Original research | Peer reviewed |
Monitoring the prevalence of Lawsonia intracellularis IgG antibodies using serial sampling in growing and breeding swine herds
Marsteller TA, Armbruster G, Bane DP, et al
TAM, GA, DPB, RM, JW: Elanco Animal Health, Indianapolis, IN 45240; CJG: University of Minnesota, 205 Veterinary Science, 1971 Commonwealth Avenue, St Paul, MN 55108BT: 903 Park Circle, Huxley, IA 50124.
Cite as: Marsteller TA, Armbruster G, Bane DP, et al. Monitoring the prevalence of Lawsonia intracellularis IgG antibodies using serial sampling in growing and breeding swine herds. J Swine Health Prod 2003;11(3):127-130. Also available as a PDF.
Summary
Objective: To determine seroprevalence of Lawsonia intracellularis and the time of seroconversion in growing and breeding swine herds using the immunoperoxidase monolayer assay (IPMA).
Methods: Blood was collected from individually identified growing pigs in 16 herds at weaning (approximately 3 weeks of age) and in those herds, plus four others, at 3-week intervals until market weight (total 20 herds). Blood was collected from individually identified breeding animals (replacement gilts) at entry into nine breeding herds and then at 3-week intervals. Samples were tested at a dilution of 1:30 using the IPMA, which identifies IgG antibodies.
Results: Fifteen of twenty growing herds (75%) were seropositive for L intracellu-laris at some time between weaning and 24 weeks of age. Within-herd seroprevalence in positive growing herds ranged from 11 to 92% and peaked near marketing (18 to 24 weeks of age). Seven of nine breeding herds (78%) were seropositive. Within-herd seroprevalence in breeding herds ranged from 5 to 61% and peaked near the time of herd entry and again late in the study.
Implications: As L intracellularis IgG serum antibodies were short-lived in this study, serial blood sampling at intervals of 3 weeks or less may prove useful in determining L intracellularis serological status in a swine production system. On the basis of these results, blood samples to detect exposure to L intracellularis should be collected from growing swine at 18 to 24 weeks of age, and from replacement gilts at entry into the breeding herd and at first lactation.
Keywords: Lawsonia intracellularis, immunoperoxidase monolayer assay, porcine proliferative enteritis, serology
Search the AASV web site for pages with similar keywords.
The obligate intracellular gram-negative bacterium, Lawsonia intracellularis, causes porcine proliferative enteritis (PPE),1-5 an economically important enteric disease that is common in pig populations throughout the world.6-14 After the causative agent was discovered, diagnosticians were able to develop laboratory methods to aid in the diagnosis of PPE.15-22 Currently, histopathology, fecal and tissue polymerase chain reaction (PCR), and serology are the most common diagnostic methods for PPE. Fecal PCR has limitations for determining exposure to L intracellularis and may be better employed to determine active shedding of the organism.23,24 Previous studies reported only 60% of animals infected orally with L intracellularis had positive fecal PCR results 3 weeks after challenge, and in some cases, this dropped to 30% by 6 weeks after challenge.25 On the other hand, in L intracellu-laris challenge studies using either the immunoperoxidase monolayer assay (IPMA) or immunofluorescent antibody (IFA) test, over 90% of infected pigs had IgG serum antibodies.24-26 Serology is very effective in determining exposure to L intracellularis in PPE field studies.27,28 The IPMA test, using a serum dilution of 1:30, has been validated to be sensitive (88.9%) and specific (100%) in its ability to detect IgG L intracellularis antibodies in challenged pigs.29-31 On the basis of these findings, the IPMA serum test was chosen to determine the prevalence of IgG L intracellularis antibodies in growing and breeding swine herds. Serial blood samples were collected from the same growing pigs and replacement gilts (cohort groups) at 3-week intervals to gain a better understanding of the time of herd exposure. Data concerning clinical evidence of PPE disease was not collected. This study was not designed to determine the interrelationship of breeding and growing herd serological status; however, a recent risk assessment study found that the L intracellularis serological status of the growing herd was strongly associated with the serological status of the breeding herd.32
Materials and methods
Collection of samples
This study was conducted in commercial swine herds located in Iowa, Nebraska, Minnesota, and Illinois. Fourteen cooperating veterinarians selected a convenience sample of 20 growing herds and nine breeding herds, and collected the blood samples. Samples were collected from approximately 20 growing pigs in a cohort age group (range 200 to 1200 pigs) in 16 herds at weaning (approximately 3 weeks of age) and then in all 20 selected herds at 3-week intervals until market (ie, seven or eight samples per pig). Eight samples per gilt were collected from 20 replacement gilts (cohort group size range 50 to 1200 gilts) upon entry into the breeding herds and subsequently every 3 weeks. Age at entry into the breeding herd was not determined. Individual animals were identified using ear tags to allow serial sampling.
Serological testing
Serum samples were submitted to the University of Minnesota and tested for L intracellularis IgG antibodies using the IPMA.30,31 The IPMA test does not require fluorescence and is a modification of the indirect serum IFA test.30,31 Acetone-fixed, 96-well sterile culture plates containing McCoy cells (mouse fibroblasts, ATCC #CRL-1696; American Type Culture Collection, Rockville, Maryland) infected with L intracellularis were rehydrated, and 50 µL of a 1:30 dilution of test serum was added to each test well. The plate was then incubated for 30 minutes at 37°C, washed, and incubated with anti-porcine IgG-peroxidase conjugate (Sigma-Aldrich Biochemical Co, St Louis, Missouri) for 45 minutes at 37°C. After another wash, chromogen (AEC [3-amino-9-ethyl-carbazole]; Sigma-Aldrich Biochemical) in hydrogen peroxide was added to each well. The plate was then incubated at room temperature for 20 minutes, washed, dried, and examined using an inverted light microscope. The serum sample was considered positive if red-labeled bacteria were observed in the cytoplasm and extracellularly in the cell culture. Specific antibody-positive and antibody-negative pig sera were included on each plate as controls.30 Positive and negative sera were validated during a previous challenge trial in which the absence and presence of PPE was confirmed at necropsy by immunohistochemistry staining of intestinal tissues from non-challenged and challenged pigs, respectively.30
Feed samples were collected at the time of serum collection and analyzed for in-feed antibiotics (Woodson-Tenet Laboratories, Des Moines, Iowa). Animal or herd therapy via injection or water-soluble antibiotics was not recorded.
No herds in this study were vaccinated against L intracellularis.
Results
Growing herd prevalence
Lawsonia intracellularis antibodies were detected by the IPMA in 15 of 20 growing herds (75%) during the 24-week study. Antibodies were detected in only one of the 16 growing herds tested at weaning (6%). All 20 growing herds were negative for L intracel-lularis serum antibodies when the pigs were 6 weeks of age. Individual pigs in eight of the 15 seropositive growing herds seroconverted over a 6-week period. The greatest increase in herd prevalence occurred when pigs were 15 to 18 weeks of age (Figure 1). Maximum growing herd prevalence at one data point was 60%, when the pigs were 24 weeks of age (Figure 1). In nine of the 15 seropositive herds, the greatest herd prevalence of L intracellularis serum antibodies occurred when the pigs were approximately 24 weeks of age, just prior to marketing.
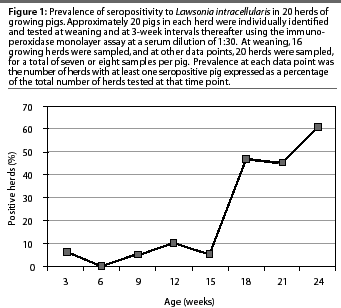
Due to lost tags or lost animals, there were fewer than 20 animals in some groups at some data points in some herds. Maximum within-herd prevalence of L intracellularis antibodies ranged from 11 to 92%, with 13 of the 15 seropositive herds having a maximum herd prevalence of less than 50%, and five herds having a maximum herd prevalence of less than 30%. At the time of maximum herd prevalence, pigs ranged in age from 18 to 24 weeks. Pigs became seropositive at 9 to 24 weeks of age, excluding two seropositive pigs in one herd at weaning: it was assumed that these positive tests represented colostral antibodies. In eight of the 15 seropositive herds, pigs became seropositive at 18 weeks of age.
Breeding herd prevalence
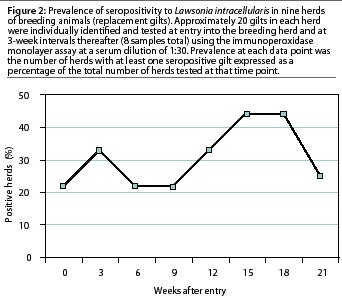
In seven of the nine breeding herds tested (78%), replacement gilts were seropositive for L intracellularis by the IPMA. Within-herd seroprevalence ranged from 5 to 61% , with five of the seven seropositive herds having a herd prevalence of less than 30%. There was no definitive time pattern in which these nine breeding herds became serologically positive to L intracellularis. Two of the nine herds (22%) were seropositive when the gilts entered the breeding herd (Figure 2). During the subsequent serial blood collections, a new breeding herd became seropositive at each time interval except between weeks 3 and 6. Individual gilts in three of the seven seropositive breeding herds seroconverted over a 6-week period. Two peaks in seroprevalence to L intracellularis occurred, at 3 weeks after entry and again 15 weeks after entry into the breeding herd (Figure 2). Maximum breeding herd prevalence at one data point was 44%, which occurred 15 to 18 weeks after the gilts had entered the breeding herds (Figure 2).
Time of seroconversion
The range in time from the first pig's seroconversion until the maximum percentage of animals became seropositive, within herds, ranged from 0 to 15 weeks for growing pigs and 0 to 21 weeks for gilts.
Duration of L intracellularis seropositive status
The serological status of 32 growing pigs changed from negative to positive to negative status during the study. The mean length of time in which these growing pigs were seropositive, when sampled at 3-week intervals, was 26.3 days.
The serological status of 38 replacement gilts changed from negative to positive to negative status during the study. The mean length of time in which these breeding pigs were seropositive, when sampled at 3-week intervals, was 26.5 days.
The trend was for only one sample from a seropositive animal to be positive: 72.9% of seropositive growing pigs and 62.0% of seropositive replacement gilts were positive at just one data point. Twelve of the 308 growing pigs in the 15 seropositive herds (3.9%) were seropositive at three of the eight data points, and no pigs were sero-positive at more than three data points (6- week interval). Two of the 140 gilts tested (1.4%) in the seven seropositive herds were seropositive at three data points, three (2.2%) were seropositiveat four data points, and none were seropositive at more than four points (9-week interval).
In-feed antibiotics
In-feed antibiotics were reported in 19 of the 20 growing herds. Only one growing herd did not use in-feed antibiotics throughout the study. This herd was IPMA-positive for L intracellularis. In-feed antibiotics were reported in six of the nine breeding herds. The three breeding herds that did not use in-feed antibiotics throughout the study were all IPMA-positive for L intracellularis.
Thirteen different in-feed antibiotic programs were used in the 20 growing herds, and a different in-feed antibiotic program was used in each of the six breeding herds that used in-feed antibiotics. This large variation in the number of in-feed antibiotic programs was associated with the large number of herds and veterinarians involved in the study. Due to the great amount of variation found in the types and levels of antibiotics used, no attempt was made to correlate L intracellularis serological status and use of in-feed antibiotics.
Discussion
This study design was unique in that it determined the prevalence of L intracellularis IgG antibodies, as detected by IPMA, in growing and breeding herds and determined the time of seroconversion by testing serum samples collected at 3-week intervals.
While 75% of the 20 growing herds and 78% of the nine breeding herds (replacement gilts) were seropositive at some time during the study, a maximum of only 60% of the growing pigs and 44% of the breeding females in these herds were seropositive at a single data point, due to the differences in the time of seroconversion between herds, the short duration of IPMA IgG serum antibody titers, and the intermittent seropositivity noted in some individual animals.
Lawsonia intracellularis antibodies were detected by IPMA in growing pigs at weaning in only one herd and in only two animals in this herd. It is reasonable to assume that these antibodies were colostral in origin. As only this one herd was seropositive at weaning, and none of the 20 growing herds were seropositive at 6 weeks of age, one might conclude that exposure to L intracellularis occurred late in finishing in these herds, and not in the farrowing or nursery stage of swine production.
Most growing pigs and replacement gilts were seropositive at just one data point. These data support the need for frequent blood sampling intervals in serological studies for L intracellularis, because animals appeared to be seropositive for only a short time period. This is in agreement with previous studies28 in which it was necessary to collect blood samples at 21-day intervals to detect L intracellularis antibodies by IFA, due to the apparently short-lived L intracel-lularis antibodies. In this study, many growing pigs seroconverted at the last data point, thus limiting the number of animals in which to determine the period of seropositivity in the growing pig population.
Thirty-two growing pigs and 38 breeding pigs were intermittently seropositive to L intracellularis. The length of time these animalswere seropositive was approximately 26 days. This suggests that re-exposure to L intracellularis may have occurred in these herds, or that the animals did not develop persistent titers, or both. The exact length of time pigs were seropositive could not be determined in this study because samples were collected only at 3-week intervals.
In this study, blood samples were collected from approximately 20 growing pigs and replacement gilts at each data point. In the growing herd (cohort age group, 200 to 1200 pigs), sampling 22 pigs would provide a 90% confidence of detecting a 10% prevalence rate in 200 pigs.33 All growing herds had a within-herd prevalence of seropositivity greater than 10%; however, two breeding herds had less than a 10% within-herd prevalence rate. Collecting more blood samples at each time point would have improvedthe power of the study in low prevalence herds. In addition, a herd was considered positive in this study when one animal tested IPMA-positive for L intracellularis. The results of this study could have been validated using PCR to detect the presence of the L intracellularis organism in fecal samples. In future studies, more than one diagnostic test for L intracellularis should be employed.
Serological tests currently available (ie, IFA and IPMA) measure IgG serum antibodies to L intracellularis, reflecting exposure to the L intracellularis organism. Because L intracellu-laris bacteria reside within the enterocyte, cell-mediated immunity, determined by a serum test for antigen-specific gamma interferon production, might be a more appropriate method to determine exposure and protection after L intracellularis challenge.34
In eight growing herds and three breeding herds, there was an interval of 6 weeks from the time when the first animal seroconverted until the maximum percentage of animals were seropositive. This suggests that exposure to L intracellularis may occur over a long period of time within a herd, due in part to the ability of the organism to survive in extracellular conditions for up to 2 weeks.35 Proper disinfectant use is important in preventing the spread of L intracellularis between groups of pigs managed in an all-in all-out (AIAO) manner. Prevention and control of PPE with intermittent therapeutic doses of in-feed antibiotics may be important in herds when pigs are exposed to L intracellularis over a relatively long time period.36,37
Implications
- In a convenience sample of swine herds in four Midwest states, 75% of growing herds and 78% of replacement gilt herds were seropositive by the IPMA for L intracellularis.
- Antibodies detected by IPMA are short-lived (<3 weeks) both in growing pigs and in replacement gilts.
- Serial blood sampling at intervals of 3 weeks or less may be necessary to determine L intracellularis serological status in a production system.
Acknowledgment
The authors thank the investigating veterinarians and the herd owners cooperating in this study.
References - refereed
1. Lawson GHK, McOrist S. The enigma of proliferative enteropathies: A Review. J Comp Pathol. 1993;108:41-46.
2. McOrist S, Jasni S, Mackie RA, McIntyre N, Neef N, Lawson GHK. Reproduction of porcine proliferative enteropathy with pure cultures of ileal symbiont intracellularis. Infect Immun. 1993;61:4286-4292.
3. Gebhart CJ, McOrist S, Lawson GHK, Collins JE, Ward GE. Specific in situ hybridization of the intra-cellular organism of porcine proliferative enteropathy. Vet Pathol. 1994;31:462-467.
4. McOrist S, Gebhart CJ, Boid R, Barns SM. Characterization of Lawsonia intracellularis gen. nov., sp. nov., the obligately intracellular bacterium of porcine proliferative enteropathy. Int J Syst Bacteriol. 1995;45:820-825.
5. Joens LA, Nibbelink S, Glock RD. Induction of gross and microscopic lesions of porcine proliferative enteritis by Lawsonia intracellularis. Am J Vet Res. 1997;58:1125-1131.
6. Gogolewski RP, Cook RW, Batterham ES. Suboptimal growth associated with porcine intestinal adenomatosis in pigs in nutritional studies. Aust Vet J. 1991;68:406-408.
7. Connor JF. Diagnosis, treatment, and prevention of porcine proliferative enteritis. Food Anim Comp. 1991;13:1172-1176.
8. Winkelman NL. Ileitis: An update. Comp Contin Educ Pract Vet. 1996;18(1):S19-S25.
9. Moore GM, Shryock TR. Lawsonia intracellularis and swine enteric disease. Comp Contin Educ Pract Vet. 1996;18(1):S11-S18.
10. Smith SH, McOrist S, Green LE. Questionnaire survey of proliferative enteropathy on British pig farms. Vet Rec. 1998;142:690-693.
11. McOrist S, Gebhart CJ. Porcine proliferative enteropathies. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:521-534.
12. Wilson JB, Pauling GE, McEwen BJ, Smart N, Carman PS, Dick CP. A descriptive study of the frequency and characteristics of proliferative enteropathy of swine in Ontario by analyzing routine animal health surveillance data. Can Vet J. 1999;40:713-717.
13. Stege H, Jensen TK, Møller K, Baekbo P, Jorsal SE. Prevalence of intestinal pathogens in Danish finishing pig herds. Prev Vet Med. 2000;46:279-292.
14. Lawson GHK, Gebhart CJ. Proliferative enteropathy. J Comp Pathol. 2000;122:77-100.
15. Lawson GHK, McOrist S, Rowland AC, McCartney E, Roberts L. Serological diagnosis of the porcine proliferative enteropathies: Implications for aetiology and epidemiology. Vet Rec. 1988;122:554-557.
16. Jones GF, Davies PR, Rose R, Ward GE, Murtaugh MP. Comparison of techniques for diagnosis of proliferative enteritis of swine. Am J Vet Res. 1993;54:1980-1985.
17. McOrist S, Gebhart CJ, Lawson GHK. Polymerase chain reaction for diagnosis of porcine proliferative enteropathy. Vet Microbiol. 1994;41:205-212.
18. Jensen TK, Møller K, Leser TD, Jorsal SE. Comparison of histology, immunohistochemistry and polymerase chain reaction for detection of Lawsonia intracellularis in natural porcine proliferative enteropathy. Eur J Vet Pathol. 1997;3:115-123.
19. Cooper DM, Gebhart CJ. Comparative aspects of proliferative enteritis. JAVMA. 1998;212:1446-1451.
20. Knittel JP, Roof M, Schwartz KJ, Jordan DM, Harris DL, McOrist S. Diagnosis of porcine proliferative enteritis. Comp Contin Educ Pract Vet. 1997;19(1):S26-S29.
21. Cooper DM, Swanson DL, Gebhart CJ. Diagnosis of proliferative enteritis in frozen and formalin-fixed, paraffin-embedded tissues from a hamster, horse, deer and ostrich using a Lawsonia-intracel-lularis-specific multiplex PCR assay. Vet Microbiol. 1997;54:47-62.
22. Wilson K. Diagnostic approach to enteric diseases of swine. Swine Health Prod. 2000;8:235-236.
24. Bane DP, Neumann E, Gebhart CJ, Gardner IA, Norby B. Porcine proliferative enteropathy: a case-control study in swine herds in the United States. J Swine Health Prod. 2001;9:155-158.
25. Knittel JP, Jordan DM, Schwartz KJ, Janke BH, Roof MB, McOrist S, Harris DL. Evaluation of antemortem polymerase chain reaction and serologic methods for detection of Lawsonia intracellularis-exposed pigs. Am J Vet Res. 1998;59:722-726.
26. Marsteller T, Winkelman N, Gebhart C, Armbruster G, Weldon W, Muller R, Weatherford J, Symanowski J. Efficacy of intramuscular tylosin for the treatment and control of porcine proliferative enteropathy caused by Lawsonia intracellularis. Vet Therapeutics. 2001;2:51-60.
27. USDA. NAHMS Swine '95: Part I. Reference of 1995 Swine Management Practices. http://www.aphis.usda.gov/vs/ceah/cahm/Swine/swine.htm . Accessed February 1, 2003.
28. Just SD, Thoen CO, Thacker BJ, Thompson JU. Monitoring of Lawsonia intracellularis by indirect serum immunofluorescence assay in a commercial swine production system. J Swine Health Prod. 2001;9:57-61.
29. Guedes RMC, Gebhart CJ, Winkelman NL, Mackie-Nuss RAC, Marsteller T, Deen J. Comparison of different methods for diagnosis of porcine proliferative enteropathy. Can J Vet Res. 2002;66:99-107.
30. Guedes RMC, Gebhart CJ, Winkelman NL, Mackie-Nuss RA. A comparative study of an indirect immunofluorescent test and an immunoperoxidase monolayer assay for the diagnosis of porcine proliferative enteropathy. J Vet Diagn Invest. 2002;14:420-423.
31. Guedes RMC, Gebhart CJ, Deen J, Winkelman NL. Validation of an immunoperoxidase monolayer assay as a serological test for porcine proliferative enteropathy. J Vet Diagn Invest. 2002;14:528-530.
32. Bronsvoort M, Norby B, Bane DP, Gardner IA. Management factors associated with seropositivity to Lawsonia intracellularis in US swine herds. J Swine Health Prod. 2001;9:285-290.
34. Guedes RMC, Gebhart C. Onset and duration of fecal shedding, cell-mediated and humoral immune responses in pigs after challenge with a pathogenic isolate or attenuated vaccine strain of Lawsonia intracellularis. Vet Micro. 2003;9:135-145.
35. Collins A, Love RJ, Pozo J, Smith SH, McOrist S. Studies on the ex vivo survival of Lawsonia intracellularis. Swine Health Prod. 2000;8:211-215.
36. Schwartz K, Knittel J, Walter D, Roof M, Anderson M. Effect of oral tiamulin on the development of porcine proliferative enteropathy in a pure-culture challenge model. Swine Health Prod. 1999;7:5-11.
37. Veenhuizen MF, Mowrey DH, Moore GM, Watkins LE. Evaluating a natural outbreak of porcine proliferative enteropathy and treatment with tylosin in the grow-finish phase. Swine Health Prod. 1998;6:67-72.
References - non refereed
23. Winkelman NL, Pauling GE, Bagg RN, Dick CP, Paradis MA, Brennan J, Wilson J. Use of a challenge model to measure the impact of subclinical porcine proliferative enteritis on growth performance in pigs. Proc AASP. Des Moines, Iowa. 1998:209-211.
33. Pointon AM, Morrison R, Hill G, Dargatz DA, Dial G. Monitoring pathology in slaughtered stock: Guidelines for selecting sample size and interpreting results. Proc AASP. 1990.
