Original research |
Peer reviewed |
Detection of porcine reproductive and respiratory syndrome virus in semen and serum of boars during the first six days after inoculation
Detección del virus del síndrome reproductivo y respiratorio del cerdo en semen y suero de sementales durante los primeros seis días después de la inoculación
Détection du virus du syndrome reproducteur et respiratoire porcin dans la semence et le sérum de verrats durant les six premiers jours suivant l'inoculation
Darwin L. Reicks, DVM; Claudia Muñoz-Zanzi, DVM, MPVM, PhD; William Mengeling, DVM, PhD, Diplomate ACVM; Jane Christopher-Hennings, DVM, MS; Kelly Lager, DVM, PhD; Dale Polson, DVM, PhD; Scott Dee, DVM, PhD, Diplomate ACVM; Kurt Rossow, DVM, PhD
DLR: Swine Veterinary Center, St Peter, Minnesota. CMZ: Department of Veterinary Population Medicine, University of Minnesota, St Paul, Minnesota. WM: Collaborative Professor, College of Veterinary Medicine, Iowa State University, Ames, Iowa. JCH: Animal Disease Research and Diagnostic Laboratory, South Dakota State University, Brookings, South Dakota. KL: National Animal Disease Center, USDA-ARS, Ames, Iowa. DP: Boehringer Ingelheim Vetmedica, Ames, Iowa. SD: Swine Disease Eradication Center, University of Minnesota College of Veterinary Medicine, St Paul, Minnesota. KR: Veterinary Diagnostic Laboratory, University of Minnesota College of Veterinary Medicine, St Paul, Minnesota. Corresponding author: Dr Darwin L. Reicks, 1608 S Minnesota Ave, PO Box 269, St Peter, MN 56082; Tel: 507-934-3970; Fax: 507-934-3968; E-mail: dreicks@swinevetcenter.com.
Cite as: Reicks DL, Muñoz-Zanzi C, Mengeling W, et al. Detection of porcine reproductive and respiratory syndrome virus in semen and serum of boars during the first six days after inoculation. J Swine Health Prod. 2006;14(1):35-41.
Also available as a PDF.
SummaryObjectives: To determine, during the first 6 days post inoculation, when porcine reproductive and respiratory syndrome virus (PRRSV) can be detected in serum or semen by polymerase chain reaction (PCR); the impact of pooling on detection of PRRSV by PCR; and the possible association between rectal temperature and detection of PRRSV in serum by PCR. Materials and methods: Forty mature boars (four groups of 10) were inoculated intranasally with PRRSV variant MN 30-100. Serum and semen samples were collected on a rotating basis from one group every 12 hours for 6 days and tested for PRRSV by PCR. Rectal temperatures were recorded for all 40 boars at 12-hour intervals. Results: Serum samples became PCR-positive before semen samples. During the first 6 days after inoculation, serum was PRRSV-positive in 36 of 40 boars, and semen was PRRSV-positive in four of 40 boars. Median time to detection was 36 and 72 hours for nested PCR and Taqman PCR, respectively. Results were inconsistent when a positive semen sample was pooled with negative semen. Elevated rectal temperature was not associated with PCR-positive serum or semen results. Implications: Under the conditions of this study, PCR is more sensitive and detects PRRSV-infected boars earlier in serum than in semen. Pooling of positive semen samples provides variable PCR results. Rectal temperatures are not correlated with PCR-positive results. Sampling techniques are needed to more easily obtain serum samples for PCR from boars once or twice weekly at the time of semen collection. | ResumenObjetivos: Determinar, durante los primeros 6 días post inoculación, cuando puede detectarse el virus del síndrome reproductivo y respiratorio porcino (PRRSV por sus siglas en inglés) en suero o semen mediante la reacción en cadena de la polimerasa (PCR por sus siglas en inglés); el impacto de agrupar las muestras en la detección del virus por PCR; y la posible asociación entre la temperatura rectal y la detección en suero del virus por PCR. Materiales y métodos: Se inocularon intranasalmente cuarenta sementales maduros (cuatro grupos de 10) con la cepa MN 30-100 del PRRSV. Cada 12 horas y durante 6 días, se colectaron muestras de suero y semen de un grupo en base rotativa y se probaron en busca del PRRSV por PCR. Cada 12 horas se registraron las temperaturas rectales de los 40 machos. Resultados: Las muestras de suero fueron positivas por PCR antes que las de semen. Durante los primeros 6 días después de la inoculación, el suero fue PRRSV positivo en 36 de los 40 machos y el semen fue PRRSV positivo en cuatro de los 40 machos. La mediana del tiempo de detección fue de 36 y 72 horas para el PCR anidado y PCR Taqman, respectivamente. Los resultados fueron inconsistentes cuando una muestra positiva de semen se agrupo con semen negativo. La temperatura rectal elevada no se asoció con resultados positivos de PCR de suero o semen. Implicaciones: Bajo las condiciones de este estudio, el PCR para el PRRSV es más sensible y detecta sementales infectados con PRRSV en suero antes que en semen. El agrupar muestras positivas de semen provee resultados de PCR variables. Las temperaturas rectales no se correlacionan con los resultados positivos de PCR. Se requiere de técnicas de muestreo para obtener con mayor facilidad muestras de suero para PCR de machos una o dos veces a la semana durante la recolección de semen. | ResuméObjectifs: Déterminer, durant les 6 premiers jours suivant l'inoculation, quand le virus du syndrome reproducteur et respiratoire porcin (VSRRP) peut être détecté dans le sérum et la semence par réaction en chaîne par polymérase (PCR en anglais); l'impact de fonctionner par pool sur la détection du virus par PCR; et l'association possible entre la température rectale et la détection du virus dans le sérum par PCR. Matériel et méthodes: Quarante verrats matures (quatre groupes de 10) ont été inoculés par voie intra-nasale avec la souche MN 30-100 du VSRRP. Des échantillons de semence et de sérum ont été prélevés sur une base rotative chez un groupe aux 12 heures, durant 6 jours, et testés par PCR pour le VSRRP. Les températures rectales ont été notées pour les 40 verrats aux 12 heures. Résultats: Les échantillons de sérum sont devenus positifs par PCR avant ceux de semence. Durant les 6 premiers jours après l'inoculation, le sérum a été positif pour le VSRRP chez 36 des 40 verrats, et la semence chez 4 des 40 verrats. Le temps médian pour la détection a été respectivement de 36 et 72 heures pour la PCR nichée et la Taqman PCR. Les résultats ont été inconsistants lorsqu'un échantillon de semence positif était utilisé en pool avec de la semence négative. Des résultats positifs par PCR sur la semence ou le sérum n'ont pas été associés avec une température rectale élevée. Implications: Dans les conditions de cette étude, la PCR pour le VSRRP est plus sensible et détecte les verrats infectés de VSRRP plus rapidement dans le sérum que dans la semence. L'utilisation de pools pour les échantillons de semence positifs produit des résultats de PCR variables. Les températures rectales ne sont pas corrélées avec des résultats positifs de PCR. Des techniques d'échantillonnage sont nécessaires pour obtenir plus facilement des échantillons de sérum des verrats une ou deux fois par semaine, au moment de la collecte de semence. |
Keywords: swine, porcine
reproductive and respiratory syndrome virus, boar, semen, polymerase chain
reaction, PRRS, PRRSV, PCR
Search the AASV web site
for pages with similar keywords.
Received: March
5, 2004
Accepted: February
14, 2005
It has been well documented that por- cine reproductive and respiratory syn- drome virus (PRRSV) can be transmitted through semen from boars to sows or gilts.1,2 To prevent infection of sow herds, biosecurity measures must first be implemented to prevent boar studs from becoming infected. Secondly, after detection of porcine reproductive and respiratory syndrome (PRRS) in a boar stud, shipment of semen must stop immediately. In order to stop shipment of infected semen before sow herds are at risk, PRRSV infection must be identified in the boar stud as soon as possible. Clinical signs such as lethargy and anorexia have been reported in boars in research settings, particularly during the first few days of infection.2-4 However, under field conditions, clinical signs are quite variable. Therefore, clinically monitoring boars in an effort to detect peracute infection is not reliable. Initial viremia is detectable as early as 12 hours after infection with PRRSV, with subsequent virus distribution to other organs.5 Therefore, a complete understanding of the sensitivity of the test used to detect PRRSV (considering various ante mortem samples) is important for monitoring purposes.
Polymerase chain reaction (PCR) is the most practical test currently available to detect early PRRSV infection in boars. Other tests are not as practical, either because of the significant delay between sampling and results (eg, swine bioassay, virus isolation) or the requirement for detectable antibodies at sampling (eg, ELISA, immunofluorescence assay). In addition, virus isolation is not as sensitive as PCR because of the cytotoxicity of semen for cells used for culture.6
Two common PCR techniques used for testing semen or serum to monitor boar studs are nested PCR and Taqman PRRSV reverse transcriptase-PCR (RT-PCR). In previous studies, the diagnostic sensitivity of the nested PCR was comparable with the swine bioassay, which detects infectious virus in semen. For an estimate of analytical sensitivity, a lower limit of 10 virions per mL was detected in serial cell culture dilutions.6 Sensitivity of the Taqman PCR is similar to that of a nested PCR assay at a detection level in the range of 0.1 to 0.01 median tissue culture infectious doses (TCID50) of virus in semen samples.7
In young pigs, virus can be detected in serum by virus isolation within 12 hours after infection.5 In one study,4 virus was detected in serum in four of four boars at 1 day post infection and in serum prior to semen. In other studies8,9 involving small numbers of boars, virus was detected in serum before semen. Prieto et al (1996)10 were unable to detect virus in semen (by virus isolation) when nine boars were collected once per week through day 70 post inoculation. In another study, Prieto et al (2004)11 reported that semen was PRRSV-positive in four of 20 boars 4 to 14 days post infection.11 No published studies have included large numbers of boars or have reported frequent sampling of boars within the first few days after infection.
Due to the ease of sample collection and sample availability, the most practical way to sample boars is to collect semen and test it for PRRSV by PCR. However, for unknown reasons, there appears to be great variation in field reports on PRRSV shedding in semen and its timing. While several studies4,8,9 have reported detection of PRRSV in semen by RT-PCR, the frequency and degree of shedding have been variable.
Because of the importance of early detection of infection and the need for studies involving a larger number of boars and frequent sampling within the first few days after infection, the objectives of this study were first to determine how soon PRRSV can be detected in serum and semen by PCR; second, to determine if pooling of a positive semen sample with negative samples affects the sensitivity of PRRSV detection by PCR; and third, to determine if there is an association between elevated rectal temperature and PRRSV detection by PCR.
Materials and methods
Animals
Forty working boars 12 to 36 months of age were PRRS-negative when tested by the Idexx PRRS 2XR ELISA (Idexx Laboratories, Westbrook, Maine). An additional four working PRRS-negative control boars served as a source of negative samples for pooling and for quality control of the test procedures. All boars were from the same source, which was considered PRRS-negative on the basis of monthly serological testing with negative results.
Housing, feeding, and management
After negative PRRS ELISA results were available, the 40 principle boars were moved to a vacant commercial facility and allowed to acclimate for 3 days. All boars were housed in individual, partially slatted stalls (1.5 m2) with nose-to-nose contact. All animals were housed in the same room with forced-air ventilation targeted to maintain a temperature of 22°C to 24°C. Water drippers activated when temperatures exceeded 26°C. Animals were fed a corn and soybean-meal diet (16% crude protein) once daily. Semen was collected in one of two identical, adjacent collection pens.
Control boars remained in the original facility, which had forced-air ventilation, totally slatted flooring, and the same target temperature, cooling guidelines, and stall size as the facility housing the principle boars. All animals at both facilities were cared for in accordance with published animal welfare guidelines.12
Experimental design
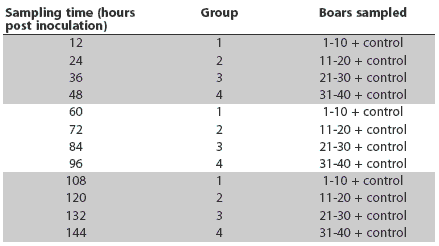
The inoculum (PRRSV variant MN 30-100) was prepared by passage of the virus in MARC-145 cells and was suspended in minimal essential medium. On Day 0, virus was administered intranasally at a concentration of 104 TCID50. Principle boars were allocated into four groups of 10 for sampling purposes, with seven Hampshire and three Large White boars per group. Semen was collected alternately on 12-hour schedules for 6 days; therefore, each boar was collected three times at 48-hour intervals. A blood sample was collected from each boar at the time of collection. Semen and serum samples were put on ice immediately after collection. A summary of the semen and serum sampling schedule is shown in Table 1. Samples were also obtained from the control boar for each principle group and submitted with the treatment-group samples.
Table 1: Sampling schedule for semen and serum collection for the purpose of detecting porcine reproductive and respiratory syndrome virus in 40 experimentally infected boars (four groups of 10 individually housed in one room) and four uninfected controls (individually housed in a different facility)
|
Serum and semen PCR
At all 12 sampling times, 2-mL aliquots of serum and semen samples were submitted to the University of Minnesota Veterinary Diagnostic Laboratory for PRRSV PCR. Samples of serum (2-mL aliquots) and semen (10-mL aliquots) were also submitted for PRRSV PCR to the South Dakota State University Veterinary Diagnostic Laboratory (SDSU-VDL) when each boar was first sampled, at 12, 24, 36, and 48 hours post inoculation for Groups 1, 2, 3, and 4 respectively. Samples from Group 4 at the remaining sampling times (96 and 144 hours) were submitted to SDSU-VDL. All PCR tests were performed 24 to 48 hours post collection. Samples were delivered by car to each location to minimize shipping effects.
All samples were coded to ensure that the laboratory technicians were blinded to treatment and sampling times. Samples were tested by Taqman PCR at the University of Minnesota Veterinary Diagnostic Laboratory and by nested PCR at SDSU-VDL.
Nested PCR was performed on serum and semen as previously described, using primers from open reading frame (ORF) 7.4,6,8,9,13 Ten mL of semen was centrifuged to concentrate the cellular fraction and remove the cell-free fraction. The cellular fraction was then used for PCR analysis, as it has been reported6 that PRRSV is found primarily within seminal macrophages in this fraction. A viral RNA mini-kit (Qiagen, Valencia, California) was used for RNA extractions from serum, and a proteinase K-guanidinium buffer and RNeasy protocol (Qiagen) were used for extraction of RNA from the cellular fraction of the semen.14
For the Taqman PCR, RNA was extracted from 0.2 mL of the original serum sample using a commercial kit (QIAamp DNA Blood BioRobot 9604 kit; Qiagen) according to the manufacturer's protocol. An automated viral purification procedure was used for the extraction,7 using duplicate samples. One-step RT-PCR was then performed on the extracted RNA using a commercial kit (One Step RT-PCR kit; Qiagen) according to the manufacturer's protocol. Semen samples were tested for US PRRSV by Taqman PCR using a 0.2-mL aliquot of raw (undiluted) semen to detect ORF 6.7 Results were determined to be positive, negative, or suspect. A suspect result was an inconclusive result, ie, one positive and one negative result on the same sample. Suspect results were considered positive for purposes of statistical analysis.
Pooled semen samples
A semen sample from each boar was pooled with negative control semen in dilutions of 1:3, 1:5, and 1:10 at collection times 12, 24, 36, 48, 96, and 144 hours. Samples were pooled at the time of collection, prior to submitting to SDSU-VDL or University of Minnesota Diagnostic Laboratory. All samples were coded, and laboratory technicians were blinded to treatment and to whether samples were pooled or individual samples.
Rectal temperatures
A digital thermometer was calibrated using a calibrated thermometer traceable to National Institute of Science and Technology (NIST) standards. Rectal temperatures were obtained on all 40 boars at 12-hour intervals. Room temperature was also obtained using a high-low thermometer accurate to within 1.0 Fahrenheit degree when checked using a thermometer certified to NIST standards. Boars with body temperature > 103.0°F were considered to have fever. A room temperature > 74.0°F had a confounding influence on rectal temperature. Rectal temperatures were not obtained on control boars because they were in a different facility. Clinical signs, such as off-feed and lethargy, were observed but not recorded.
Serum ELISA
Serum samples were tested for PRRSV antibodies using an ELISA test (Idexx PRRS 2XR ELISA; Idexx Laboratories) 3 days prior to inoculation and on Days 6 and 13 post inoculation. Samples were tested at Boehringer Ingelheim Vetmedica, Inc (Ames, Iowa). Laboratory technicians were blinded to treatment. The same lot of ELISA reagents was used for testing all samples. Control boars were tested 4 weeks after completion of the study to verify negative status. A sample-to-positive (S:P) ratio >= 0.4 was considered positive.
Statistical analyses
Descriptive statistics were initially performed to summarize and describe the data on diagnostic testing, pooling, and rectal temperature. Subsequently, survival analysis methods, specifically the Cox proportional hazards regression model, were used to model the time-to-first detection of PRRSV by PCR in serum and semen.15 This type of analysis was used to estimate the median time and 90% confidence interval (CI) to the first PCR-positive test for a given type of sample and PCR test and to obtain the cumulative probability of PRRSV detection at a given sampling time (1- survival function). A 90% CI was used due to the relatively small sample size.The sampling schedule for each boar was taken into consideration in the analysis by including the actual time when samples were collected and results were available. For a given analysis of serum or semen samples and type of PCR test, when results for some boars at some sampling times were missing, results for those boars were considered until the time of the last available result. Variables in the model included occurrence of fever (yes or no; used as a time-dependent variable) and breed (Hampshire or Large White). Assumption of proportional hazards was evaluated on the basis of the plot of the log(-log S ), where S is the survival function, and of the Schoenfeld residuals. Plots of the Cox-Snell residuals were used to assess overall goodness-of-fit for the final model.15
The association between rectal temperature and the probability of a positive PCR test was evaluated for Taqman PCR in serum using a mixed effects logistic regression model.16,17 A random effect was included in the model to account for the repeated testing of boars over time, and fixed effects terms were added for occurrence of fever and sampling time. Room temperature was added into the model to adjust for its potential effect on increasing rectal temperature. Results of the model indicated whether there was an association between rectal temperature and a PCR-positive test over time.
All statistical analyses considered a P value < .05 as statistically significant. Survival analysis and logistic regression were performed using the statistical software S-Plus 6.2 (Insightful Corporation, Seattle, Washington) and MIXNO (University of Illinois, Chicago, Illinois), respectively.
Results
All boars were sampled as scheduled with the exception of one boar that did not successfully mount the collection dummy at 48 or 96 hours, but did collect at 144 hours post inoculation.
Serum and semen PCR
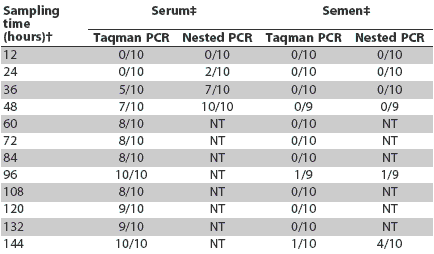
PRRS virus was first detected in serum samples by nested PCR at 24 hours post inoculation and by Taqman PCR at 36 hours post inoculation (Table 2). Control boars were PCR-negative (both serum and semen) throughout the study. All serum samples that tested positive by Taqman PCR were also positive by nested PCR. Of the 40 inoculated boars, 36 had at least one positive serum PCR result during the first 6 days after inoculation. Semen was positive by Taqman PCR in only one boar (at 96 and 144 hours post inoculation), and semen for this boar was also positive by nested PCR at these sampling times. In a second boar, the semen Taqman PCR result at 120 hours post inoculation was reported as suspect. In three other boars, semen tested positive by nested PCR at 144 hours post inoculation (Table 2). In all four boars with PCR-positive semen tests, semen tested positive 96 hours later than serum had tested positive.
Table 2: Summary of polymerase chain reaction (PCR) results in semen and serum of boars experimentally infected with porcine reproductive and respiratory syndrome virus*
* Forty boars total (four groups of 10) were sampled on a rotational basis as described in Table 1. † Time post inoculation. ‡ Number PCR-positive/number tested. NT = Sample not tested. |
Results of the survival analyses of time to first PRRSV detection showed that median time to detection of PRRSV in serum (ie, the time post inoculation by which 50% of the boars had tested positive) was 48 hours (90% CI; 36 - 60 hours) for Taqman PCR and 36 hours (90% CI; 36 - 48 hours) for nested PCR. The results of nested-PCR testing of semen samples were considered as of the last available result for each boar. Because of the few semen samples that tested PCR-positive, median time to first detection could not be estimated for semen testing.
Breed was not significantly associated with detection of PRRSV in serum by nested PCR; however, results showed an effect of breed on the probability of detection of PRRSV in serum by Taqman PCR (P < .01). Median time to detection by Taqman PCR was 84 hours (90% CI; 72 - 84 hours) in Hampshire and 48 hours (90% CI; 48 - 72 hours) in Large White. Large Whites were 3.08 times (90% CI; 1.43 - 6.70) more likely to have a positive serum Taqman-PCR result than Hampshires.
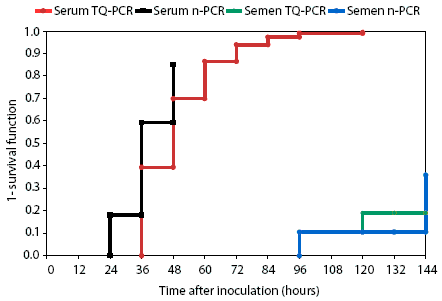
The survival model predicted a 39.4% probability that boars tested positive at least once by 48 hours post inoculation when serum was tested by Taqman PCR. The probability using nested PCR was 59.3% by 48 hours. Predictions from the limited data on semen testing indicated that the probability of a positive test was 0 by 48 hours for both tests; however, the probability by 144 hours was 19% using Taqman PCR and 35.9% using nested PCR (Figure 1).
Figure 1: Forty boars were experimentally infected with porcine reproductive and respiratory syndrome virus (PRRSV; Day 0). Serum and semen samples from four groups of 10 boars were tested for PRRSV at 12-hour intervals using the Taqman polymerase chain reaction (TQ-PCR) and nested PCR (n-PCR). The schedule of testing is shown in Table 1. One negative control boar was tested with each principle group. A Cox proportional hazards regression model was used to calculate the cumulative probability (1- survival function) of identifying serum or semen samples as positive at a given sampling time.
|
Pooled semen samples
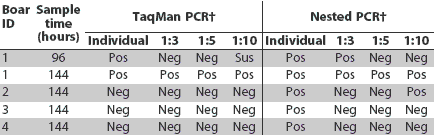
All semen samples collected at the various sampling times that tested negative individually by nested PCR and Taqman PCR also tested negative when pooled with known-negative semen samples in pools of three, five, and 10 samples (results for pooled samples shown in Table 3). Estimations of the probability of a pool testing positive or negative when there was a positive sample in the pool were not calculated because of the small number of positive semen samples.
Table 3: Results of testing semen pools* for porcine reproductive and respiratory syndrome virus (PRRSV) by Taqman and nested polymerase chain reaction (PCR)
* Boars were either inoculated with PRRSV or were separately housed, PRRSV-negative controls, and postinoculation semen and serum samples were collected (Table 1). Pools were created by combining one PCR-positive semen sample with negative control semen samples. For example, the 1:3 pool was created using two parts control boar semen and one part inoculated boar semen. † PCR tests were performed in duplicate, and results were defined as negative if both tests were negative (Neg), positive if both tests were positive (Pos), and suspect (Sus) if there was one positive and one negative result on the same sample. |
Rectal temperatures
Fever was first detected at 12 hours post inoculation. Only 21 of the 40 boars had a fever at any time during the trial. Room temperature exceeded 74.0°F at two testing periods (72 hours and 144 hours). Fever was never detected in three of the four boars that tested positive on semen PCR. Fever had no significant association with detection of PRRSV in serum for either PCR test (P > .05).
PRRS ELISA
All boars tested at the start of the trial (Day 0) and at Day 6 were negative for antibodies by PRRS ELISA. The highest S:P ratio at Day 0 was 0.11 and the highest at Day 6 was 0.08. On Day 13, twenty-nine of 40 boars were seropositive. Of the 11 negative boars, five had S:P ratios between 0.20 and 0.40, and the other six had S:P ratios < 0.20. Ten of the seronegative boars on Day 13 were of the Hampshire breed. The one seronegative Large White boar had an S:P ratio of 0.37. All four boars that were serum PCR-negative at all sampling times during the first 6 days post inoculation were seronegative at Day 13, with S:P ratios of 0.00, 0.00, 0.10, and 0.20, respectively. One of these boars was necropsied on Day 13 and was PCR-positive for PRRSV on pooled tissue homogenate and seminal vesicle fluid.
Discussion
The serum PCR test was much more sensitive at detecting infected boars (36 of 40 positive) during the first 6 days post inoculation than the semen PCR test (one of 40 positive on the Taqman PCR and four of 40 positive on nested PCR), suggesting that even individually tested semen samples are inferior to serum for early detection of a new PRRSV infection in a boar stud. All four inoculated boars that were serum PCR-negative during the first 6 days post inoculation were negative on the ELISA test at Day 13. The reason for this might be operator error in failing to infect these boars on Day 0, or resistance to infection in these boars. It is interesting to note that all of the PCR-negative boars were of the Hampshire breed. Because of the significant difference between breeds in the probability of detecting PRRSV in serum using the Taqman PCR, one could speculate that the Hampshire boars had more resistance to the virus and thus would take longer than 6 days to become infected or might not become infected at all. Ten of the 11 ELISA-negative boars on Day 13 were of the Hampshire breed. This leads one to speculate that there may be a breed difference in resistance during early infection with PRRSV in boars. This has been observed in field experience by one of the authors (DR). However, it may also just have required more than 13 days for seroconversion to occur in these boars. Alternatively, they might not have received the same amount of infectious virus as the boars that seroconverted. One of the seronegative boars, necropsied on Day 13, was PCR-positive for PRRSV on pooled tissue homogenate and seminal vesicle fluid, suggesting that infection had occurred but did not result in detectable serum virus or antibody through Day 13 post inoculation.
In contrast to some previous studies,4,8,9 a low percentage of boars were semen PCR-positive during the first 6 days post inoculation. The MN 30-100 strain of PRRSV used in this study has not been used in other published semen-shedding studies, making a direct comparison with other studies difficult. One possible explanation for the small number of positive semen samples is that strain MN 30-100 may take longer to "traffic" into the semen, ie, to be shed in the semen. Pathogenesis studies have shown that after initial viremia, the virus enters various tissues.13 There was a delay of 96 hours between detection of PCR-positive serum results and detection of PCR-positive semen results for the four boars with semen PCR-positive results. This confirms that there is a delay between detection of PRRSV in serum and detection in semen, and also suggests compartmentalization between the systemic circulation and the reproductive tract. Likely, a breakdown of the blood-testis barrier must occur before virus enters the reproductive tract, or it may just take longer to traffic from the systemic circulation to the reproductive tract. In our experience, under field conditions, variation exists pertaining to the shedding and detection of PRRSV in semen during a PRRS outbreak in a boar stud. Strain differences may explain in part why this happens, and therefore may explain why more virus is detected in semen in some studies than in others. Other contributors to variation might be dose and animal susceptibility.
As serum PCR-positive boars were detected earlier and with a higher probability by nested PCR, and three additional positives were found at 144 hours post inoculation in semen by nested PCR, the data from this study suggest that nested PCR may be more sensitive than Taqman PCR. However, there were also differences in the samples (ie, the cellular fraction from a 10-mL semen sample was used for nested PCR, and 1 mL of whole semen for Taqman PCR), and different RNA extraction methods were used for serum and semen.
For PCR-positive semen samples collected within 6 days after infection, results were variable when samples were pooled. This may indicate a high viral load in the individual sample, allowing detection when positive samples were pooled with negative samples. Two individual samples that were PRRSV-positive both by nested PCR and Taqman PCR were negative at the 1:3 and 1:5 dilutions, but either suspect or positive at the 1:10 dilution. This may have been due to the nonhomogenous nature of the semen sample and demonstrates the unpredictability of detecting PRRSV in pooled semen samples. Four individual semen samples that were PRRSV-positive by nested PCR were positive either at the 1:3 dilution alone or at none of the dilutions. This may be because the viral load in the individual sample was insufficient for PRRSV to be detected when it was diluted with PRRSV-negative samples. It has been previously suggested that PCR results may vary when samples contain small amounts of virus,18 and the variable results in the pooled samples may simply have occurred by chance. Also, for a largely negative population undergoing a new PRRSV infection, the larger the size of the pool, the greater the dilution effect on the nucleic acid present, and the greater the reduction in sensitivity of the test.
An important question is whether the analytical sensitivities (detection limits) of these tests are greater than the level of virus in semen needed to infect a sow or gilt. Previous studies have shown that the amount of PRRSV in the infected boar's semen may vary, and a minimum dose may be required for transmission to females.2,3,6,19-21 In the field, there is a probability-driven relationship between dose and infection: as the dose of virus to the sow increases, the probability of infection increases with each insemination. Semen PCR tests are currently performed on raw ejaculate. An ejaculate is usually extended at a dilution of 1:10 to 1:15. In commercial studs, that extended ejaculate is then pooled with three or four other ejaculates, so the final dilution would range from 1:40 to 1:75. However, the owner of a negative herd may not want to take the chance that an individual sow may become infected with semen containing a level of virus undetectable by current PCR testing procedures. Thus, early detection is critical to ensure complete protection for the sow herd.
Implications
- Under the conditions of this study, serum PCR is more sensitive than semen PCR for PRRSV detection during the first 6 days post inoculation.
- Boars are detected as PRRSV-positive by serum PCR before semen PCR.
- Pooling of semen samples provides variable PCR results during the first 6 days post inoculation.
- Elevated rectal temperatures are not correlated with serum or semen PCR-positive results in boars.
- Sampling techniques are needed to more easily obtain serum samples from boars at the time of each semen collection (once or twice weekly).
Acknowledgements
The project was funded by Boehringer Ingelheim Vetmedica, Inc.
References
1. Robertson IB. Transmission of blue-eared pig disease. Vet Rec. 1992;130:478-479.
2. Yaeger MJ, Prieve T, Collins J, Christopher-Hennings J, Nelson E, Benfield D. Evidence for transmission of porcine reproductive and respiratory syndrome (PRRS) virus in boar semen. Swine Health Prod. 1993;1(5):7-9.
3. Swenson SL, Hill HT, Zimmerman JJ, Evans LE, Wills RW, Yoon KJ, Schwartz KJ, Althouse GC, McGinley MJ, Brevik AK. Artificial insemination of gilts with porcine reproductive and respiratory syndrome virus-contaminated semen. Swine Health Prod. 1994;2:19-23.
4. Christopher-Hennings J, Nelson EA, Hines RJ, Nelson JK, Swenson SL, Zimmerman JJ, Chase CCL, Yaeger MJ, Benfield DA. Persistence of porcine reproductive and respiratory syndrome virus in serum and semen of adult boars. J Vet Diagn Invest. 1995;7:456-464.
5. Rossow KD, Collins JE, Goyal SM, Nelson EA, Christopher-Hennings J, Benfield DA. Pathogenesis of porcine reproductive and respiratory virus in gnotobiotic pigs. Vet Pathol. 1995;32:361-373.
6. Christopher-Hennings J, Nelson EA, Nelson JK, Hines RJ, Swenson SL, Hill HT, Zimmerman JJ, Katz JB, Yaeger MJ, Chase CCL, Benfield DA. Detection of porcine reproductive and respiratory syndrome virus in boar semen by PCR. J Clin Micro. 1995;33:1730-1734.
*7. Molitor TW, Tune KA, Shin J. Application of Taqman PCR in the detection of porcine reproductive and respiratory syndrome virus. Proc AD Leman Swine Conf. 1997;173-175.
8. Christopher-Hennings J, Holler LD, Benfield DA, Nelson EA. Detection and duration of porcine reproductive and respiratory syndrome virus in semen, serum, peripheral blood mononuclear cells and tissues from Yorkshire, Hampshire, and Landrace boars. J Vet Diagn Invest. 2001;13:133-142.
9. Christopher-Hennings J, Nelson EA, Nelson JK, Benfield DA. Effects of a modified-live virus vaccine against porcine reproductive and respiratory syndrome virus. Am J Vet Res. 1997;58:40-45.
10. Prieto C, Suarez P, Bautista JM, Sanchez R, Rillo SM, Simarro J, Solana A, Castro M. Semen changes after experimental infection with porcine reproductive and respiratory syndrome (PRRS) virus. Theriogenology. 1996;45:383-395.
11. Prieto C, Garcia C, Simarro I, Castro JM. Temporal shedding and persistence of porcine reproductive and respiratory syndrome in boars. Vet Rec. 2004;154:824-827.
12. Federation of Animal Science Societies. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. 1st rev ed. Savoy, Illinois: Federation of Animal Science Societies; 1999.
13. Christopher-Hennings J, Nelson E, Nelson J, Rossow K, Shivers J, Yaeger M, Chase C, Garduno R, Collins J, Benfield D. Identification of porcine reproductive and respiratory syndrome virus in semen and tissues of vasectomized and non-vasectomized boars. Vet Path. 1998;35:260-267.
14. Wasilk A, Callahan J, Christopher-Hennings J, Gay T, Fang Y, Dammen M, Reos M, Torremorell M, Polson D, Mellencamp M, Nelson E, Nelson W. Detection of U.S., Lelystad, and European-like porcine reproductive and respiratory syndrome viruses and relative quantitation in boar semen and serum samples by real-time PCR. J Clin Microbiol. 2004;42:4453-4461.
15. Therneau T, Grambsch P. Modeling Survival Data: Extending the Cox Model. New York, New York: Springer; 2000.
16. Collet D. Modeling Binary Data. London, England: Chapman & Hall; 1991.
17. Pinheiro J, Bates D. Mixed-effects Models in S and S-Plus. New York, New York: Springer; 2000.
18. Mengeling WL, Wesley RD, Lager KM, Vorwald AC, Clouser DF. Effect of concurrent infections on persistence and shedding of porcine reproductive and respiratory syndrome virus and transmissible gastroenteritis virus. Swine Health Prod. 2002;10:67-73.
19. Gradil C, Dubuc C, Eaglesome MD. Porcine reproductive and respiratory syndrome virus: seminal transmission. Vet Rec. 1996;138:521-522.
20. Swenson SL, Hill HT, Zimmerman JJ, Evans LE, Landgraf JG, Wills RW, Sanderson TP, McGinley MJ, Brevik AK, Ciszewski DK, Frey ML. Excretion of porcine reproductive and respiratory syndrome virus in semen after experimentally induced infection in boars. JAVMA. 1994;204:1943-1948.
21. Christopher-Hennings J, Nelson EA, Benfield DA. Detecting porcine reproductive and respiratory syndrome virus in boar semen. Swine Health Prod. 1996;4:37-39.
* Non-refereed reference.