Brief communication |
Peer reviewed |
Immunostimulatory effects of an anionic alkali mineral complex solution (Barodon®) on porcine lymphocytes
Byung Woo Yoo, DVM, MPH, PhD; Soo Il Choi, PhD; So Hyun Kim, DVM, MS; Soo Jin Yang, DVM, MS; Hye Cheong Koo, DVM, MS; Nam Hoon Kwon, DVM; Sang Hoon Seo, MS; Bong Kyun Park, DVM, MS, PhD; Han Sang Yoo, DVM, MS, PhD; Yong Ho Park, DVM, MS, PhD
BWY, SHS: Agribrands Purina Korea Inc, Seoul 135-280, Korea; SIC: Barodon-SF Corp, Ansung, Gyounggi, 456-880, Korea; SHK, SJY, HCK, NHK, BKP, YHP: Department of Microbiology, College of Veterinary Medicine and School of Agricultural Biotechnology, Seoul National University, Suwon, Gyounggi, 441-744, Korea; HSY: Department of Infectious Disease, College of Veterinary Medicine and School of Agricultural Biotechnology, Seoul National University, Suwon, Gyounggi, 441-744, Korea; Corresponding author: Yong Ho Park, Department of Microbiology, College of Veterinary Medicine and School of Agricultural Biotechnology, Seoul National University, Seo-Doon Dong 103, Kwon-Sun Gu, Suwon, Gyounggi, 441-744, Korea. Tel: +82-31-290-2735; Fax: +82-31-295-7524; E-mail: yhp@snu.ac.kr.
Yoo BW, Choi SI, Kim SH, et al. Immunostimulatory effects of an anionic alkali mineral complex solution (Barodon®) on porcine lymphocytes. J Swine Health Prod. 2002;10(6):265-270. Also available as a PDF
Note: Figure 1 has been updated per errata published in January 2003.
Summary
The anionic alkali mineral complex solution, Barodon®, increased expression of porcine leukocyte subpopulations in treated pigs, determined by use of specific monoclonal antibodies and flow cytometry. This immunostimulatory effect supports use of Barodon as an alternative to antimicrobials for improving productivity in the swine industry.
Keywords: swine, Barodon®, immunostimulator, CD4+CD8+ double-positive population
Search the AASV web site for pages with similar keywords.
Received: December 1, 2001
Accepted: May 23, 2002
There has been an increasing demand in the food animal industry for drugs which leave no residue in meat, because of concern about antibiotic-resistance problems in humans.1 Alternatives to antimicrobials, such as nonspecific immunostimulators, synthetic peptides, natural herbs, and fermentative microorganisms, are being evaluated with new interest.2-9 Dietary supplements, such as probiotics, vitamins, amino acids, and minerals, are relatively well established in the feed industry, show considerable potential for improving growth rate of animals, and may enhance immunity, alter microbial flora, and reduce the occurrence of diseases associated with pathogens.9-13
Recently, an anionic alkali mineral complex solution, Barodon (Barodon-SF, Ansung, Gyounggi, Korea), was introduced to improve productivity of food-producing animals in Korea. Barodon's properties are based on its mineral composition, including silicon, silver, sodium, and potassium ions in an alkaline solution (pH 13.5). Although Barodon was patented as an anionic solution in the US,14 and also in Korea,15 the exact mechanism of its effects are unknown. This study was designed to evaluate Barodon as a nonspecific immunostimulating agent in pigs. A set of monoclonal antibodies specifically reactive with porcine leukocyte differentiation antigens were used with flow cytometry to determine the proportions of leukocyte subpopulations. To investigate the specific cell types which may respond to Barodon, two-color fluorescence flow cytometry was used with monoclonal antibodies (mAbs) of different isotypes that reacted with lymphocytes from peripheral blood and lymphoid tissues from treated feeder pigs.
Materials and methods
Animals and housing
A total of 50 mixed-breed (Yorkshire-Landrace-Duroc), healthy feeder pigs at 15 weeks of age were used for the study. Pigs were randomly divided into a Barodon-treated group (40 pigs) and a control group (10 pigs). Pigs were housed ten per pen in five 4 x 4.2-m pens (16.8 m2) with concrete floors in an environmentally-controlled building.
Feeding and treatment with Barodon
The control group was fed a grower diet (Leantec Grower; Agribrands Purina Korea, Seoul, Korea) based on corn and soybean oil meal, with 17% crude protein, 6% fat, 3% fiber, and digestible energy 3500 kcal per kg. Minerals (including calcium, phosphorus, iron, magnesium, copper, and zinc) and vitamins (including vitamins A, D3, and E) were added at levels commonly found in grower diets. The Barodon-treated group was fed the same diet sprayed with Barodon at a rate of 0.05% of the total amount of feed. Composition of Barod-on is shown in Table 1. Barodon contains sucrose, which was present in the treated feed at 15.97 g per 1000 kg of feed, for a difference in energy of 0.16 kcal per day per pig in the Barodon-treated group.
Experimental design
The pigs were allowed 1 week of acclimatization in the housing facility, and had free access to feed and water. During Study Weeks 1 to 9 of the 13-week study, the four treated pens received the grower diet with 0.05% Barodon, and the control pen received the same diet without Barodon. During Study Weeks 10 through 13, both Barodon-treated and control groups received untreated feed. Daily weight gain and feed consumption were measured and feed conversion rate was calculated on an individual animal basis in each group until the end of Week 5. Blood samples were collected at the beginning of the study and at Study Weeks 3, 8, 11, and 13. During each of Study Weeks 8 and 11, two pigs from the control group and four from the Barodon-treated group were sacrificed for collection of mesenteric lymph nodes (a total of 12 pigs). The remaining pigs in both groups were weighed at the end of the study period (Week 13).
Preparation of peripheral blood leukocytes and mesenteric lymph node leukocytes
Approximately 20 mL of blood was collected from each animal using Vacutainers (Becton Dickinson Vacutainer System, Rutherford, New Jersey). Peripheral blood leukocytes were separated by the method of Davis et al (1987).16 Briefly, blood was mixed with an equal volume of acid-citrate-dextrose-EDTA and leukocytes were separated using density gradient centrifugation in Hypaque Ficoll (specific gravity 1.086; Sigma Chemical, St Louis, Missouri) at 800g for 30 minutes. Mesenteric lymph node leukocytes were also separated by the method of Davis et al16 using Hypaque Ficoll. Cells were counted by the trypan blue exclusion technique16 and final concentration was adjusted to 1x107 cells per mL.
Monoclonal antibodies specific to porcine leukocyte differentiation antigens
A panel of mAbs (Veterinary Medical Research and Development, Inc, Pullman, Washington) specifically reactive with porcine leukocyte differentiation antigens is shown in Table 2. The mAbs specific to major histocompatibility complex (MHC)-class I, MHC-class II, porcine (Po)CD2, PoCD4, PoCD8, surface (s)IgM, nonT/nonB ([gamma]dT-cell receptor), granulocyte, and monocyte antigens were used to examine the proportions of leukocyte subpopulations in peripheral blood or mesenteric lymph nodes. Mouse anti-pig CD4-fluorescein isothio-cyanate (FITC) conjugate (isotype IgG2a; Southern Biotechnology Associates, Birmingham, Alabama) and CD8-phycoerythrin (PE) conjugate (isotype IgG2b; Southern Biotechnology Associates) were used in dual color analysis.
Flow cytometry analysis
The proportions of leukocyte subpopulations were determined by flow cytometry using the CellQuest program (FACS Calibur, Becton Dickinson Immunocyto-metry System, San Jose, California). Approximately 50 mL of each mAb (15 mg per mL) was reacted with 100 mL of cells (1x107 cells per mL) in a V-bottomed, 96-well microplate (Costar, Corning, Corning, New York). Plates were first incubated on ice for 30 minutes, then washed three times with PBS-ACD buffer with horse serum (PBS, 450 mL; acid-citrate-dextrose (ACD), 50 mL; 20% sodium azide, 5 mL; gamma globulin-free horse serum [Sigma], 10 mL; 250 mM EDTA, 20 mL; and 0.5% phenol red, 1 mL), with centrifugation at 700g for 5 minutes. Pellets were disrupted by vortexing and mixed with 100 mL of FITC-conjugated goat anti-mouse IgG+IgM antibody (Caltag Laboratories, Burlingame, California) that had been diluted 1:200 in buffer and incubated on ice for 30 minutes in the dark. Pellets were then washed three times with PBS-ACD buffer without horse serum with centrifugation at 700g for 5 minutes. After the final washing, pellets were mixed with 200mL of 2% PBS-formaldehyde (37% formaldehyde, 20 mL; PBS, 980 mL) and stored at 4°C for flow cytometry analysis.
For dual color analysis, a pair of FITC- or PE-conjugated PoCD4 or PoCD8 mAbs of different isotypes were used as second step reagents.
Statistical analysis
The proportions of lymphocytes carrying the various antigens in peripheral blood and the proportions of CD4+CD8+ double positive T lymphocytes in peripheral blood and mesenteric lymph nodes collected during the 13 weeks of the study were compared in pigs in the Barodon-treated and control groups using the Kruskal-Wallis one-way ANOVA by ranks. Analyses were performed using Microcal Origin 5.0 (Microcal Software, Northampton, Maine), with the level of significance set at P<.05.
Results
Feed efficiency, weight gain, and productivity
Pigs remained clinically healthy during the study. During the first 5 study weeks, Barodon-treated pigs gained 890.48 g per day (SD 41.92 g), and control pigs gained 842.86 g per day (SD 61.42 g); feed intake was 2769.05 g per day for Barodon-treated pigs and 2713 g per day for control pigs; and feed-to-gain was 3.11 for the Barodon-treated pigs and 3.22 for control pigs. At the end of the 13-week study, mean weight was 108.75 kg (SD 5.13 kg) for the remaining 32 Barodon-treated pigs and 106.20 kg (SD 8.38 kg) for the remaining six control pigs.
Flow cytometry analysis
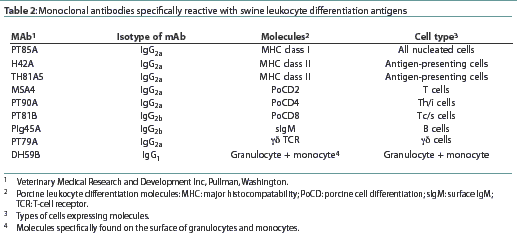
The proportion of peripheral blood lymphocytes expressing CD4 antigen was higher in the Barodon-treated group than in the control group at Study Weeks 8, 11, and 13 (Figure 1A). The proportions of peripheral blood lymphocytes expressing CD8 antigen were higher in the Barodon-treated group than in the control group at Study Weeks 3, 8, 11, and 13 (Figure 1B). The proportions of peripheral blood lymphocytes expressing MHC class II antigen were higher in the Barodon-treated group than in the control group at Study Weeks 3, 8, 11, and 13 (Figure 1C). In addition, the proportions of non T/non B cells were higher in the Barodon-treated group than in the control group at Study Weeks 3, 11, and 13, but not at Study Week 8 (Figure 1D). There were no differences between the Barodon-treated group and the control group in the proportions of peripheral blood CD2+ cells, B cells, monocytes, or granulocytes during the experiment (data not shown).
Proportion of CD4+CD8+ double-positive T lymphocytes in peripheral blood and mesenteric lymph nodes
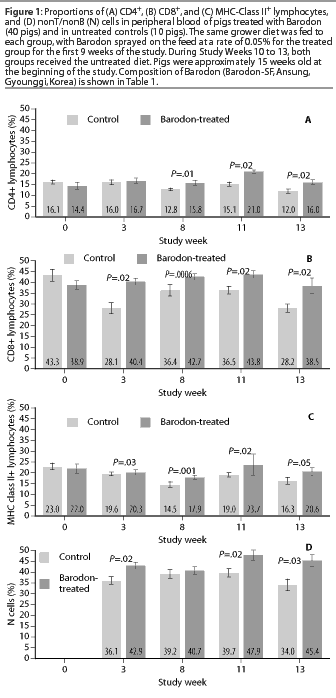
At Study Week 8, a higher proportion (P<.05) of CD4+CD8+ double-positive T lymphocytes was observed in the Barodon-treated group than in the control group, both in peripheral blood lymphocytes (Figure2A) and in mesenteric lymph node lymphocytes (Figure 2B). At Study Week 11, a higher proportion (P<.05) of double-positive lymphocytes was observed in mesenteric lymph node lymphocytes in the Barodon-treated group than in the control group (Figure 2C).
Discussion
The recent development of mAbs specific to leukocyte differentiation antigens of various animals, including pigs, makes it possible to define the host immune system more completely.17-27 By monitoring the animal immune system, the efficacy of vaccines and new drugs can be evaluated in vivo by comparing the host response before and after application of reagents.20,22,28 The porcine immune system is unique in the expression of extrathymic CD4+CD8+ double-positive lymphocytes.21,27,29-31 This double-positive population is found in the peripheral blood and secondary lymphoid organs of swine,21,30,31 and the proportion increases with age.21,30-33 In 1-month-old piglets, 1% of T cells are CD4+CD8+, while in 3-year-old pigs, up to 60% of T lymphocytes express these antigens.21 The age-associated increase in the proportion of CD4+CD8+ cells suggests that this type of T cell may have an important role in host defense. In fact, the CD4+CD8+ population does play a major role in protective immunity.30 Several studies have showed that these cells possess immunological memory function.21,31 Zuckermann and Husmann31 showed that CD4+CD8+ T lymphocytes have a specific memory cell marker, CD29; thus, the double-positive population may play a role in inducing secondary immune responses in the host.
Although the total CD2+ T lymphocyte population was not larger in the Barodon-treated group, the CD4+ and CD8+ T lymphocyte populations were larger in peripheral blood. In addition, dual color flow cytometry analysis revealed a high proportion of CD4+CD8+ cells in lymphocytes from peripheral blood and mesenteric lymph nodes in the Barodon-treated group, whereas a very small proportion of double-positive cells was observed in the control group. These results show that most CD4+ T lymphocytes coexpressed the CD8 antigen in pigs treated with Barodon. The larger double-positive population in peripheral blood and mesenteric lymph nodes of Barodon-treated pigs provides evidence of Barodon's effects on the porcine immune system. The difference in the double-positive population was greater in lymphoid organs, suggesting that Barodon may enhance antigenic stimulation in the immune tissues. Further studies using purified CD4+CD8+ double-positive cell populations will be necessary to elucidate the activity of Barodon more specifically.
Barodon's adjuvant properties have been demonstrated in pigs vaccinated against classical swine fever. Higher antibody titers, and greater proportions of MHC-class II+ and CD8+ immune cells and surface IgM+ B lymphocytes were observed in pigs treated with Barodon, compared to untreated controls.34 Silica, among several candidate substances in Barodon, is likely responsible for these immuno-enhancing effects. Antonini et al35 (2000) showed that subchronic silica exposure enhances respiratory defense mechanisms, for example, by increasing proportions of neutrophils, T lymphocytes, and natural killer cells, and increasing macrophage activation and pulmonary clearance of Listeria mono-cytogenes.
Other substances in Barodon, including minerals, may affect vital biological processes such as immune responses. When administered in an anionic aqueous solution such as Barodon, these substances may penetrate into body fluids more easily than similar products in powder formulations. This may contribute to Barodon's ability to enhance productivity and activation of immune responses in pigs. Although further studies are needed to elucidate the exact mechanism of Barodon and its synergistic effect with vaccines or antibiotics in the porcine immune system, this study shows that Barodon may be a candidate immunostimulator and alternative to antimicrobial feed additives for improving productivity and host immune responses.
Implications
- Barodon has nonspecific immuno-stimulatory effects on porcine immune cells.
- Barodon may be an alternative to antimicrobials for improving productivity in the swine industry.
Acknowledgements
This study was supported by the Agriculture Special Fund, and further support was provided by the Research Institute of Veterinary Science, College of Veterinary Medicine, Seoul National University. The study was also supported by the Brain-Korea 21 project in Agricultural Biotechnology.
References - refereed
1. Pedersen KB, Aarestrup FM, Jensen NE, Bager F, Jensen LB, Jorsal SE, Nielsen TK, Hansen HC, Meyling A, Wegener HC. The need for a veterinary antibiotic policy. Vet Record. 1999;145:50-53.
2. Abo KA, Ogunleye VO, Ashidi JS. Antimicrobial potential of Spondias mombin, Croton zambesicus and Zygotritonia crocea. Phytother Res. 1999;13:494-497.
3. Berg RD. Probiotics, prebiotics or conbiotics. Trends Microbiol. 1998;6:89-92.
4. Chattopadhyay D, Maiti K, Kundu AP, Chakraborty MS, Bhadra R, Mandal SC, Mandal AB. Antimicrobial activity of Alstonia macrophylla: a folklore of bay islands. J Ethnopharmacol. 2001;77:49-55.
5. Dunier M, Vergnet C, Siwicki AK, Verlhac V. Effect of lindane exposure on rainbow trout (Oncorhynchus mykiss) immunity. IV. Prevention of nonspecific and specific immunosuppression by dietary vitamin C (ascorbate-2-polyphosphate). Ecotoxicol Environ Safety. 1995;30:259-268.
6. Gonser S, Weber E, Folkers G. Peptides and polypeptides as modulators of the immune response: thymopentin - an example with unknown mode of action. Pharmaceutica Acta Helvetiae. 1999;73:265-273.
7. Guarner F, Schaafsma GJ. Probiotics. Int J Food Microbiol. 1998;39:237-238.
8. Siwicki AK, Klein P, Morand M, Kiczka W, Studnicka M. Immunostimulatory effects of dimerized lysozyme (KLP-602) on the nonspecific defense mechanisms and protection against furunculosis in salmonids. Vet Immunol Immunopathol. 1998;61:369-378.
9. Spacciapoli P, Buxton D, Rothstein D, Friden P. Antimicrobial activity of silver nitrate against periodontal pathogens. J Periodontal Res. 2000;36:108-113.
10. Bonneau M, Laarveld B. Biotechnology in animal nutrition, physiology and health. Livest Prod Sci. 1999;59:223-241.
11. Brassart D, Schiffrin EJ. The use of probiotics to reinforce mucosal defense mechanisms. Trends Food Sci Technol. 1997;8:321-326.
12. Brown KM, Pickard K, Nicol F, Beckett GJ, Duthie GG, Arthur JR. Effects of organic and inorganic selenium supplementation on selenoenzyme activity in blood lymphocytes, granulocytes, platelets and erythrocytes. Clin Sci. 2000;98:593-599.
13. Erf GF, Bottje WG, Bersi TK, Headrick MD, Fritts CA. Effects of dietary vitamin E on the immune system in broilers: altered proportions of CD4 T cells in the thymus and spleen. Poult Sci. 1998;77:529-537.
14. Choi SI, Choi HS, Chun KS, Yoo BW, Park YH, inventors. Barodon S-F Corp, assignee. Composition of multipurpose high functional alkaline solution composition, preparation thereof, and for the use of nonspecific immunostimulators. US patent 09 755 020. January 8, 2001.
15. Choi SI, Choi HS, Chun KS, Yoo BW, Park YH, inventors. Barodon S-F Corp, assignee. Composition of multipurpose high functional alkaline solution composition, preparation thereof, and for the use of nonspecific immunostimulators. Korean patent 0 331 952. March 26, 2002.
16. Davis WC, Maruisic S, Lewin HA, Splitter GA, Perryman LE, McGuire TC, Gorham JR. The development and analysis of species specific and cross reactive monoclonal antibodies to leukocyte differentiation antigens and antigens of the MHC for use in the study of the immune system in cattle and other species. Vet Immunol Immunopathol. 1987;15:337-376.
17. Biddison WE, Shaw S. CD4 expression and function in HLA class II-specific T cells. Immunol Rev. 1989;109:5-15.
18. Bolin SR, McClurkin AW, Coria MF. Effects of bovine viral diarrhea virus on the percentages and absolute numbers of circulating B and T lymphocytes in cattle. Am J Vet Res. 1985;46:884-886.
19. Creemers PC. Determination of co-expression of activation antigens on proliferating CD4+, CD4+CD8+ and CD8+ lymphocyte subsets by dual parameter flow cytometry. J Immunol Methods. 1987;97:165-171.
20. Erf GF, Bottje WG, Bersi TK. CD4, CD8 and TCR defined T-cell subsets in thymus and spleen of 2- and 7-week old commercial broiler chickens. Vet Immunol Immunopathol. 1998;62:339-348.
21. Hernandez J, Garfias Y, Nieto A, Mercado C, Montano LF, Zenteno E. Comparative evaluation of the CD4+CD8+ and CD4+CD8- lymphocytes in the immune response to porcine rubulavirus. Vet Immunol Immunopathol. 2001;79:249-259.
22. Higgins DA. Markers for T and B lymphocytes and their application to animals. Vet Bull. 1981;51:925-963.
23. Lunney JK, Pescovitz MD. Phenotypic and functional characterization of pig lymphocyte populations. Vet Immunol Immunopathol. 1987;17:135-144.
24. Muraille E, Leo O. Revisiting the Th1/Th2 paradigm. Scand J Immunol. 1998;47:1-9.
25. Siwicki AK, Anderson DP, Dixon OW. Comparisons of nonspecific and specific immunomodulation by oxolinic acid, oxytetracycline and levamisole in salmonids. Vet Immunol Immunopathol. 1989;23:195-200.
26. Woldehiwet Z. Lymphocyte subpopulations in peripheral blood of sheep experimentally infected with tick-borne fever. Res Vet Sci. 1991;51:40-43.
27. Zuckermann FA. Extrathymic CD4/CD8 double positive T cells. Vet Immunol Immunopathol. 1999;72:55-66.
28. Ober BT, Summerfield A, Mattlinger C, Wiesmuller KH, Jung G, Pfaff E, Saalmuller A, Rziha HJ. Vaccine-induced, pseudorabies virus-specific, extrathymic CD4+CD8+ memory T-helper cells in swine. J Virol. 1998;72:4866-4873.
29. Pescovitz MD, Sakopoulos AG, Gaddy JA, Husmann RJ, Zuckermann FA. Porcine peripheral blood CD4+/CD8+ dual expressing T cells. Vet Immunol Immunopathol. 1994;43:53-62.
30. Summerfield A, Rziha HJ, Saalmuller A. Functional characterization of porcine CD4+CD8+ extrathymic T lymphocytes. Cell Immunol. 1996;168:291-296.
31. Zuckermann FA, Husmann RJ. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology. 1996;87:500-512.
32. Westermann J, Pabst R. Lymphocyte subsets in the blood: a diagnostic window on the lymphoid system? Immunol Today. 1990;11:406-410.
33. Zuckermann FA, Pescovitz MD, Aasted B, Dominguez J, Trebichavsky I, Novikov B, Valpotic I, Nielsen J, Arn S, Sachs DH, Lunney JK, Boyd P, Walker J, Lee R, Davis WC, Barbosa IR, Saalmuller A. Report on the analyses of mAb reactive with porcine CD8 for the second international swine CD workshop. Vet Immunol Immunopathol. 1998;60:291-303.
34. Park BK, Park YH, Seo KS. Lymphocyte subpopulations of peripheral blood in pigs treated with an ionized alkali mineral complex. Seoul Univ J Vet Sci. 1999;24:67-74.
35. Antonini JM, Yang HM, Ma JY, Roberts JR, Barger MW, Butterworth L, Charron TG, Castranova V. Subchronic silica exposure enhances respiratory defense mechanisms and the pulmonary clearance of Listeria monocytogenes in rats. Inhal Toxicol. 2000;12:1017-1036.
