Case study |
Peer reviewed |
Concurrent pasteurellosis and classical swine fever in Indian pigs
Pasteurelosis concurrente y fiebre porcina clásica en cerdos de India
Pasteurellose et peste porcine classique rencontrées simultanément chez des porcs en Inde
H. Kumar, MVSc; V. Mahajan, MVSc; S. Sharma, MVSc; Alka, MVSc; R. Singh, MVSc; A. K. Arora, PhD; H. S. Banga, PhD; S. Verma, MVSc; Kamalpreet Kaur, MVSc; P. Kaur, MVSc; Meenakshi, MVSc; K. S. Sandhu, PhD
HK, VM, SS, A, RS, SV, KK, M, KSS: Department of Epidemiology and Preventive Veterinary Medicine, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, Punjab, India. AKA, PK: Department of Veterinary Microbiology, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, Punjab, India. HSB: Department of Veterinary Pathology, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, Punjab, India. Corresponding author: Dr Hardeep Kumar, Department of Epidemiology and Preventive Veterinary Medicine, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana-141004, Punjab, India; Tel: 91-161-2414030; E-mail: drhardeep@rediffmail.com
Cite as: Kumar H, Mahajan V, Sharma S, et al. Concurrent pasteurellosis and classical swine fever in Indian pigs. J Swine Health Prod. 2007;15(5):279–283.
Also available as a PDF.
SummaryOutbreaks of classical swine fever (CSF) occurred in four herds in Punjab state. All herds were located in the same geographical area, but were approximately 100 km apart. Overall morbidity, mortality, and case fatality rate were 88.2%, 77.5%, and 87.8%, respectively, in pigs ≤ 3 months of age, and 20.5%, 8.2%, and 40.0%, respectively, in older pigs. Clinical signs included high fever; erythema of the skin of the ears, abdomen, and medial thighs; and greenish watery diarrhea. Postmortem lesions observed were intestinal ulcers; congestion and multifocal hemorrhages of the spleen; enlarged, edematous, and hemorrhagic lymph nodes; and petechial hemorrhages on the kidneys. Subcapsular hemorrhages in kidneys and chronic necrotic enteritis were the significant histological lesions. Testing by agar gel immunodiffusion in all four outbreaks identified CSF virus antigen. Pasteurella multocida isolates from two o utbreaks were characterized morphologically and biochemically. Serotype B:2 was identified from both outbreaks by polymerase chain reaction using P multocida-specific primers (KMT1T7, KMT1 SP6) and B:2-type-specific primers (KTSP61, KTT72). Pneumonic lesions were more marked in cases from which P multocida was isolated. | ResumenEn el estado de Punjab ocurrieron brotes de fiebre porcina clásica (CSF por sus siglas en ingles) en cuatro piaras. Todas las piaras se encontraban en la misma área geográfica, pero estaban separadas aproximadamente por 100 km. Los índices totales de morbilidad, mortalidad, y los casos de muerte fueron de 88.2%, 77.5%, y 87.8%, respectivamente, en cerdos ≤ de 3 meses de edad, y 20.5%, 8.2%, y 40.0%, respectivamente, en cerdos mayores. Los signos clínicos incluyeron fiebre alta; eritema de la piel de las orejas, abdomen, y muslos mediales; y diarrea verde acusosa. Las lesiones observadas después de la muerte fueron úlceras intestinales; congestión y hemorragias multifocales del bazo; nódulos linfáticos aumentados de tamaño, edematosos, y hemorrágicos; y petequias en los riñones. Las hemorragias subcapsulares de los riñones y la enteritis necrótica crónica fueron las lesiones histológicas importantes. Al hacer la prueba de inmunodifusión en gel de agar en los cuatro brotes, se identificó el antígeno del virus CSF. Los aislamientos de Pasteurella multocida de dos brotes se caracterizaron morfológicamente y bioquímicamente. El serotipo B:2 se identificó de ambos brotes por medio de la a reacción en cadena de polimerasa utilizando los primers específicos del P multocida (KMT1T7, KMT1 SP6) y tipo B:2 (KTSP61, KTT72). Las lesiones de neumonía fueron más marcadas en los casos de donde se aisló la P multocida. | ResuméDes poussés de cas de peste porcine classique (CSF) se sont produites dans quatre troupeaux dans l’état du Punjab. Tous les troupeaux étaient situés dans la même région géographique, mais étaient distancés l’un l’autre d’environ 100 km. Les taux de morbidité globale, de mortalité, et de fatalité étaient respectivement de 88.2%, 77.5%, et 87.8% chez les porcs âgés de ≤ 3 mois, et de 20.5%, 8.2%, et 40.0%, respectivement, chez les porcs plus âgés. Les signes cliniques incluaient une fièvre élevée; un érythème de la peau des oreilles, de l’abdomen et de l’aspect médial des cuisses; de même qu’une diarrhée aqueuse verdâtre. Les lésions post-mortem observées incluaient des ulcères intestinaux; une congestion et des hémorragies multifocales au niveau de la rate; des ganglions lymphatiques hypertrophiés, oedémateux, et hémorragiques; et des pétéchies hémorragiques sur les reins. Des hémorragies rénales subcapsulaires et une entérite nécrotique chronique représentaient les lésions histologiques significatives. Une épreuve d’immunodiffusion en gélose effectuée lors des quatre épisodes a permis d’identifié le virus du CSF. Les isolats de Pasteurella multocida obtenus lors de deux des poussés de cas ont été caractérisés morphologiquement et biochimiquement. Le sérotype B:2 a été identifié lors des deux épisodes par réaction d’amplification en chaîne par la polymérase utilisant des amorces spécifiques à P multocida (KMT1T7, KMT1SP6) et des amorces spécifiques au type B:2 (KTSP61, KTT72). Les lésions de pneumonie étaient plus évidentes lors des cas à partir desquels on isolait également P multocida. |
Keywords: swine, classical
swine fever, Pasteurella multocida, pestivirus, pneumonic pasteurellosis
Search the AASV web site
for pages with similar keywords.
Received: September
25, 2006
Accepted: April
2, 2007
The swine industry in Punjab, currently in its infancy, is in the hands of people having little or no awareness about pig diseases. A livestock census conducted by the government of India in 1997 showed that the swine population in Punjab was 96,000.1 A second swine census conducted in 2003 identified a total of 29,000 animals, a decrease of 69.8%.1 This drastic decrease is of major concern. Causes may be multifactorial, including less acceptability of pork in the region, lack of awareness among the farmers and pig raisers regarding management practices, disease prevention, and control measures, and above all, a high incidence of fatal diseases, eg, classical swine fever (CSF) and pneumonic pasteurellosis.
Classical swine fever, caused by a member of the genus Pestivirus of the family Flaviviridae, affects pigs of all age groups. It is a devastating disease, associated with very high morbidity and mortality, mummification of fetuses, and abortions,2 and responsible for huge economic losses to pig farmers and pig raisers in India. The disease is worldwide in distribution and has been reported frequently from various parts of Punjab and other regions of India.3,4
Outbreaks of acute and subacute swine pasteurellosis have also been reported by various workers in India and abroad,5 but concurrent infection with CSF and pneumonic pasteurellosis has rarely been reported. In India, pneumonic pasteurellosis is usually caused by Pasteurella multocida type B, a strain prevalent both in swine and cattle.5,6 In contrast, P multocida types A and D have been isolated from North American swine,7 but are less prevalent in India. Pasteurella multocida type A causes bronchopneumonia7 and type D is a well-known cause of atrophic rhinitis.8 In this study, pneumonic pasteurellosis caused by P multocida type B occurred concurrently with CSF in two of four CSF outbreaks in Indian pigs.
Case description
Disease outbreaks were investigated in four swine herds in Punjab, India, located approximately 100 km apart. Herds were housed in relatively modern barns with concrete floors. Half of the nutritional requirement was met using a formulated ration and the rest consisted of vegetable waste and kitchen surplus. Owners of these herds had little knowledge of pig diseases, and the animals were not vaccinated against either CSF or pasteurellosis. There was no movement of human beings from one farm to the other, but pigs were continually purchased without prior monitoring or screening. All in-all out management was not used: animals of all age groups were housed together. Disease first appeared in piglets, causing high mortality, and later, older pigs were affected. Initially, there was a rise in body temperature to 42.2°C. Affected animals were dull, depressed, and anorectic, and exhibited a staggering gait. Greenish watery diarrhea was a prominent clinical sign observed 2 to 3 days after the onset of disease. The skin was highly erythemic, especially on the ears, abdomen, and medial thighs, and became cyanotic prior to death. Animals succumbed within 7 to 10 days. In the first and third outbreaks, labored respiration, coughing, and high respiratory rate were observed.
Animals that died were subjected to a full postmortem examination. Tissues for histological examination were collected into 10% buffered formalin, including lung, liver, spleen, kidney, lymph node, intestine, and brain.9 For virological studies, samples of spleen, lymph node, and kidney were aseptically collected into 50% glycerol saline. Samples were submitted to the Central Animal Disease Research and Diagnostic Laboratory (Indian Veterinary Research Institute, Izatnagar, Uttar Pradesh) for identification of the CSF virus. Samples were tested by agar gel immunodiffusion (AGID) using hyperimmune sera raised against CSF virus antigen.10 For bacterial isolation, heart blood and lung swabs were collected into nutrient broth. Pasteurella multocida isolates were obtained from the first and third outbreaks. In these cases, 0.5 mL of heart blood was inoculated intraperitoneally into Swiss albino mice for pathogenicity studies.
Mouse studies were performed in the Department of Veterinary Microbiology of Guru Angad Dev Veterinary and Animal Sciences University (Ludhiana, Punjab, India). The animal use protocol was approved by the Animal Ethics Committee of India. Isolation was attempted from visceral organs (lungs and heart) of mice that died after inoculation, and isolates obtained were subjected to morphological and biochemical analysis. Multiplex polymerase chain reaction (PCR) was performed using two sets of primers simultaneously: primers KMT1T7 and KMT1SP6, specific for P multocida, and primers KTSP61 and KTT72, specific for P multocida serotype B:2.11 The specificity of this PCR to detect serotype B:2 using these primers has been confirmed and validated in other laboratories.6
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS for Windows version 11.0.1; SPSS Inc, Chicago, Illinois). Overall mortality, morbidity, and case fatality rate for all four outbreaks were compared for pigs categorized as ≤ 3 months of age and > 3 months of age, and relative risk and odds ratios were calculated using the chi-square statistic.
Immunological and bacteriological test results
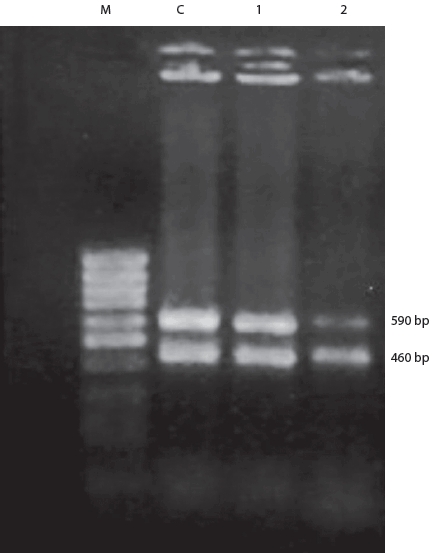
Results of the AGID were positive for lymph node, spleen, and kidney tissues, ie, CSF virus antigen was identified. Heart blood samples from the first and third outbreaks produced pure, small, circular, glistening, and dewdrop-like colonies on blood agar plates. All mice died within 24 hours of inoculation with heart blood collected from these two outbreaks, indicating a highly pathogenic organism, which was re-isolated from the heart blood and lungs of the mice. Smears made from the heart blood of mice and stained with methylene blue revealed bipolar organisms characteristic of P multocida. No other bacterial pathogens were isolated from the heart blood or lungs of pigs that died during the outbreaks. Polymerase chain reaction amplification produced two products, 460 bp and 590 bp, from the swine heart-blood isolates (Figure 1).
| Figure 1: Polymerase chain reaction amplification
showing two amplified products (460 bp and 590 bp) from heart blood bacterial
isolates collected during an outbreak of classical swine fever. Appearance
of 460 bp bands indicates that all isolates are Pasteurella multocida, whereas
590 bp bands identify P multocida serotype B. Lane M: molecular
marker; Lane C: positive control; Lanes 1 and 2: samples from outbreaks.
|
Morbidity, mortality, and case fatality rates in CSF outbreaks
Overall morbidity, mortality, and case fatality rate were much higher in young pigs (< 3 months) than in pigs > 3 months of age (Table 1). In pigs ≤ 3 months of age, risk of having the disease was 4.3 times higher than in pigs > 3 months old (chi square = 81.22; P < .001 at 95% confidence), and death rate was nine times higher (chi square = 81.64, P < .001).
Case fatality rate was significantly higher (chi square, P = .04) in the first and third outbreaks (mixed infections) than in the second and fourth outbreaks (classical swine fever alone) (Table 1). The highest fatality rates (> 90% in piglets ≤ 3 months of age) occurred in the first and third outbreaks, in which concurrent infection with P multocida and CSF was confirmed.
Table 1: Epidemiological data from four outbreaks of classical swine fever (CSF) occurring in Punjab, India
* Overall morbidity: Relative risk = 4.29 (95% CI, 2.72-6.78); Chi square uncorrected, 81.22; Mantel Haenszel, 81.22; Yates corrected, 78.43. † Mortality: Relative risk = 9.42 (95% CI, 4.35-20.43); Chi square uncorrected, 81.64; Mantel Haenszel, 81.17; Yates corrected, 78.89. ‡ Comparison of overall case fatality rate and risk of death in the first and third outbreaks (mixed infection of Pasteurella multocida and CSF) with second and fourth outbreaks (no Pasteurella multocida infection identified): P = .04, Chi square uncorrected = 4.19. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Necropsy and histological observations
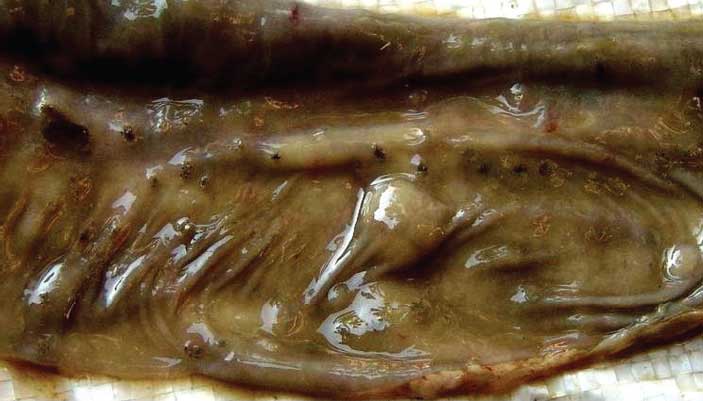
Grossly, cutaneous lesions were characterized by vascular changes, including hyperemia, edema, and cyanosis. Button ulcers, a characteristic lesion of CSF,12 were observed on the mucosal surface of the large intestine, ie, small circular necrotic areas (approximately 0.5 to 1.0 cm diameter) with raised edges and depressed centers (Figure 2). Mesenteric lymph nodes were enlarged, edematous, severely congested, and hemorrhagic. Petechial hemorrhages and pale infarcts gave the kidneys a turkey-egg appearance. Kidney pelvises were congested, and spleens were severely congested, enlarged, and dark red, with pale infarcts. Lungs were edematous, congested, and hemorrhagic. In the first and third outbreaks (concurrent CSF and P multocida infection), there was a layer of fibrin on the lungs, and cut sections revealed purulent exudate and froth.
| Figure 2: Section of caecum collected from a pig < 3
months of age during an outbreak of classical swine fever in Punjab, India,
showing small circular lesions (diameter approximately 0.5 cm) with the
appearance of button ulcers.
|
On histopathological sections, the intestinal mucosal epithelium had sloughed off in some areas. A heavy infiltration of mononuclear cells in the mucosa and submucosa formed necrotic areas. Infarcts and shrinkage of glomerular tufts were observed in the kidneys. Diffuse hemorrhages were observed in the spleen and lymph nodes. In the liver, an infiltration of mononuclear leukocytes was observed in the periportal areas. Brain sections revealed perivascular cuffing, particularly in the cerebrum. In the lungs, alveolar edema, hemorrhages, and infiltration of polymorphonuclear cells were observed. In cases from which PÂ multocida was isolated, findings typical of pneumonic pasteurellosis were observed. Pleura were thickened due to deposition of fibrin, edema, and infiltration of polymorphonuclear cells. Septae were thickened with fibrin, and this, combined with cellular infiltration and edema, imparted a marbled appearance to the lungs. Alveoli were filled with edematous fluid, erythrocytes, and polymorphonuclear cells.
Discussion
A higher CSF incidence and mortality rate (90% to 100%) has been reported in pigs ≤ 3 months of age than in older pigs (50%),3,13 indicating that young pigs are highly susceptible to CSF infection, probably because of the immature immune system. Age is therefore the most important risk factor associated with the severity of this disease.
Pasteurella multocida type B:2 is considered a commensal organism14 in the upper respiratory tract and tonsils and causes disease outbreaks in swine, cattle, buffalo, sheep, and goats under extreme environmental conditions, or in animals immunosuppressed by viral infections. This organism invades already damaged lungs15 and is the most common pathogen isolated from pigs housed under poor husbandry conditions, eg, overcrowding and poor ventilation.14 The high mortalities observed in the first and third outbreaks in this study reflect the role played by P multocida type B:2, invading already damaged lungs in animals immunosuppressed by the CSF virus. Hence, in these cases, CSF acted as a predisposing factor for establishment of pneumonic pasteurellosis, observed as coughing, lethargy, and respiratory distress.16,17
Many outbreaks have been recorded of acute swine pasteurellosis causing mortality in India.5 The same organism, P multocida type B:2, is responsible for hemorrhagic septicemia in dairy cattle and buffalo, and many outbreaks are reported every year from all over India,18 suggesting that this serotype is transmitted between bovine species and swine. Therefore, pigs that recover from the disease act as a reservoir of PÂ multocida type B:2 not only for other swine, but also for nearby dairy herds.
Pig farmers in this study had not vaccinated their pigs against CSF or hemorrhagic septicemia mainly because of a lack of awareness concerning the vaccines, despite the endemicity of both diseases. In addition, as these farmers were unaware of the requirement to report outbreaks of disease to the appropriate authorities, many outbreaks remained unchecked or unreported. These farmers may have encountered tremendous financial losses, which might have discouraged them from continuing to raise pigs, ultimately resulting in a decrease in the pig population in the state. Postmortem findings and pathological changes suggestive of CSF, and similar to those observed in these outbreaks, have also been reported by other workers.3,4,19 Thus, due to its highly contagious nature and high mortality rate, CSF may have played a major role in reducing the pig population of India since the previous animal census.1
Implications
- As young pigs are so severely affected by CSF, all in-all out management would be expected to protect against severe CSF outbreaks.
- Better vaccines are needed in India to control CSF.
References
1. 17th Indian Livestock Census. All India Summary Report. Livestock, Poultry, Machinery and Implements and Fishery Statistics – 2003. Available at: http://mospi.nic.in/mag_rept_pubn.htm. Accessed 30 June 2007.
2. Van Oirschot JT. Classical swine fever (hog cholera). In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames Iowa: Iowa State University Press. 1999:159–172.
3. Saini SS, Dhand NK, Sharma DR, Sood NK. An outbreak of swine fever in Punjab. Indian J Vet Pathol. 2000;24:135–136.
4. Bhattacharya A. An outbreak of swine fever in a quarantine pig farm in Tripura. Indian J Anim Health. 2001;40:85–86.
5. Murty DK, Kaushik RK. Studies on an outbreak of acute swine pasteurellosis due to Pasteurella multocida Type B (Carter, 1955). Vet Rec. 1965;77:411–416.
6. Arora AK, Virmani S, Jand SK, Oberoi MS. Isolation, characterization and antibiogram of Pasteurella multocida isolates from different animal species. Indian J Anim Sci. 2005;75:749–752.
7. Zhao G, Pijoan C, Murtaugh MP. Epidemiology of Pasteurella multocida in farrow-to-finish swine herd. Can J Vet Res. 1992;57:136–138.
8.Martineau-Doize B, Dumas G, Larochelle R, Frantz JC, Martineau GP. Atrophic rhinitis caused by Pasteurella multocida type D: Morphometric analysis. Can J Vet Res. 1991;55:224–228.
9. Luna LG, ed. Routine staining procedure. In: Manual of Histologic Staining Methods of the Armed Forces. 3rd ed. New York, New York: McGraw-Hill Book Company, Blakiston Division; 1968:32–39.
10. Ruckerbour GM, Appel M, Gray DP, Bannister GL, Boulanger P. Hog cholera II. Reliability of the agar double diffusion precipitation test for the differentiation of H.C. virus from other infectious agents in swine tissue. Can J Comp Med Vet Sci. 1965;29:157–163.
11. Townsend KM, Frost AJ, Lee CW, Papadimitrion JM, Dankins HJS. Development of PCR assay for species and type specific identification of Pasteurella multocida isolates. J Clin Micro. 1998;36:1096–1100.
12. Vegad JL, Katiyar AK, eds. Diseases caused by pestiviruses, swine fever. In: A Textbook of Veterinary Special Pathology (Infectious Diseases of Livestock and Poultry). 1st ed. Lucknow, India: Army Printing Press; 2001:28–32.
13. Barman NN, Nath AJ, Hazarika MP, Barman B, Thakuria, D. Outbreak of classical swine fever in regularly vaccinated pig herd. Ind J Comp Microbiol Infect Dis. 2003;24:89–90.
14. Aiello SE, ed. Merck Veterinary Manual. 6th ed. Whitehouse Station, New Jersey: Merck and Co Inc; 1998:509-512.
15. Pijoan C, Ochoa G. Interaction between a hog cholera vaccine strain and Pasteurella multocida in the production of porcine pneumonia. J Comp Pathol. 1978;88:167–170.
16. Glasser K. Untersuchungen uber die Schweineseuche mit besonderer Berucksichtigung ihrer Aetiologic und Pathologie [Studies on swine fever especially regarding etiology and pathology of the disease]. Deutsche Tierarztliche Wochenschrift. 1910;18:729–733.
17. Hutyra F. Zur Atiologie der Schweinepest und der Schweineseuche.Zeitshrift fur Infektionskrankheiten [About the etiology of swine fever]. Parasitare Krankheiten und Hygiene der Haustiere. 1907;2:281–309.
18. Kumar H, Sharma S, Mahajan V, Verma S, Arora AK, Kaur P, Sandhu KS. Pathology and PCR based confirmation of haemorrhagic septicemia outbreaks in bovines. Indian J Vet Pathol. 2006;30(1):5–8.
19. Choi C, Chae C. Glomerulonephritis associated with classical swine fever virus in pigs. Vet Rec. 2003;153:20–22.