Brief communication |
Peer reviewed |
Feral swine exposure to selected viral and bacterial pathogens in southern Texas
Exposición del cerdo salvaje a patógenos bacterianos y virales en el sur de Texas
Exposition de porcs sauvages à certains virus et bactéries pathogènes dans le sud du Texas
Tyler A. Campbell, MS, PhD; Randy W. DeYoung, MS, PhD; Erin M. Wehland, MS; Lon I. Grassman, MS, PhD; David B. Long, MS; Johanna Delgado-Acevedo, MS
TAC, DBL: United States Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, National Wildlife Research Center, Texas A&M University-Kingsville, Kingsville, Texas. RWDY, EMW, LIG, JDA: Caesar Kleberg Wildlife Research Institute, Texas A&M University-Kingsville, Kingsville, Texas. Corresponding author: Dr Tyler A. Campbell, National Wildlife Research Center, Texas A&M University-Kingsville, 700 University Boulevard, MSC 218, Kingsville, TX 78363; Tel: 361-593-2426; Fax: 361-593-4311; E-mail: tyler.a.campbell@aphis.usda.gov.
Cite as: Campbell TA, DeYoung RW, Wehland EM, et al. Feral swine exposure to selected viral and bacterial pathogens in southern Texas. J Swine Health Prod. 2008;16(6):312–315.
Also available as a PDF.
SummaryBlood samples were obtained from feral swine in Texas to determine the seroprevalence of selected pathogens. Exposures rates were 35%, 1%, and 1% for pseudorabies virus, Brucella, and porcine reproductive and respiratory syndrome virus, respectively. Simple modifications to enclosures may provide adequate biosecurity for swine at risk within this region. | ResumenSe obtuvieron muestras de sangre de cerdos salvajes en Texas para determinar la seroprevalencia de ciertos patógenos seleccionados. Los índices de exposición fueron de 35%, 1%, y 1% para Pseudorabia, Brucella, y síndrome reproductivo y respiratorio del cerdo respectivamente. Modificaciones sencillas en los corrales pueden proveer una bioseguridad adecuada para los cerdos en riesgo dentro de esta región. | ResuméDes prélèvements sanguins ont été obtenus de porc sauvage au Texas afin de déterminer la séro-prévalence d’agents pathogènes spécifiques. Les taux d’exposition étaient respectivement de 35%, 1%, et 1% pour le virus de la pseudo-rage, Brucella, et le virus du syndrome reproducteur et respiratoire porcin. Des modifications mineures aux enclos permettraient de fournir une protection bio-sécuritaire adéquate aux porcs à risque dans cette région. |
Keywords: swine, feral, Brucella, porcine
reproductive and respiratory syndrome, pseudorabies
Search the AASV web site
for pages with similar keywords.
Received: April 3, 2008
Accepted: May 21, 2008
Invasive feral swine (Sus scrofa) occur across much of the United States, where they often come into conflict with agricultural and livestock producers.1 Estimates of feral swine damage to ecosystems and agricultural resources exceed $200 per animal annually or approximately $800 million per year.2 Much of this damage is caused by the aggressive rooting activities of feral swine.1 Approximately 2 million feral swine exist within Texas alone, where opinions among farmers and ranchers regarding feral swine are largely negative.3
An additional source of conflict that is more difficult to quantify involves the role feral swine play in porcine disease ecology. For decades, feral swine have been recognized as reservoirs of swine diseases such as pseudorabies virus (PRV) and swine brucellosis (Brucella suis).4-6 These diseases are presently regarded as significant threats to US agriculture.7 This is particularly true because of the successful eradication in 2004 of PRV from domestic swine herds, and the potential for feral swine to transmit pathogens back into domestic populations. Consequently, disease surveillance activities involving feral swine have been intensified throughout the country in recent years.8-11
Herein, we report findings from a regional pathogen surveillance effort involving feral swine. Our objectives were to determine exposure rates of feral swine to PRV, Brucella, and porcine reproductive and respiratory syndrome virus (PRRSV) within nine counties in southern Texas and one county in central Texas. A previous report from counties in southern Texas during 1985 found PRV and Brucella exposure rates in feral swine of 0% to 85% and 0% to 31%, respectively.5 Consequently, we hypothesized that exposure of feral swine to these pathogens also would occur in these and surrounding unstudied counties, particularly given that PRV persists naturally in feral swine populations over long periods (≥ 25 years).10
Materials and methods
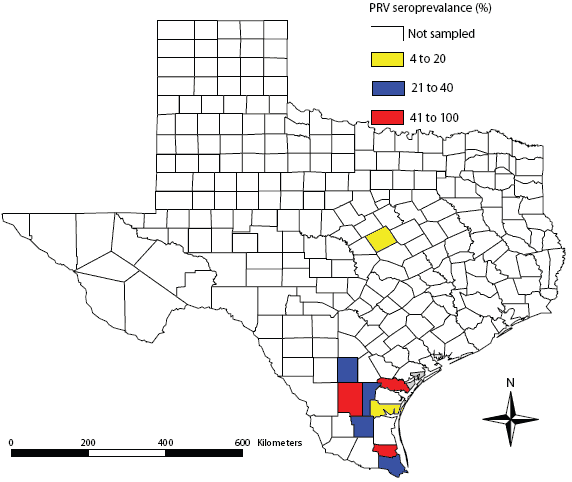
From May 2006 to June 2007, we obtained blood samples from feral swine collected as part of routine feral-swine damage-abatement activities in Aransas, Brooks, Cameron, Duval, Jim Wells, Kleberg, McMullen, San Patricio, and Willacy counties in southern Texas, and Coryell County in central Texas (Figure 1). We trapped swine using 2.5 × 1.2 × 1-m portable box traps with fermented corn as bait. Upon capture, swine were euthanized using humane methods,12 and a blood sample was collected from each animal via cardiac puncture. All procedures were approved by the Institutional Animal Care and Use Committee at the National Wildlife Research Center (Permit No. QA-1309).
| Figure 1: Geographic distribution of pseudorabies virus
(PRV) seroprevalence in feral swine from 10 counties in Texas
between May 2006 and June 2007.
|
Serum was separated by centrifugation and samples were stored at -20°C prior to analysis. We used the Pseudorabies Virus Antibody Test Kit (Viral Antigens Incorporated, Memphis, Tennessee), which uses latex agglutination, to test for antibodies against PRV; the buffered Brucella antigen card test (United States Department of Agriculture, Animal and Plant Health Inspection Service, Veterinary Services, Washington, DC) to test for antibodies against Brucella; and the Idexx 2XR enzyme-linked immunosorbent assay (HerdCheck PRRS 2XR Antibody Test Kit; Idexx Laboratories, Westbrook, Maine) to test for antibodies against PRRSV. For PRV, results were interpreted as positive or negative at a 1:4 dilution. For Brucella, results were interpreted as positive or negative using undiluted sera. For PRRSV, we considered animals positive if the sample:positive ratio was ≥ 0.4. Pathogen exposure rates were reported at the county level.
Results
We obtained samples from 387 feral swine from the nine southern Texas counties and 22 samples from feral swine from Coryell County in central Texas (Table 1). We found overall feral swine exposures rates to PRV of 35%, with peak seroprevalence occurring in San Patricio county (33 of 45 positive) and Willacy county (22 of 49 positive). We determined overall exposure rates to Brucella and PRRSV of 1% and 1%, respectively, with antibody detection only in feral swine from McMullen and Willacy counties.
Table 1: Summary of feral swine survey data for exposure to selected pathogens* in nine southern Texas counties and Coryell County in central Texas between May 2006 and June 2007
* Pseudorabies Virus Antibody Test Kit (Viral Antigens Incorporated, Memphis, Tennessee), using latex agglutination, was used to test for antibodies against pseudorabies virus (PRV); the buffered Brucella antigen card test (United States Department of Agriculture, Animal and Plant Health Inspection Service, Veterinary Services, Washington, DC) was used to test for antibodies against Brucella; and the Idexx 2XR enzyme-linked immunosorbent assay (HerdChek PRRS 2XR Antibody Test Kit; Idexx Laboratories, Westbrook, Maine) was used to test for antibodies against porcine reproductive and respiratory syndrome virus (PRRSV). For PRV, results were interpreted as positive or negative at a 1:4 dilution. For Brucella, results were interpreted as positive or negative using undiluted sera. For PRRSV, animals were considered positive if the sample:positive ratio was ≥ 0.4. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Discussion
The exposure rates of feral swine to PRV in this study were similar to others reported from across the United States. For example, feral swine PRV exposure rates were 35% in Florida,13 29% among adult swine on Ossabaw Island, Georgia,14 and 61% among adult swine in coastal South Carolina.9 Additionally, feral swine exposure rates to PRRSV in this study were also consistent with others reported from the United States. For example, exposure rates to PRRSV were 1% and 2% in Texas and Oklahoma, respectively.8,15 Additionally, antibodies against PRRSV have been detected in feral swine in Texas (3% prevalence)16 and Mississippi (1% prevalence).17 However, exposure rates for Brucella in this study were inconsistent with others reported from the United States. For example, feral swine exposure rates to Brucella were 18% in coastal South Carolina4 and 4% in California.18 Additionally, we found variability at the county level in exposure rates to all three pathogens. However, we detected antibodies against PRV in all counties sampled, with seroprevalence rates ranging from 5% to 100%. Interestingly, previous reports from Aransas and Cameron counties did not identify antibodies against PRV within feral swine populations,5 whereas we found exposure rates to be 7% and 31%, respectively. It is unclear if this increase in PRV seroprevalence is due to inadequate sampling in 1985 (13 and 11 samples were evaluated in Aransas and Cameron counties, respectively)5 or is indicative of PRV dissemination in this region. Likely modes of PRV spread include natural dispersal and artificial dispersal (eg, unauthorized releases) of PRV-positive feral swine. The Texas Animal Health Commission has strict regulations for the trapping, movement, and release of feral swine for hunting purposes, but clandestine releases are known to occur.
We detected antibodies against Brucella and PRRSV only in McMullen and Willacy counties, two counties from which feral swine exposure rates have not previously been reported. A prior study did not detect antibodies against Brucella with feral swine from Aransas and Cameron counties.5 Although we found antibodies against Brucella at comparatively low seroprevalence, we caution hunters to wear gloves when handling and processing carcasses to reduce the possibility of exposure to this debilitating zoonosis. Given that the population of feral swine in Texas is estimated at approximately 2 million animals, we acknowledge that we sampled only a small fraction of animals occurring in any one county and that we likely missed some Brucella- and PRRSV-positive swine because the proportion of animals sampled was small. Furthermore, because there are no reliable means of accurately determining feral-swine numbers, it is not possible to know the proportions of the feral-swine populations sampled within counties.
In Texas, there are approximately two times more feral swine than domestic swine. However, many of the domestic swine herds are transitional, small-scale operations, where domestic swine regularly come in direct contact with feral swine.16 In these situations, improved biosecurity measures may be needed to reduce the risk of pathogen transmission to domestic swine herds.19 Our pathogen exposure rates suggest that, in southern Texas, the greatest feral swine disease threat may be from PRV, though we also detected antibodies against Brucella and PRRSV. Feral swine are believed to transmit PRV to both feral and domestic swine venereally.20 Therefore, simple modifications to existing swine enclosures, such as adding two strands of electrified polywire21 or double fencing,20 may provide an adequate barrier to many domestic swine facilities at risk within this region.
Implications
- In southern Texas, exposure rates of feral swine to PRV are greater than exposure rates to Brucella or PRRSV, suggesting that PRV is a more common threat to domestic swine.
- Simple changes to domestic swine facilities could make them adequately secure against the threat of PRV transmission from feral swine.
- As feral swine now occur across North America, it is important that disease surveillance activities continue, particularly in areas near domestic swine facilities or within recently discovered feral populations with unknown histories of disease exposure.
Acknowledgements
We are grateful to Garrett R. Timmons and Matthew M. Reidy for field assistance. Financial support was provided by the United States Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, National Wildlife Research Center. We thank the Caesar Kleberg Wildlife Research Institute at Texas A&M University-Kingsville for providing logistical support. We thank numerous landowners for providing access for this survey, including the Texas Parks and Wildlife Department, United States Fish and Wildlife Service, United States Army, Rob and Bessie Welder Wildlife Foundation, and Texas A&M University-Kingsville.
References
1. Seward NW, VerCauteren KC, Witmer GW, Engeman RM. Feral swine impacts on agriculture and the environment. Sheep Goat Res J. 2004;19:34–40.
2. Pimentel D, Zuniga R, Morrison D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ. 2005;52:273–288.
3. Adams CE, Higginbotham BJ, Rollins D, Taylor RB, Skiles R, Mapston M, Turman S. Regional perspectives and opportunities for feral hog management in Texas. Wildl Soc Bull. 2005;33:1312–1320.
4. Wood GW, Hendricks JB, Goodman DE. Brucellosis in feral swine. J Wildl Dis. 1976;12:579–582.
5. Corn JL, Swiderek PK, Blackburn BO, Erickson GA, Theirmann AB, Nettles VF. Survey of selected diseases in wild swine in Texas. JAVMA. 1986;189:1029–1032.
6. Stoffregen WC, Olsen SC, Wheeler CJ, Bricker BJ, Palmer MV, Jensen AE, Halling SM, Alt DP. Diagnostic characterization of a feral swine herd enzootically infected with Brucella. J Vet Diagn Invest. 2007;19:227–237.
*7. Witmer GW, Sanders RB, Taft AC. Feral swine – are they a disease threat to livestock in the United States? Proc Wildl Damage Manage Conf. 2003;10:316–325.
8. Saliki JT, Rodgers SJ, Eskew G. Serosurvey of selected viral and bacterial diseases in wild swine from Oklahoma. J Wildl Dis. 1998;34:834–838.
9. Gresham CS, Gresham CA, Duffy MJ, Faulkner CT, Patton S. Increased prevalence of Brucella suis and pseudorabies virus antibodies in adults of an isolated feral swine population in coastal South Carolina. J Wildl Dis. 2002;38:653–656.
10. Corn JL, Stallknecht DE, Mechlin NM, Luttrell MP, Fischer JR. Persistence of pseudorabies virus in feral swine populations. J Wildl Dis. 2004;40:307–310.
11. Hartin RE, Ryan MR, Campbell TA. Distribution and disease prevalence of feral hogs in Missouri. Human-Wildl Confl. 2007;1:186–191.
12. AVMA Guidelines On Euthanasia. Schaumburg, Illinois: American Veterinary Medical Association; 2007.
13. van der Leek ML, Becker HN, Pirtle EC, Humphrey P, Adams CL, All BP, Erickson GA, Belden RC, Frankenberger WB, Gibbs EPJ. Prevalence of pseudorabies (Aujeszky’s disease) virus antibodies in feral swine in Florida. J Wildl Dis. 1993;29:403–409.
14. Pirtle EC, Sacks JM, Nettles VF, Roller EA III. Prevalence and transmission of pseudorabies virus in an isolated population of feral swine. J Wildl Dis. 1989;25:605–607.
*15. Lawhorn B. Texas Rolling Plains feral swine disease survey. Proc Nat Feral Swine Conf. 1999;1:124–127.
16. Wyckoff AC. Movements, habitats, interactions, and disease prevalence of feral swine in Texas [master’s thesis]. Kingsville, Texas: Texas A&M University-Kingsville; 2007.
17. Drew ML, Jessup DA, Burr AA, Franti CE. Serologic survey for brucellosis in feral swine, wild ruminants, and black bears of California, 1977 to 1989. J Wildl Dis. 1992;28:355–363.
*18. Craven MB, Rickard LG, Minnis RB, Rosypal A, Lindsay DS. Seroprevalence of selected infectious agents in Mississippi feral hogs (Sus scrofa). Proc Nat Conf Wild Pigs. Mobile, Alabama. 2006;12.
19. Amass SF, Clark LK. Biosecurity considerations for pork production units. J Swine Health Prod. 1999;7:217–228.
20. Romero CH, Meade PN, Shultz JE, Chung HY, Gibbs EP, Hahn EC, Lollis G. Venereal transmission of pseudorabies viruses indigenous to feral swine. J Wildl Dis. 2001;37:289–296.
21. Reidy MM, Campbell TA, Hewitt DG. Evaluation of electric fencing to inhibit feral pig movements. J Wildl Manage. 2008;72:1012–1018.
* Non-refereed references.