Production tool |
Peer reviewed |
Carbon dioxide for emergency on-farm euthanasia of swine
Bióxido de carbono para la eutanasia de emergencia de cerdos en una granja
Bioxyde de carbone pour la euthanasie d'urgence dans les fermes porcines
Robert E. Meyer, DVM, Diplomate American College of Veterinary Anesthesiologists; W. E. Morgan Morrow, BVSc, MS, PhD
REM: Department of Clinical Sciences, College of Veterinary Medicine, Mississippi State University, Mississippi State, Mississippi. WEMM: Department of Animal Science, North Carolina State University, Raleigh, North Carolina. Corresponding author: Dr Robert E. Meyer, Department of Clinical Sciences, College of Veterinary Medicine, PO Box 6100, Mississippi State University, Mississippi State, MS 39762-6100; Fax: 662-325-4596; E-mail: meyer@cvm.msstate.edu
Cite as: Meyer RE, Morrow WEM. Carbon dioxide for emergency on-farm euthanasia of swine. J Swine Health Prod. 2005;13(4):210-217.
Also available as a PDF.
SummaryIn the event of a foreign animal disease outbreak in the United States, a rapid and humane method of on-farm swine euthanasia will be required. Given the extraordinary number of animals involved and the design of currently used swine confinement buildings, methods relying on the handling and restraint of individual animals will likely prove much too slow to stem the spread of disease. Humanely depopulating commercial swine production facilities may be accomplished by using enclosed dump bed trucks or trailers as on-farm carbon dioxide euthanasia chambers. We review the principles governing the use of carbon dioxide in enclosed spaces and show that adherence to the 2000 Report of the American Veterinary Medical Association (AVMA) Panel on Euthanasia recommendation for carbon dioxide flow rate is appropriate for humane euthanasia within any enclosed volume or space. In addition, we demonstrate the feasibility of applying the AVMA guidelines for on-farm carbon dioxide euthanasia to groups of adult pigs. | ResumenEn caso de que hubiera un brote de alguna enfermedad exótica animal en los Estados Unidos, se requerirá de un método de eutanasia de cerdos rápido y humanitario dentro de la granja. Debido al gran número de animales involucrados y al diseño de las construcciones de cerdos utilizadas actualmente, los métodos que dependen del manejo y restricción individual de animales probablemente serán demasiado lentos para detener la diseminación de la enfermedad. El despoblar humanitariamente las instalaciones comerciales de producción de cerdo puede lograrse utilizando cajas cerradas de trailers ó de camiones de carga como cámaras de eutanasia con bióxido de carbono dentro de la granja. Revisamos los principios que rigen el uso de bióxido de carbono en espacios cerrados y demostramos que el respeto al Reporte de la Comisión de la Asociación Médica Veterinaria Americana (AVMA por sus siglas en inglés) 2000, de las recomendaciones del índice de flujo del bióxido de carbono, es apropiada para la eutanasia humanitaria dentro de cualquier área o espacio cerrado. Además, demostramos la viabilidad de la aplicación de las indicaciones de la AVMA para la eutanasia con bióxido de carbono para grupos de cerdos adultos dentro de una granja. | ResuméEn cas de la présentation de une maladie exotique animale dans les États-Unis, une méthode d'euthanasie de porc, rapide et humaine dans la ferme sera exigée. Étant donné le nombre extraordinaire d'animaux impliqués et le dessin de bâtiments utilisés actuellement, les méthodes qui comptent la manipulation et contrôle individuel d'animaux seront probablement trop lents pour contenir l'étendue de la maladie. Dépeupler humainement des installations commerciales de production porcine peut être accompli en utilisant des remorques ou des camions clos comme chambres d'euthanasie de bioxyde de carbone dans les fermes. Nous examinons les principes qui gouvernent l'usage de bioxyde de carbone dans les espaces clos et montrons que l'adhésion au Rapport du Comité de l'Association Américain Médicale Vétérinaire (AVMA par ses sigles en anglais) 2000, sur la recommandation du taux de flux du bioxyde de carbone pour l'euthanasie, est approprié pour l'euthanasie humaine dans tout espace ou surface clos. Aussi nous avons également démontré la faisabilité de appliquer les indications de l'AVMA pour l'euthanasie pour groupes de cochons adultes avec le bioxyde de carbone dans la ferme. |
Keywords: swine, euthanasia,
carbon dioxide, time constant, biosecurity
Search the AASV web site
for pages with similar keywords.
Received: April
6, 2004
Accepted: August
24, 2004
The 2000 Report of the American Veterinary Medical Association (AVMA) Panel on Euthanasia1 states that "under unusual conditions, such as disease eradication and natural disasters, euthanasia options may be limited. In these situations, the most appropriate technique that minimizes human and animal health concerns must be used. These options include, but are not limited to, carbon dioxide and physical methods such as gunshot, penetrating captive bolt, and cervical dislocation."
In the case of foot-and-mouth disease (FMD), infected animals are to be humanely killed and disposed of within 24 hours of diagnosis to limit viral replication and subsequent disease spread, and all susceptible animals on adjacent farms within a specified radius are to be humanely killed and disposed of within 48 hours.2 These goals were not achieved in the response by the Department for the Environment, Food, and Rural Affairs (DEFRA) to the 2001-2002 United Kingdom FMD outbreak. If all infected animals had been killed within 24 hours, as recommended, the extent of the UK epidemic might have been reduced by 40%.2 Further, if animals on contiguous farms had been killed within 48 hours as recommended, then the UK epidemic might have been reduced by 66%.2 Clearly, timely euthanasia would likely have greatly limited the spread of the disease and the period during which the country was designated as non-FMD-free.
According to DEFRA, 4,220,000 animals were killed during the 2001-2002 UK FMD outbreak,3 with an average of 10,000 to 12,000 animals being killed each day of the outbreak. As sobering as these numbers are, the potential situation for the United States in the event of an outbreak of a foreign animal disease is much, much worse due to greater numbers of animals and extensive interstate animal movement. For example, at a slaughter rate of 12,000 animals per day, it would take nearly 2 years to depopulate the nine million pigs currently in production in North Carolina, without taking into account infected ruminants or wildlife.
Animals identified for slaughter during the 2001-2002 UK FMD outbreak were euthanized by government-licensed slaughter teams, each of which included at least one veterinarian, using a combination of accepted humane methods, including captive bolt accompanied by pithing rod, gunshot to the brain, and lethal injection. Captive bolt, gunshot, and lethal injection each require that individual animals be handled and restrained, and that operators are properly trained in the correct application of each technique. Given that large US swine operations commonly have 1000 or more animals in each building and very few animal workers, handling individual animals would greatly slow the euthanasia process and increase the potential for viral replication and spread. Worker safety, as well as emotional trauma, will be significant issues. Clearly, faster, less labor intensive, but equally humane euthanasia methods would be required if the goals of humane slaughter and timely disposal were to be met in the event of an FMD or other foreign animal disease outbreak in the United States.
Carbon dioxide and humane euthanasia
Depopulation of commercial swine operations would likely take place on-site to reduce disease spread. One suggestion made by the swine industry for humanely killing large numbers of swine on-farm is to utilize enclosed dump-bed trucks or trailers as carbon dioxide (CO2) euthanasia chambers. Potential advantages to this method over captive bolt, gunshot, or lethal injection of individual animals include the ability to rapidly move animals out of buildings using existing walkways and chutes, the ability to deposit the carcasses at the disposal site, and lower manpower requirements. Implementation of this method will require proactive establishment of protocols ensuring humane conditions while conserving resources and protecting personnel.
Carbon dioxide is one of several asphyxiating gases recommended by the 2000 Report of the AVMA Panel on Euthanasia1 for humane euthanasia of swine. Although the gases CO2, nitrogen, and argon all kill by displacing oxygen and causing fatal hypoxemia, the rapid central nervous system depressant, analgesic, and anesthetic effects of CO2 are well recognized.1 Other advantages of CO2 as a euthanasia agent include its ready availability and relatively low cost, and its nonflammable and nonexplosive properties. Toxic effects due to accidental exposure of personnel to CO2, in contrast to effects of carbon monoxide exposure, can be readily reversed by prompt removal from the area, and CO2 poses minimal hazard when used with properly designed equipment. Disadvantages of CO2 include its heavier-than-air density, such that incomplete filling of a chamber could permit animals to climb or raise their heads to avoid exposure.1
Carbon dioxide stunning (ie, swine to be humanely slaughtered are first rendered unconscious through exposure to high concentrations of CO2) has been extensively studied. Exposure to 80% to 90% CO2 in air produces unconsciousness in swine within 13 to 30 seconds without signs of pain and suffering, as determined by behavior, physical signs, and electroencephalographic activity; unconsciousness equivalent to stage 2 barbiturate anesthesia occurs prior to myoclonic activity.4-6 The mechanism by which CO2 stunning causes unconsciousness in swine is not hypoxia,5 and plasma cortisol levels are not further increased, compared with levels observed during awake transport, indicating that no additional emotional strain is imposed by CO2 inhalation.7 It is important to understand that once ataxia and loss of righting reflex occur, subsequently observed activities, such as convulsions, vocalization, reflex struggling, breath holding, and tachypnea, can be attributed to Guedel's second stage of anesthesia, which by definition, lasts from loss of consciousness to the onset of a regular breathing pattern.5,8
The 2000 AVMA euthanasia guidelines recommend displacement with CO2 of 20% of the chamber volume per minute as an optimal flow rate for euthanasia.1 The recommendation is based on the work of Hornett and Haynes,9 in a study that examined the effect of various CO2 gas flow rates on the behavior and death of rats. In that study, a CO2 flow rate of 19.5% of the chamber volume per minute was empirically determined to achieve a quiet delivery into unconsciousness, with death occurring between 6 and 9 minutes after inflow of CO2 begins. Similar findings in rats were reported by Smith and Harrap,10 using a CO2 flow rate equivalent to 22% of the chamber volume. The physical principles underlying the choice of this particular flow rate were not described in Hornett and Haynes,9 Smith and Harrap,10 or in the 2000 AVMA guidelines.1
The change in gas concentration within an enclosed space involves two physical processes: the "wash-in" of new gas (or "wash-out" of existing gas) and the time constant required for that change to occur within the container for a known flow rate. These processes are commonly combined in the practice of anesthesia to predict how quickly a change in concentration of an inhaled anesthetic will occur within a circle rebreathing circuit. A review of how gases wash-in to an enclosed space provides the explanation for the AVMA CO2 flow rate recommendation as well as insight into how the process can be adapted for on-farm use.
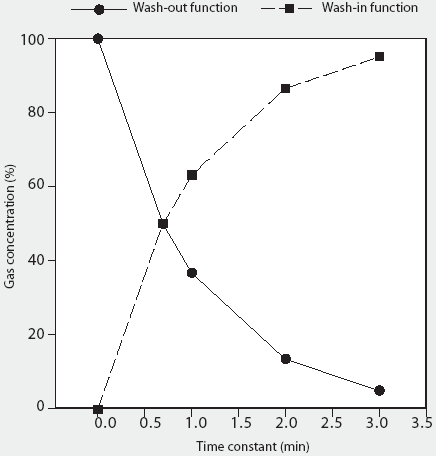
The rate of change of gas concentration within any enclosed space is a special form of nonlinear change known as an exponential process, and as such can be derived from the wash-in and wash-out exponential functions, where the wash-in function is y = y() (1-e-kt) (expressed as percent of y()) and the wash-out function is y = y0 e-kt (expressed as percent of y0). In this equation, e is the base of natural logarithms, k is a constant which defines the speed of the wash-in or wash-out function and is the reciprocal of the time constant for the process, and t is time.11 Briefly, for the wash-in exponential function, the quantity under consideration rises towards a limiting value at a rate which progressively decreases in proportion to the distance it still has to rise. In theory, the quantity approaches, but never reaches, 100% (Figure 1). Conversely, for the wash-out exponential function, the quantity under consideration falls at a rate which progressively decreases in proportion to the distance it still has to fall; again, in theory, the quantity approaches, but never reaches, zero (Figure 1).
Figure 1: Graphic representation of the wash-in and wash-out exponential functions, using a hypothetical example of a closed container, originally filled with Gas A, into which Gas B is introduced. It demonstrates the exponential decline in Gas A (wash-out) and the rise in Gas B (wash-in), where wash-out is described by y = y0 e-kt (expressed as percent of y0) and wash-in is described by y = y() (1-e-kt) (expressed as percent of y()), where e is the base of natural logarithms, k is a constant which defines the speed of the wash-in or wash-out function and is the reciprocal of the time constant for the process, and t is time.11 Both gases theoretically reach asymptotic final concentrations, illustrating the diminishing rate-of-change dynamic of exponential curves.
|
The exponential wash-in and wash-out equations are used to derive the time constant (t) for an enclosed volume or space. The time constant is mathematically equal to the enclosed volume or space undergoing wash-in or wash-out divided by the flow rate into the space, where t = volume flow rate,11,12 and represents the time at which the wash-in or wash-out process would have been complete had the initial rate of change continued as a linear function rather than an exponential function.11 The time constant is similar in concept to the half-life, although they are neither identical nor interchangeable.12
For the wash-in function, 1t is required for the concentration of any inflowing gas to rise to 63.2%, 2t are required for the concentration to rise to 86.5%, and 3t are required for the concentration to rise to 95%, with ()(t) required for the gas concentration to rise to 100% (Figure 1). Conversely, for the wash-out function, 1t is required for the remaining gas concentration to fall to 36.8% of the original value, 2t are required for gas concentration to fall to 13.5%, and 3t are required for gas concentration to fall to 4.98%, with ()(t) required for gas concentration to fall to 0%. (Figure 1). The flow rate therefore determines the time constant for any given enclosed volume, such that increasing the flow rate will result in a proportional reduction of the wash-in and wash-out time constants for any size chamber (and vice versa).
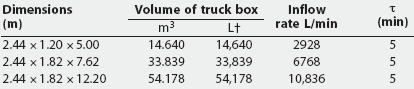
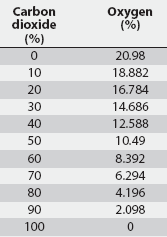
On this basis, it can be shown that the AVMA-recommended CO2 inflow rate of 20% of the chamber volume per minute represents a time-constant value of 5 minutes regardless of chamber volume (1 volume divided by 0.2 volume per minute; Table 1). Thus, a CO2 inflow rate equivalent to 20% of the chamber volume per minute is predicted to increase CO2 concentration within any enclosed space from 0% to 63.2% in 5 minutes (1t), to 86.5% in 10 minutes (2t), and to 95% in 15 minutes (3t) (Figure 1). An examination of the experimental data of Smith and Harrap10 confirms this, where CO2 supplied at an inflow rate of 22% of chamber volume increased CO2 concentration to approximately 64% in 4.5 minutes (1t for their chamber). Similarly, using the wash-out function, a CO2 inflow rate of 20% of chamber volume per minute will result in the fall of oxygen concentration from standard atmospheric concentration of 20.98% initially to 7.72% in 5 minutes (1t), further decreasing to 2.83% in 10 minutes (2t), and to 1.04% in 15 minutes (3t) (Table 2).
Table 1: Example container volumes and required flow rates to fill 20% of a truck box volume with carbon dioxide when the time constant (t)* is 5 minutes
* Time constant (t) = volume inflow rate. 1 m3 = 1000 L. |
Table 2: Concentration of oxygen in a closed chamber with increasing percent of carbon dioxide (CO2) when inflow rate of CO2 is 20% of chamber volume per minute
|
Thus, by knowing the container volume and the flow rate, one can easily determine the duration of gas inflow required to obtain a specific gas concentration as a function of the time constant for that container. An inflow rate greater than 20% per minute will reduce the time constant required to achieve the target gas concentration for a given chamber size. For example, increasing the inflow rate from 20% to 40% of the chamber volume per minute will decrease the time constant from 5 minutes to 2.5 minutes (1 volume divided by 0.4 volume per minute) and therefore will decrease the time necessary to achieve a 63% CO2 atmosphere from 5 minutes to 2.5 minutes.
Generating recommended CO2 inflow rates
Large containers suitable for euthanizing several dozen adult pigs in a single batch will require very high CO2 flow rates to meet the AVMA recommendation. For example, a box of dimensions 2.44 m x 5.00 m x 1.20 m, with a floor area of approximately 12 m2 and a volume of approximately 14.6 m3 (14,600 L), could hold up to 32 adult pigs at a trucking-load density of 0.37 m2 per 100 kg.13 Meeting the nominal 20% per minute flow rate requirement to produce a 63% CO2, 8% oxygen atmosphere inside this box will require approximately 2900 L of CO2 each minute during a 5-minute (1t) exposure (14,600 L x 20%; Table 1).
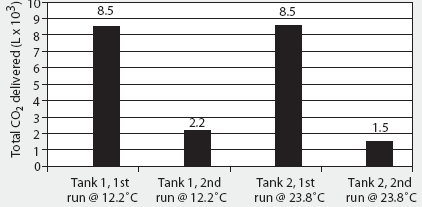
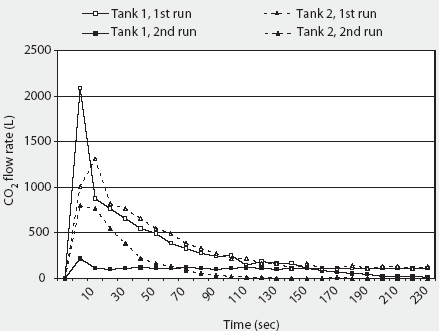
The 2000 AVMA euthanasia guidelines recommend compressed CO2 gas in cylinders as the only acceptable source of CO2, because the flow can be regulated precisely.1 The specific volume of CO2 is 0.55 m3 per kg at 20°C. Commonly available "G" size cylinders (20-cm diameter x 130 cm; 44 kg empty) contain 22.6 kg (12.4 m3) CO2 as a liquefied gas at 5800 kPa (838 psig).14 Although approximately 8.5 m3 of CO2 is available from a single G cylinder during the first 4 minutes of unregulated flow (Figure 2), gas flow is not linear, and rapid evaporation causes the remaining liquid within the cylinder to freeze into dry ice at -79°C within 2 minutes (Figure 3). Because of this nonlinearity, it is difficult to accurately estimate the flow rate, and therefore to predict concentration, during a timed release. Additionally, after a single unregulated discharge, very little CO2 is available even after the cylinder has thawed (Figure 2).
Figure 2: Total carbon dioxide (CO2) flow during a 4-minute discharge from unregulated "G" cylinders at 12.2°C and 23.8°C. Full tanks contain 12.4 m3 (12,400 L) of CO2. Tanks were placed on an electronic scale (weight = 44 kg empty) and the change in weight during discharge was converted to cubic meters of CO2 (0.55 m3/kg at 20°C) and then to liters 1m2 = 1000 L. Although approximately 8500 L of CO2 are available from each cylinder on the first 4-minute discharge, rapid evaporation causes the remaining liquid within the cylinder to freeze into dry ice at -79°C within 2 minutes, and only 1500 to 2000 L are available for subsequent discharge after the cylinder has thawed.
|
Figure 3: Carbon dioxide (CO2) flow during a 4-minute discharge from unregulated "G" cylinders at 12.2°C and 23.8°C. Tanks were placed on an electronic scale (weight = 44 kg empty) and the change in weight during discharge was converted to cubic meters of CO2 (0.55 m3/kg at 20 degrees C) and then to liters (1 m3 = 1000 L). Although initial flow is high, flow rapidly decreases by 120 seconds due to content freezing. |
Although not specifically AVMA-approved at this time, low pressure liquid CO2 sources can provide large measured quantities of gaseous CO2 at a defined flow rate and may prove to be an acceptable alternative to compressed gas cylinders for field euthanasia. An extensive liquid CO2 delivery infrastructure exists throughout the US to service the food and restaurant industries, as well as the welding and oil-well recovery industries. Local delivery trucks contain up to 3175 kg (1746 m3), and over-the-road tank trailers contain up to 18,150 kg (9854 m3) of low-pressure liquid CO2 at 750 kPa (108 psig) and -18°C. Liquid CO2 is converted within the delivery hose to gaseous CO2 by exogenous environmental heat and delivered at the nominal flow rate of 36.3 kg per minute (19.8 m3 per minute) (Figure 4). Delivered CO2 volume is easily monitored using the Sponsler flow meter present on commercial equipment. The AVMA-recommended flow rate of 20% of the chamber volume per minute could thus be met in a container with an internal volume up to 99 m3 (3.35 m x 2.42 m x 12.2 m). The time required to reach 63% CO2 for smaller enclosed spaces would be substantially shorter than 5 minutes due to reduced time constant at the higher flow rate.
Figure 4: Carbon dioxide (CO2) gas release from a commercial liquid CO2 delivery truck. Local delivery trucks contain up to 3175 kg (1746 m3) of liquid CO2 at 750 kPa (108 psig) and -18°C. Liquid CO2 is converted to gaseous CO2 within the delivery hose by exogenous environmental heat and is delivered at the nominal flow rate of 36.3 kg (19.8 m3) per minute. The gas plume is greater than 7.5 m wide. |
Field tests
Two demonstration-of-principle field tests, one using CO2 cylinders and one using low pressure liquid CO2, were performed at the request of the swine industry, using casualty pigs on cooperating industry-owned farms. All efforts were made to apply current AVMA recommendations1 on humane euthanasia of swine using CO2. Each farm provided the container to be used as the euthanasia chamber. As the principles governing the wash-in of gases and time constants can be applied to a container of any size, the only requirements were hydraulic dump capability and absence of large gaps or openings on the sides or bottom that could not be sealed.
Farm 1: July 10, 2001. A hydraulic dump body truck with box dimensions of 2.42 m x 5.00 m x 1.20 m was used (floor area approximately 12 m2; volume approximately 14.5 m3). The bottom and sides of the box were covered with plywood sheathing. The top was open but was subsequently covered with plastic sheeting. The tailgate was modified by means of a sliding plywood panel to accept the load-out chute, and a plastic observation window was installed. A video camera was mounted inside the truck. For this container volume of 14,500 L, a CO2 flow rate of 2900 L per minute (20% of 14,500 L) is required to produce a 63% CO2 atmosphere within the first 5 minutes (1t).
Industrial-grade CO2 was supplied to the truck box from three unregulated G cylinders. Each cylinder was fitted with a compressed gas fitting specific for CO2 (Compressed Gas Association 320; National Welders Supply Inc, Charlotte, North Carolina) and attached to 12.7-mm copper tubing by means of flexible rubber hose and screw-type hose clamps.
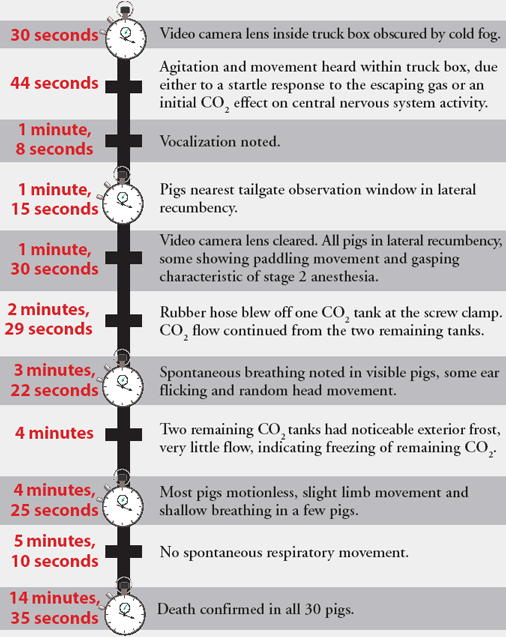
The time to load 30 casualty pigs from a holding pen was 1 minute 16 seconds. An additional 2 minutes was required to secure heavy plastic sheeting over the open top of the truck with duct tape. The valves of the three tanks were fully opened simultaneously, and the chronology of subsequent events is shown in Figure 5. Although direct visual observation of the pigs was obscured by CO2 fog, on the basis of observed signs, the onset of unconsciousness likely occurred 30 seconds to 1 minute following CO2 introduction into the truck box.
Figure 5: Flow chart detailing the timeline of events observed during a field test (Farm 1) of a method proposed for humanely killing a large number of swine using carbon dioxide (CO2). A hydraulic dump body truck was used as a euthanasia chamber , with three unregulated "G" cylinders providing the source of CO2. The time to load 30 casualty pigs from a holding pen was 1 minute 16 seconds. An additional 2 minutes was required to secure heavy plastic sheeting over the open top of the truck with duct tape. Events are described after the valves of the three tanks were simultaneously opened.
|
Volume of CO2 used for this test was approximately 25.5 m3 (estimated at 8500 liters x 3 tanks; Figure 2), equivalent to a CO2 flow rate of 2125 L per minute per tank. However, it must be noted that one of the tanks was lost when the delivery hose came off due to excessive pressure, and that gas flow from unregulated cylinders is not linear, making accurate estimation of gas use and rate of delivery difficult. Cost of CO2 was US$10 per cylinder plus drayage charges.
Farm 2: November 29, 2001. A hydraulic dump body trailer with a box having dimensions 3.15 m x 2.40 m x 1.20 m (9.07 m3) was used. The bottom and sides of the box were covered with plywood sheathing, the top was open, and the tailgate was modified by means of a sliding plywood panel to accept the load-out chute. A video camera was mounted inside the truck and thermistors (Fisher Brand Digital Dual Thermo, part number 862302; Fisher Scientific, Atlanta, Georgia) were placed to monitor interior temperature. A sampling line for an oxygen analyzer (Datex Engstrom Capnomac II; Datex Instruments, Helsinki, Finland) was also placed inside the truck. As in the Farm 1 test, the trailer was covered with plastic sheeting secured with duct tape after the pigs were loaded.
Carbon dioxide was supplied to the box through a surface-mount, stainless steel liquid-CO2 filling box (Taylor-Wharton BC04-8C26; Taylor-Wharton/Harsco, Theodore, Alabama; Figure 6). The filling box is required by the liquid CO2 supplier for personnel safety and is designed to securely hold the filling hose during gas transfer. Carbon dioxide gas from a commercial liquid CO2 bulk tanker (National Welders Supply Co, Charlotte, North Carolina; Figure 4) was introduced into the test trailer at a nominal flow rate of 36.3 kg per minute (19.8 m3 per minute). This flow rate produced a time constant of 27.5 seconds for the test trailer (9.07 m3 19.8 m3 per minute).
Figure 6: In the event of an emergency situation requiring on-farm euthanasia of large numbers of swine using carbon dioxide, a surface-mount stainless steel carbon dioxide (CO2) filling box is required for personnel safety when connecting to the commercial low pressure liquid CO2 source. The box illustrated here, Taylor-Wharton BC04-8C26 (Taylor-Wharton/Harsco, Theodore, Alabama) or equivalent may be used.
|
In the first batch of 14 casualty pigs, CO2 gas was supplied for 90 seconds (approximately 3t for this size trailer with the available flow rate). Temperatures within the trailer quickly fell following CO2 gas inflow, from > 38°C to 0°C. As in the Farm 1 test, the video camera lens was obscured by fog at the start of CO2 gas inflow and did not clear for several minutes.
All 14 pigs were quickly stunned, but gradually recovered consciousness over the subsequent 15-minute exposure period. A 4-inch gap in the trailer tailgate was covered with additional plastic sheeting, sealed with duct tape, and the 90-second CO2 application (3t) was repeated. Measured oxygen levels within the trailer fell to 7%. Of the 14 exposed pigs, 12 died and two were alive but unconscious after the second 15-minute CO2 exposure. The unconscious pigs were both in the right front quadrant of the trailer and were subsequently humanely killed, without regaining consciousness, using a penetrating captive bolt. A total of 80.7 kg CO2 (43.9 m3) was used in the two exposures.
In the second batch of eight casualty pigs, 36.3 kg CO2 (19.8 m3) was supplied over 70 seconds (approximately 2t for this size trailer and flow rate). Monitored oxygen levels within the trailer fell to 6%. Oxygen gradually increased to 15% during the subsequent 15-minute exposure period. Of the eight pigs, seven had died and one was alive but unconscious after the 15-minute exposure. The unconscious pig was humanely killed using a penetrating captive bolt. Again, the stunned pig was in right front quadrant of trailer, suggesting an air leak at that position.
A total of 117 kg of liquid CO2 was used, at a cost of $90.09 ($0.77 per kg). Rental for the CO2 truck and driver was $110 per hour and the cost of the liquid-CO2 filling box was $120 (all currency in $US).
Discussion
We have reviewed the principles governing the use of CO2 in enclosed spaces and shown that adherence to the 2000 Report of the AVMA Panel on Euthanasia1 recommendation for CO2 flow rate is appropriate for humane euthanasia within any enclosed volume or space. In addition, we have demonstrated the feasibility of applying the AVMA guidelines for on-farm carbon dioxide euthanasia to groups of adult pigs. Procedural costs and labor requirements will be directly related to container size and the ability to provide adequate CO2 flow into those containers, as well as to the number of containers utilized per farm. Further studies to confirm mass CO2 euthanasia as an effective euthanasia tool, and to define engineering and performance standards, will be necessary prior to wide-scale adoption of this method for on-farm use in the event of a foreign animal disease outbreak in the United States.
Presently, the only AVMA-accepted source of CO2 is compressed CO2 gas in cylinders, because the flow can be regulated precisely.1 In the event of a foreign animal disease outbreak, field use of CO2 cylinders will be problematic, in light of their nonlinear output at the high flow rates necessary in large containers, as well as the large number of cylinders required. Conceivably, a sequential or ganged arrangement of regulated G cylinders operating at subfreezing flow rates could meet the AVMA gas flow requirement for small enclosed chambers, but the number of individual cylinders required for a chamber large enough for several dozen adult pigs would be very high. For example, to meet the AVMA-recommended flow rate of 2900 L per minute for a 14.5-m3 box, 64 individual cylinders would be required, each equipped with a nonfreezing CO2 regulator operating at the maximum rated flow of 45 L per minute (model SG 9012-CGA; Advanced Specialty Gas Equipment, Middlesex, New Jersey).
Low-pressure liquid CO2, although not specifically AVMA-approved, does meet the AVMA requirement for regulated CO2 flow. Gaseous CO2 outflow is 19.8 m3 per minute, and as previously shown, flow into containers can be controlled by varying the inflow time to achieve the desired CO2 concentration on the basis of the time constant. An issue to be resolved with low-pressure liquid CO2 is the potential for freeze-burning the animals with cold gas or liquid CO2 during the euthanasia process. This will likely be a problem during prolonged high-flow situations where insufficient environmental heat is available to convert the -18°C liquid CO2 into gaseous CO2 within the delivery hose. Passing the cold liquid CO2 through a heat exchanger prior to delivery would ensure that only CO2 gas is delivered during high flow periods.15 In Field Test 2, approximately 10% of casualty pigs survived 15 minutes of hypoxia, demonstrating the absolute necessity of leak-proof containers for on-farm CO2 euthanasia of pigs. Measured oxygen levels within the test container fell to 7% for the first batch of 14 casualty pigs and to 6% for the second batch of eight casualty pigs. These levels are higher than the 1% oxygen predicted for a 3t CO2 wash-in exposure, and this indicates that air leaks were present in the container even after the gap at the tailgate was resealed. We used dump-bed equipment already in place on each farm to demonstrate that the principles of CO2 euthanasia could be applied to a variety of containers. Leak testing on the day of the test was limited to visual inspection. Ideally, containers to be used for CO2 euthanasia should be dynamically leak tested prior to use. Should CO2 euthanasia be adopted for emergency on-farm use, it is absolutely imperative that individual animal death is verified at the disposal area and that any pigs that may have survived CO2 exposure be humanely killed using an alternate AVMA-approved method, such as captive bolt.
On the basis of our findings, we can speculate as to potential CO2 costs for on-farm euthanasia. Using low-pressure liquid CO2 and a flow rate of 19.8 m3 per minute, the AVMA-recommended 20% inflow rate can be met in a container with dimensions 3.6 m x 2.25 m x 12.2 m having an internal floor area of 44 m2 and an enclosed volume of approximately 99 m3. At the recommended truck-loading density of 0.37 m2 per 100 kg body weight,13 approximately 120 pigs could thus be humanely killed in each batch. Based on a CO2 cost of $0.77 ($US) per kg of product, cost per batch for a container of this size is estimated to be $138.60 for each 5-minute (1t) CO2 exposure (180 kg CO2). At 120 pigs per batch, approximately 6000 kg of CO2 will be required to depopulate a 4000-head farm at a cost $4620, plus driver and truck charges and farm labor costs. Local delivery trucks have a capacity for up to 3175 kg (1746 m3), and over-the-road tank trailers for up to 18,150 kg (9854 m3) of liquid CO2. The time to process each batch is estimated at 30 minutes and consists of moving the pigs out of the building and into the container, application of the plastic tarpaulin cover (5 minutes), and CO2 fill for at least 1t (5 minutes; 63% CO2 atmosphere), after which the container is left undisturbed for 15 minutes of CO2 exposure. The container is then moved to the disposal area, dumped, and recycled for the next batch. In practice, although the loading density could be doubled, several euthanasia chambers will likely be required on an individual farm to speed the depopulation process. It would be prudent for the swine industry to enter into negotiations with regional carbon dioxide suppliers prior to a disease outbreak to ensure availability and adequate supplies.
Implications
- Emergency on-site CO2 euthanasia of swine is feasible using or exceeding the AVMA-recommended CO2 flow rate of 20% of the chamber volume per minute.
- Although CO2 cylinders are presently the only AVMA-recommended source of CO2 gas, bulk low-pressure liquid CO2 sources meet the AVMA requirement of metered flow and have significant advantages in generating the high gas flows required for large containers.
- Container size and loading density will determine the actual number of animals that can be processed in a given duty cycle.
- Should CO2 euthanasia be adopted for emergency on-farm use, it is absolutely imperative that containers be leak checked prior to use, that individual animal death is verified at the disposal area, and that any pigs surviving CO2 exposure be humanely killed using an alternate AVMA-approved method, such as captive bolt.
References
1. 2000 Report of the AVMA Panel on Euthanasia. JAVMA. 2001;218:669-696.
2. Ferguson NM, Donnelly CA, Anderson RM. Transmission intensity and impact of control policies on the foot and mouth epidemic in Great Britain. Nature. 2001;413:542-548.
3. Department for Environment, Food, and Rural Affairs (DEFRA). Statistics on Foot and Mouth Disease. Available at: http://www.defra.gov.uk/footandmouth/cases/statistics /generalstats.htm. Accessed April 6, 2005.
4. Forslid A. Transient neocortical, hippocampal and amygdaloid EEG silence induced by one minute inhalation of high concentration CO2 in swine. Acta Physiol Scand. 1987;130:1-10.
5. Erhardt W, Ring C, Kraft H, Schmid A, Weinmann HM, Ebert R, Schlager B, Schindele M, Heinze R, Lomholt N, Kallweit E, Henning M, Unselm J, Berner H, Blumel G. CO2-stunning of swine for slaughter from the anesthesiological viewpoint. Deutsche Tierarztliche Wochenschrift. 1989;96:92-99.
6. Martoft L, Lomholt L, Kolthoff C, Rodriguez BE, Jensen EW, Jorgensen PF, Pedersen HD, Forslid A. Effects of CO2 anaesthesia on central nervous system activity in swine. Lab Anim. 2002;36:115-126.
7. Forslid A, Augustinsson O. Acidosis, hypoxia and stress hormone release in response to one-minute inhalation of 80% CO2 in swine. Acta Physiol Scand. 1988;132:223-231.
8. Thurmon JC, Tranquilli WJ, Benson GJ, eds. Lumb and Jones' Veterinary Anesthesia. 3rd ed. Baltimore: Lippincott Williams and Wilkins; 1996:11-16.
9. Hornett TD, Haynes AR. Comparison of carbon dioxide/air mixture and nitrogen/air mixture for the euthanasia of rodents. Design of a system for inhalation euthanasia. Anim Tech. 1984;35:93-99.
10. Smith W, Harrap SB. Behavioural and cardiovascular responses of rats to euthanasia using carbon dioxide gas. Lab Anim. 1997;31:337-346.
11. Nunn JF. Nunn's Applied Respiratory Physiology. 4th ed. Oxford: Butterworth-Heinemann; 1993:583-593.
12. Davis PD, Parbrook GD, Kenny GNC. Basic Physics and Measurement in Anaesthesia. 4th ed. Oxford: Butterworth-Heinemann; 1995:61-76.
13. Swine Care Handbook. National Pork Producers Council; 1992:15.
14. Dorsch JA, Dorsch SE. Understanding Anesthetic Equipment: Construction, Care and Complications. 3rd ed. Baltimore: Williams and Wilkins; 1994:3-20.
*15. Mobile vaporizer for liquefied gases. Purgit Emission Control Systems, Houston TX. Available at: http://www.purgit.com/truck.html. Accessed March 17, 2005.
* Non-refereed references.