was repeated. At the end of the 7-day incubation period, or sooner if CPE were evident, the cells were fixed with acetone-methanol and stained with PRRSV fluorescent monoclonal antibody conjugate SDOW17 (David Benfield, South Dakota State University, Brookings, South Dakota).
Results
Trial One
All five Principals were viremic on Day 6 PI, and two of the five were viremic on Day 20 PI. Virus was not isolated from the serum of any Principal after Day 20 PI. All Principals seroconverted by Day 20 PI and remained seropositive for the remainder of the trial.
The first two Sentinels were both seropositive and viremic on Day 20 PI, the last day of the exposure period. They were seropositive but not viremic on Day 34 PI, the last day of the isolation period. Evidence of transmission is summarized in Table 1.
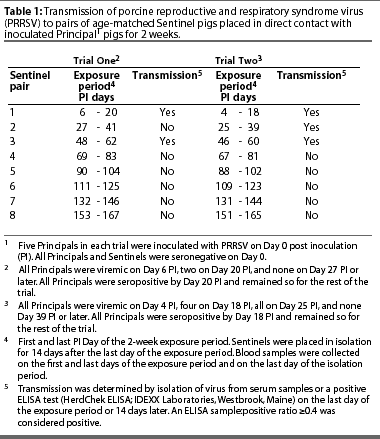
The second pair of Sentinels, exposed to the Principals from Day 27 PI until Day 41 PI, were seronegative on Day 41 (the last day of the exposure period) and on Day 55 PI (the last day of the isolation period), and were not viremic on Days 41 and 55 PI.
The third Sentinel pair was exposed to the Principals from Day 48 PI until Day 62 PI. Both Sentinels were seropositive on Day 62 (the last day of the exposure period) and Day 76 PI (the last day of the isolation period). Neither pig was viremic on Day 62 or 76 PI.
The third Sentinel pair was the last one to become infected. The experiment was ended after five more consecutive exposure cycles without evidence of virus transmission.
Trial Two
All five Principals were viremic on Days 4 and 25 PI, and four of the five were viremic on Day 18 PI. Virus was not isolated from serum of any Principals after Day 25 PI. All Principals seroconverted before Day 18 PI and remained seropositive for the remainder of the trial.
The first two Sentinels were seropositive on Day 18 PI, the last day of the exposure period, and on Day 32 PI, the last day of the isolation period. They were not viremic on Day 18 nor on Day 32 PI.
Only one of the second pair of Sentinels, placed in contact with the Principals from Day 25 PI until Day 39 PI, was seropositive on Day 39 PI (the last day of the exposure period). However, both pigs were viremic on Day 39 PI. Both pigs were seropositive but neither was viremic Day 53 PI, the last day of the isolation period.
The third Sentinel pair was exposed to the Principals from Day 46 PI until Day 60. Both pigs were seropositive on Day 60 (the last day of the exposure period) and on day 74 PI (the last day of the isolation period). Neither pig was viremic on Day 60 or 74 PI.
The third Sentinel pair were the last pigs to become infected. The experiment was ended after five more consecutive exposure cycles without evidence of virus transmission.
Discussion
After the Sentinels had been exposed to the Principals for 2 weeks, they were isolated for another 2 weeks to allow time for seroconversion. The exposure period was selected so that if transmission occurred, the Sentinels would be seropositive, viremic, or both when they were removed from the Principals' room. Previous research demonstrated that pigs became serologically positive within 13 days after inoculation when tested by the assay used in this experiment.6 Therefore, it was felt that if transmission occurred near the end or even on the last day of the exposure period, the Sentinels would have sufficient time to seroconvert during their 2-week isolation period. If, however, seroconversion did not occur before the end of the isolation period, it was expected that, in cases where transmission had occurred, viremia would be detected.
The Principals in Trial One, as a group, remained contagious through Day 62 PI but did not transmit the virus after Day 69 PI, and the Principals in Trial Two remained contagious through Day 60 PI but did not transmit the virus after Day 67 PI. In general, when transmission was demonstrated, both of the Sentinels were seropositive at the end of the exposure period. In two of the five cases where PRRSV was transmitted, the Sentinels were both viremic and seropositive at the end of the exposure period, but were not viremic at the end of the isolation period, 2 weeks later. In the other three cases, Sentinels became seropositive, but viremia was not detected. These pigs had ceased to be viremic within 14 days of initial exposure to the Principals. Other researchers have shown that the duration of viremia is shorter in sentinel pigs than in inoculated principals.5 In a study in which blood samples were collected daily from eight pigs for 15 days PI, pigs became serologically positive 9 to 13 days after inoculation (Herdchek ELISA, S:P ratio >=0.4 considered positive).6 Collectively, these results indicate that when transmission occurred, the pigs were infected soon after initial contact with the Principals.
The absence of virus transmission between the Principals and the second sentinel group in Trial One suggests that either the level of virus shed by the Principals during the exposure period was less than required for transmission, or the contact between Principals and Sentinels was not intimate enough for transmission to occur. It is also possible that transmission occurred but was not detected. Failure to detect transmission might have occurred because viremia was undetectable by the virus isolation methods used, and serum antibodies measurable by ELISA developed too slowly to be identified within the 2-week isolation period. In the second trial, both pigs in the second group of Sentinels were viremic at the end of the exposure period, but only one was seropositive. Both were seropositive 14 days later. Although this may be explained by normal variation in the time required for the development of a positive ELISA response, it may indicate that transmission did not occur immediately after exposure, and that, as in Trial One, transmissibility was depressed approximately 3 to 6 weeks after the Principals had been inoculated. The apparent decrease in contagiousness at this time might have been due to an increase in immune system activity and effectiveness resulting in decreased viral load and shedding by the Principals. Yoon et al5 showed that sentinel pigs placed in contact with principals inoculated 24 days earlier were less likely to become infected than sentinel pigs placed in contact with principals 3 days or 10 days after inoculation, even though the first sets of sentinel pigs were still in the room with the principals. However, their study did not extend long enough to determine if transmission stopped completely after that time. Additional trials with a higher turnover rate of sentinel pigs are needed to more precisely characterize the transmission rate during this period.
Possible explanations for the successful transmission between the Principals and the third Sentinel groups might include a resurgence in the levels of virus being shed by the Principals due to immunological changes, such as immunosuppression induced by endogenous corticosteroid release as a result of the stress of commingling of pigs. Alternatively, more intimate contact through increased fighting might result in transmission, ie, viral load might not have changed but the degree of contact between Principals and Sentinels might have increased. The lack of transmission which was evident after exposure of the third Sentinel pairs in both trials might have been due to decreased viral shedding by the Principals, decreased susceptibility with increasing age of the Sentinels, or both.
The two virus isolates used in the trials were selected on the basis of their origin from unrelated swine herds and their characterization (different RFLP patterns). There was no evidence to suggest that differences in virus isolates affected transmissibility. There also was no evidence to suggest that differences in gender of the pigs affected transmissibility. However, it must be noted that there were insufficient replications of the trials to provide a statistically valid assessment of these factors.
In other research, principal pigs remained capable of transmitting PRRSV beyond the approximate 2-month period that was seen in this study. Pigs that were the offspring of dams inoculated at 85 to 90 days of gestation remained contagious up to 112 days4 and 154 days3 of age, respectively. These results suggest that pigs infected through vertical transmission from their dams may remain capable of transmitting PRRSV longer than pigs infected through horizontal, pig-to-pig transmission. It should be noted that in the study in which the principals were contagious at 154 days of age, they were infected with a European virus isolate and were treated with prednisolone prior to the observance of transmission.3 Either or both of these factors might be responsible for differences in duration of contagiousness.
Transmission studies with an arterivirus in mice, lactate dehydrogenase-elevating virus (LDV), indicate that the quality of direct contact influences the likelihood of transmission. Review of these studies provides insight into the transmission of PRRSV, which is also an arterivirus. The minimum infectious dose (MID) when mice were inoculated by oral, vaginal, rectal, or ocular routes was 103 to 105 times greater than if mice were inoculated by intraperitoneal or tail cartilage injections.7 In other studies, LDV was more readily transmitted when male mice of a strain prone to fighting were inoculated and put in contact with susceptible mice than when mice of a strain of a less aggressive nature were used.8,9 Studies in which the incisors of inoculated or susceptible mice or both were removed indicated that LDV could be transmitted by either injection of saliva or ingestion of blood and tissue.9 The degree of intimate contact might also influence the likelihood of transmission of PRRSV. Although some fighting occurred in this study when age-matched Sentinels were introduced to the Principals, severe fighting was not noted. Transmission might be more likely to occur as a result of the more vigorous fighting that occurs between pigs of different ages establishing social dominance. This may explain the discrepancy between the results of this study, in which the pigs were of the same age, and one in which PRRSV was transmitted from a convalescent sow to finisher pigs 99 days after inoculation of the sow.1
The experiment was terminated before the Principals had returned to seronegative status. Therefore, it was not possible to determine if seronegative pigs were still capable of transmitting PRRSV. However, the Principals remained seropositive for at least 3 months after they were last shown to be contagious to age-matched Sentinels. Extrapolation of these results would suggest that pigs previously infected with PRRSV that have returned to seronegative status are not likely to be contagious.
In previous research, PRRSV was recovered from the tonsil scraping sample of a pig inoculated 157 days previously.10 Other research has demonstrated the presence of PRRSV RNA in serum from 210-day-old pigs from dams inoculated with virus4 and in serum from pigs inoculated 251 days previously.11 Although these studies demonstrate that some pigs may remain persistently infected by PRRSV for months, it is not clear how readily the persistently infected pigs are able to transmit the virus. The current study suggests that inoculated pigs do not routinely remain contagious to other pigs beyond 2 months. However, further research is needed to determine how transmission is affected by various factors, such as mode of transmission (vertical or horizontal), gender, age of principals, age of sentinels, host genetics, physiological state of host, degree of contact, environmental factors, and strain of virus. Until the effects of these factors are better understood, it is risky to assume that the relatively short contagious period found in this study is typical of transmission between pigs of similar ages. Because of the large size of modern swine herds, there is potential for resurgence of the disease even if shedding by a persistently infected pig is a relatively rare event.
A swine bioassay, in which naive pigs rather than tissue cultures are inoculated with serum collected from sentinels, might have provided a more sensitive test of viremia, and allowed detection of viremia in Sentinels with virus levels too low to be detected by the virus isolation methods used in the study. Increasing the length of time Sentinels remained in the seroconversion isolation room would have better ensured that adequate time was allowed for the Sentinels to develop a detectable immune response. However, in the five cases in which transmission was detected, one or more Sentinels were seropositive when removed from the Principals' room, indicating that transmission generally occurred early in the exposure period.
Even if relatively short periods of contagiousness are the rule, the influx of susceptible animals into a herd will allow persistence of virus within the herd. A continu- ing supply of susceptible pigs, which in turn become "principals" that transmit the virus to other susceptible pigs, makes it difficult to eliminate PRRSV from the herd. On the other hand, the results of this study suggest that infected grower-age pigs used as a source of PRRSV for natural infection of gilts of similar age in young-gilt acclimatization programs may only be relied upon to transmit PRRSV for a maximum of 60 days.
Implications
- Under the conditions of this study, 5-week-old pigs inoculated with PRRSV transmitted the virus for up to 62 days after inoculation to age-matched sentinel pigs.
- Seropositive, non-viremic, grower-aged pigs may be the source of spread of PRRSV in grow-finish units.
- Infected grower-aged pigs used as a source of PRRSV for natural infection of similarly aged gilts in young-gilt acclimatization programs may only be relied upon to transmit PRRSV for a maximum of 60 days.
Acknowledgements
The authors thank Judy Galeota, Blaine Clowser, and L Powell for their technical assistance. They also thank Dr William Mengeling for providing one of the PRRSV isolates used in the study. This research was funded by the National Pork Producers Council on behalf of the National Pork Board.
References - refereed
3. Albina E, Madec F, Cariolet R, Torrison J. Immune response and persistence of the porcine reproductive and respiratory syndrome virus in infected pigs and farm units. Vet Rec. 1994;134:567-73.
6. Yoon K-J, Zimmerman JJ, Swenson SL, McGinley MJ, Eernisse KA, Brevik A, Rhinehart LL, Frey ML, Hill HT, Platt KB. Characterization of the humoral immune response to porcine reproductive and respiratory syndrome (PRRS) virus infection. J Vet Diagn Invest. 1995;7:305-312.
7. Cafruny WA, Hovinen DE. The relationship between route of infection and minimum infectious dose: studies with lactate dehydrogenase-elevating virus. J Virol Methods. 1988;20:265-268.
8. Notkins AL, Shochat SJ. Studies on the multiplication and the properties of the lactic dehydrogenase agent. J Exp Med. 1963;117:735-747.
9. Notkins AL, Scheele C, Scherp HW. Transmission of the lactic dehydrogenase agent in normal and partially edentulous mice. Nature. 1964; 202:418-419.
10. Wills RW, Zimmerman JJ, Yoon KJ, Swenson SL, McGinley MJ, Hill HT, Platt KB, Christopher-Hennings J, Nelson EA. Porcine reproductive and respiratory syndrome virus: a persistent infection. Vet Microbiol. 1997;55:231-240.
References - non refereed
1. Zimmerman J, Sanderson T, Eernisse KA, Hill HT, Frey ML. Transmission of SIRS virus in convalescent animals to commingled penmates under experimental conditions. AASP Newsletter. 1992;4(4):25.
2. Terpstra C, Wensvoort G, van Leengoed LAMG. Persistence of Lelystad virus in herds affected by porcine epidemic abortion and respiratory syndrome. Proc IPVS. The Hague, the Netherlands. 1992;118.
4. Benfield DA, Christopher-Hennings J, Nelson EA, Rowland RRR, Nelson JK, Chase CCL, Rossow KD. Persistent fetal infection of porcine reproductive and respiratory syndrome (PRRS) virus. Proc AASP. Quebec City, Canada. 1997; 455-458.
11. Wills RW, Doster AR, Galeota J, Sur JH, Osorio FA. Infection duration and proportion of persistently infected pigs. Proc 80th Ann Meet Conf Res Work An Dis. Chicago, Illinois. 1999; Abstract 178.










The AASV website and The Journal of Swine Health and Production are made possible by the generous support of the AASV Industry Support Council, including: .
Copyright (C) 1996-2002 American Association of Swine Veterinarians. Please send your suggestions about this site to Dave Brown, webmaster@aasv.org.
This page last updated April 19, 2012.
