What's your interpretation? |
Non refereed |
When is the right time to vaccinate against Erysipelas rhusiopathiae in growing pigs?
E. Johnson, DVM; J. Lowe, DVM, MS
The Maschhoffs, Inc, Carlyle, IL 62231
Cite as: Johnson E, Lowe J. When is the right time to vaccinate against Erysipelas rhusiopathiae in growing pigs? J Swine Health Prod. 2005;13(2):114-115.
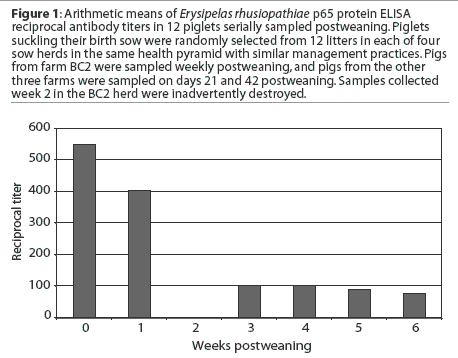
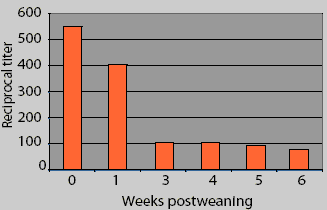 This figure represents the mean Erysipelas
rhusiopathiae p65 protein ELISA antibody titers in piglets from four sow
herds, serially sampled postweaning. What does this data suggest as the optimal
vaccine timing? What additional information about these data is necessary to
make the correct decision about vaccine timing? What might influence the level
of maternal antibody in
piglets postweaning?
This figure represents the mean Erysipelas
rhusiopathiae p65 protein ELISA antibody titers in piglets from four sow
herds, serially sampled postweaning. What does this data suggest as the optimal
vaccine timing? What additional information about these data is necessary to
make the correct decision about vaccine timing? What might influence the level
of maternal antibody in
piglets postweaning?
Management of immunization strategies relative to maternal antibody decay is important in their success, particularly when the dam is also vaccinated against the same agent. Often clinicians assay serum antibody in pigs postweaning to estimate maternal antibody decay. Testing strategy needs to be carefully planned to allow the broad interpretation of results. The data presented here were gathered to determine the rate of Erysipelas rhusiopathiae maternal antibody decay in pigs postweaning and to compare the differences in maternal antibody among pigs from different farms.
Thirteen weeks prior to the beginning of the study, all breeding females in four herds (farms BC2, BC3, BC4, and ER) in the same health pyramid had been vaccinated against E rhusiopathiae over a 1-week period, using a commercial bacterin (ER Bac/Leptofirm-5; Pfizer Animal Heath, Kalamazoo, Michigan) at label dosage. In each of the four sow herds, 12 pigs from different litters were randomly selected for E rhusiopathiae maternal antibody testing. All litters had been born on the same day and all pigs had remained with the birth dam until weaning at 14 to 17 days of age. Pigs were tagged prior to weaning and recorded by tag number, sow ID, and herd of origin. Mean parities of the selected pigs' dams were 3.2 for farm BC2, 3.1 for farm BC3, 2.3 for farm BC4, and 3.6 for farm ER. No pigs were selected from primiparous sows.
The tagged pigs were placed at a power-ventilated, total-concrete-slatted wean-to-finish facility at weaning. Blood samples were collected from BC2 pigs at placement and every 7 days thereafter through day 42 post placement. Samples were collected from BC3, BC4, and ER pigs 21 and 42 days post placement. Samples were collected into serum separator tubes using standard venipuncture technique. Serum samples were separated, frozen, and submitted in one accession to Benchmark Biolabs, Inc (Omaha, Nebraska) for ELISA testing for antibodies directed against the p65 protein of E rhusiopathiae. Samples collected from BC2 pigs 14 days postweaning were inadvertently destroyed prior to sample submission.
Titers were determined by an end-point, serial-dilution method and converted with a log2 procedure to normalize the data. Converted titers were used to calculate means, which were compared by analysis of variance (ANOVA) using PROC GLM of SAS (SAS Institute, Cary, North Carolina). Rate of maternal antibody decay was measured using a multivariate regression model (PROC REG of SAS) that measured the interaction of time and titer while controlling for source herd.
Mean antibody titers decayed from 0 to 6 weeks postweaning in pigs from BC2 (Figure 1). Mean antibody titers 3 weeks postweaning were compared among herds. Titers (mean +/- SEM) were greater in BC2 pigs (134.8 +/- 1.20) than in pigs from the other herds (56.2 +/- 1.15; F = 13.3, P < .001), but there was no difference in mean titers 6 weeks postweaning between the BC2 pigs (64.5 +/- 1.20) and the other pigs (69.2 +/- 1.09; F = 0.12, P = .72). When source herd was controlled, mean antibody titers were lower at 42 days postweaning than at 21 days postweaning (F = 4.18, P = .03). and when accounting for time, BC2 had higher titers generally than did the other farms (F = 12.41, P < .001). In addition, the rate of decay was different between BC2 and the other farms (F = 16.99, P < .001). In BC2 pigs, maternal antibody decay occurred between 3 weeks (mean titer 134.8 +/- 1.20) and 6 weeks postweaning (mean titer 64.5 +/- 1.20; F = 12.91, P < .001), while there was no significant change in mean titers between 3 weeks (mean titer 56.2 +/- 1.15) and 6 weeks postweaning (69.2 +/- 1.10) for the pigs from the other three farms (F = 1.61, P = .21).
Discussion
There appear to be large differences in the results between Figure 1 and Figure 2. These differences result from an improper calculation of central tendency in Figure 1. Titers are not interval data and are not normally distributed; conversion with the log2 allows the calculation of arithmetic means that accurately reflect the central tendency of the group. Calculating arithmetic means on non-normally distributed data results in mean values that are greater than the true values. Alternatively, titer (serial dilution) data could be summarized using a geometric mean to achieve a number that might be easier to understand. In addition, the differences within and between farms are not appreciated in Figure 1. An inaccurate interpretation might be made if Figure 1 alone were utilized for the analysis.
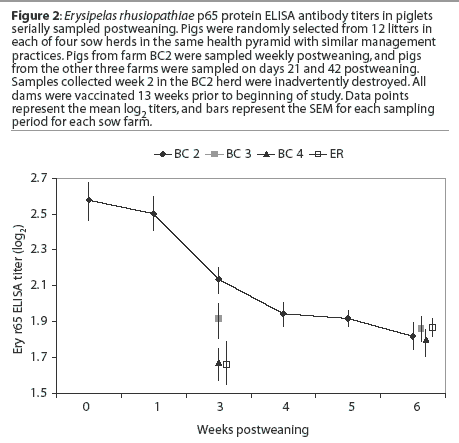
There is a steady decline in maternal antibody that is below presumed protective levels (Pfizer Animal health, unpublished data) by 6 weeks postweaning. The differences in maternal antibody levels are greater between herds than within a given herd. There are several possible explanations for these differences, including weaning age, dam parity (ie, number of vaccinations and colostrum quality), and quantity of colostrum ingestion by the pig.
In this study, pigs in a given herd were all weaned at the same age, between 14 and 17 days of age. Dam vaccination schedules were identical in all herds. In addition, dam parity was similar on all farms. No pigs from primiparous sows were sampled. These data show that in a production system with similar herd characteristics, colostrum intake may differ in different herds. This suggests that management factors at the herd level, not the system level, influenced the quantity of colostrum consumed by piglets. Monitoring maternal antibody decay on similar farms could be a useful tool for monitoring the effectiveness of management practices in increasing colostrum uptake.
The differences in mean E rhusiopathiae titers among herds also suggests that in pigs from multiple-source farms in the same system and weaned at the same age, it is necessary to monitor titers in order to understand the optimum time for vaccinating piglets when the dams are also vaccinated using the same antigen(s). These data suggest that vaccination against E rhusiopathiae should not be attempted until 5 weeks postweaning to minimize the risk that maternal antibody interferes with immunization. Strategies to improve the uniformity of colostrum consumption across herds would be beneficial to reduce the number of susceptible pigs prior to the time when the population can be vaccinated against many diseases.
