| Original research | Peer reviewed |
Cite as: Tucker AL, Widowski TM, Friendship RM. Associations between dental and oral conditions at weaning and future growth. J Swine Health Prod. 2010;18(2):68–74.
Also available as a PDF.
SummaryObjectives: To examine prevalence of abnormal dental and oral conditions in weaned piglets and associations with weight gain, and to determine if premolar eruption status remains stable within a population. Materials and methods: Study One: In February, March, and April of 2009, oral exams were performed on 735 piglets at weaning. Deciduous teeth were recorded as being erupted or not. Occlusion between premolars was noted. A superscript (or subscript) number indicated position within the maxilla (or mandible) of incisors, canines, and premolars (i, c, and p, respectively). Prevalences of tooth damage, oral lesions, and staining or caries were determined. Pig weights were recorded at weaning and 3 weeks later. Study Two: Premolar eruption and occlusion data from 2009 (208 piglets) were compared to 2007 data (180 piglets). Results: Study One: Eruption of p3 and p4 were positively associated with weight gain at 3 weeks post weaning (p3, P < .0001; p4, P = .048), with eruption of i1 showing a similar trend (P < .01). Dental caries or staining on i1 were negatively associated with weight gain (P < .05). Study Two: Dentition was more advanced at 2 weeks (p3, P < .01; p4, P < .05; occlusion of p3 and p4, P < .01), 3 weeks (p3, P < .01), and 4 weeks of age (occlusion of p3, p4, and p4, P < .01) in 2009 than in 2007. Implications: Tooth eruption and condition at weaning are associated with future weight gain. Herd premolar eruption and occlusion status changes over time. |
ResumenObjetivos: Examinar la prevalencia de problemas orales y dentales anormales en lechones destetados y las asociaciones con la ganancia de peso, y determinar si el estado de erupción premolar permanece estable en una población. Materiales y métodos: Estudio Uno: En febrero, marzo, y abril de 2009, se realizaron exámenes orales a 735 lechones al destete. Se registró si los dientes deciduos brotaron o no. Se registro la oclusión de premolares. Un número superíndice (ó subíndice) indicaba posición en el maxilar (ó mandíbula) de los incisivos, caninos y premolares (i, c, y p, respectivamente). Se determinó la prevalencia de dientes dañados, lesiones orales, y manchas ó caries. Se registró el peso de los cerdos al destete y 3 semanas después. Estudio Dos: Se comparó la información de oclusión y brote premolar de 2009 (208 lechones) con la información de 2007 (180 lechones). Resultados: Estudio Uno: El brote de p3 y p4 se asoció positivamente con la ganancia de peso a las 3 semanas post destete (p3, P < .0001; p4, P = .048), con el brote de i1 mostrando una tendencia similar (P < .01). La caries dental ó el manchado en i1 se asociaron negativamente con la ganancia de peso (P < .05). Estudio Dos: La dentición fue más avanzada a las 2 semanas (p3, P < .01; p4, P < .05; oclusión de p3 y p4, P < .01), 3 semanas (p3, P < .01), y 4 semanas de edad (oclusión de p3, p4, y p4, P < .01) en 2009 que en 2007. Implicaciones: El brote de dientes y su condición al destete se asocian con la ganancia de peso futura. El brote premolar del hato y el estado de la oclusión cambian con el tiempo. |
ResuméObjectifs: Examiner la prévalence de conditions dentaires et orales anormales chez des porcelets sevrés et les associations avec le gain de poids, et déterminer si le statut de l’éruption des prémolaires demeure stable à l’intérieur d’une population. Matériels et méthodes: Étude 1: En février, mars, et avril de 2009, des examens oraux ont été effectués sur 735 porcelets au moment du sevrage. Les dents temporaires ont été notées comme étant sorties ou non de même que l’occlusion entre les prémolaires. Un nombre avec un exposant (ou un indice) indiquait la position maxillaire (ou mandibulaire) des incisives, canines, et prémolaires (respectivement i, c, et p). Les prévalences de dommages aux dents, de lésions orales, et de taches ou de caries ont été déterminées. Le poids des animaux a été enregistré au moment du sevrage et 3 semaines plus tard. Étude 2: Les données d’éruption des prémolaires et d’occlusion pour l’année 2009 (208 porcelets) ont été comparées à celles de 2007 (180 porcelets). Résultats: Étude 1: L’éruption de p3 et p4 était associée positivement avec le gain de poids 3 semaines post-sevrage (p3, P < .0001; p4, P = .048), et l’éruption de i1 montrait une tendance similaire (P < .01). Les caries dentaires ou les taches sur i1 étaient associées négativement avec le gain de poids (P < .05). Étude 2: La dentition était plus développée à 2 semaines (p3, P < .01; p4, P < .05; occlusion de p3 et p4, P < .01), 3 semaines (p3, P < .01), et 4 semaines d’âge (occlusion de p3, p4, et p4, P < .01) en 2009 comparativement à 2007. Implications: L’éruption et la condition des dents au sevrage sont associées avec le gain de poids futur. Le statut d’éruption et d’occlusion des prémolaires dans un troupeau change dans le temps. |
Keywords: swine, dentition, oral health, teeth,
weaning
Search the AASV web site
for pages with similar keywords.
Received: August 17, 2009
Accepted: October 12, 2009
The oral and dental condition of commercial swine continues to be an overlooked area of research, even though studies indicate a high prevalence of deleterious oral conditions among market-age hogs and breeding-sow populations.1-5 Evidence also indicates significantly higher rates of culling among sows that have damaged or worn teeth, regardless of parity, presumably because of the discomfort that is associated with these conditions.5
Recent studies involving piglets have noted the occurrence of several dental and oral conditions that could affect both comfort and willingness to feed, including broken teeth, inflammation, and oral lesions.6,7 In one study, pronounced dental staining and dental caries (cavities) also affected the primary incisors of some individuals from every litter.8 Although histological examination of the affected dental tissue was not used to determine the nature of the staining, piglets were examined at regular intervals from birth onwards, suggesting that staining occurred prior to eruption and during tooth development (ie, was intrinsic). Given that both staining and early onset of dental caries have been linked to systemic developmental abnormalities and poor growth rates in human children,9-13 there is the question as to whether piglets displaying these characteristics may also be predisposed to poor health or suboptimal growth rates as they age. To date, the prevalence of dental staining and caries among piglets has not been reported.
Weaner pigs often face challenges in consuming sufficient feed after weaning, and normal tooth eruption has been shown to influence the development of feeding behavior.6 During the preweaning period, piglets < 17 days of age were inhibited from feeding when their premolars first erupted, while piglets 21 days of age and older with a more advanced dentition were more attracted to feed.6 When weaned at 28 days of age, those same piglets having a more advanced dentition did not perform more feeding behavior or have higher growth rates; however, the authors note that the postweaning husbandry conditions applied in that study were not typical of those used in commercial rearing and may therefore not adequately reflect industry norms.6
Although evidence exists for differences among populations in the age at which piglets achieve eruption and occlusion of their premolars,7 it is not yet known whether individual populations remain stable over time. This would be useful information for producers. Knowing the age at which the majority of piglets within a population develop the tools used for mastication of solid feed is beneficial for determining appropriate weaning ages.
Therefore, the two objectives of these studies were to examine the prevalences of abnormal dental and oral conditions in newly weaned piglets and determine whether they were associated with weight gain over the following 3 weeks, and to examine the eruption and occlusion status of weaned piglets to determine whether this remains consistent within a population over time.
Materials and methods
The University of Guelph Animal Care Committee approved the experimental protocols for both studies.
Study One
This study was conducted in February, March, and April of 2009 in a commercial 600-sow farrow-to-grow pig operation where Yorkshire-Landrace sows were crossed with Duroc boars. A total of 735 weaned piglets from 70 litters (345 gilts, 390 barrows) were examined across three replicates (weaning age, 14 to 27 days). Piglets had all erupted needle teeth clipped within 24 hours of birth and were processed (tail-docked, injected with iron, boars castrated) prior to 7 days of age. The day prior to weaning, all piglets were individually ear-tagged and weighed on an electronic scale accurate to 0.1 kg. All pigs were weighed again 3 weeks later.
At weaning, piglets were moved into three on-site nursery rooms, each containing eight pens, and were housed at a density of approximately 30 pigs per pen. All pens were equipped with plastic-coated slatted flooring, one three-hole stainless steel feeders, and two standard nipple drinkers.
Within 24 hours of piglets being moved into the nursery, a complete oral exam was performed on each piglet. Each individual was held in dorsal recumbency in a v-restrainer. Each piglet’s mouth was gently held open with a speculum to allow the researcher visual examination of all quadrants of the dental arches (right and left maxilla, right and left mandible). In addition, the gingiva, tongue, cheeks, and throat were also examined.
Deciduous incisors, canines, and premolars are referred to by a lowercase i, c, and p, respectively, with a superscript (or subscript) number indicating the tooth’s position within the maxilla (or mandible).8 For example, p3 is the third maxillary premolar while p4 is the fourth mandibular premolar. Eruption was considered to have occurred when any portion of the tooth crown had penetrated the gingiva.14
For each pig, every deciduous tooth within the oral cavity was recorded on a diagram as being erupted or not (Figure 1). Three measures of tooth condition were also recorded, including damage to either i1 or i1, the presence of oral lesions, and dental staining or the presence of caries on i1. Caries was diagnosed by visual examination of the dental surface and recorded on the basis of demineralization of the enamel and pitting or cavitation of the deeper dentinal tissues. Because both caries and staining were often seen together, with coloration often masking the carious lesions, these conditions were combined in a single category.
Figure 1: Deciduous dentition of the commercial pig. Reprinted with permission from the American Society of Animal Science.8 All incisors, canines, and premolars are referred to by a lowercase i, c, or p, respectively, with a superscript (or subscript) number indicating the position within the maxilla (or mandible). First premolars (p1, p1) often fail to erupt entirely.6 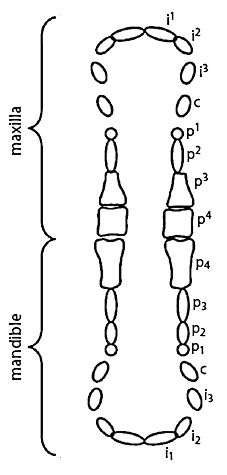 |
Study Two
To examine population changes over time for premolar eruption and occlusion, data from 2009 (Study One) were compared to data obtained from the same commercial farm in 2007. Tooth eruption, weaning weight, sow parity, number of live births, and number of stillbirths per litter were compared between time periods. As weaning age varied widely in 2009 (range, 14 to 27 days), data were utilized only from individuals that were within 1 day of 2, 3, or 4 weeks of age at the time of weaning, in order to be consistent with the data obtained in 2007.7 On the basis of this age requirement, data from a total of 388 piglets were used: 208 piglets from 2009 (104 gilts, 104 barrows) and 180 piglets from 2007 (89 gilts, 91 barrows).7 Management and feeding practices, herd genotype, experimental protocol, and weighing equipment remained constant between the time periods when piglets were examined. Examination of sow identification cards and farm records confirmed that piglets originating from the same sow were not compared between 2007 and 2009.
Statistical analysis
All data were analyzed using the Statistical Analysis System package version 9.1.3 (SAS Institute Inc, Cary, North Carolina). Data were formally examined for normality using the UNIVERIATE procedure, with comprehensive residual analyses being conducted to assess the ANOVA assumptions. No parameters were transformed prior to analysis. Analysis of covariance was performed using the MIXED procedure to assess the effects on piglet weight gain of dental condition (presence versus absence of teeth, occlusion versus no occlusion between premolars) and oral condition (presence versus absence of oral lesions, dental stains or caries, and broken incisors). Gender, weaning age, and weight at weaning were used as covariates in the model, as were sow parity, number of live-born piglets, and number of stillbirths. Pair-wise differences between means were assessed using t-tests.
To test whether any oral condition was indicative of a specific weight class or pattern of growth, chi-square (goodness of fit) tests were used. For both weaning weight and overall average daily gain (ADG), each piglet was assigned to one of three categories (small, medium, or large). Piglets designated as “large” weighed (or gained) 1 SD above the population mean (mean weaning weight, 7.48 ± 0.065 kg; mean ADG, 0.350 ± 0.004 kg per day; n = 106) while “small” piglets weighed (or gained) 1 SD below the mean (mean weaning weight, 3.10 ± 0.053 kg; mean ADG, 0.108 ± 0.004 kg per day; n = 111). All other piglets were considered “average,” with a mean weaning weight of 5.20 ± 0.034 kg and mean ADG of 0.223 ± 0.002 kg per day (n = 500).
To test whether the age at which piglets obtained premolar eruption (p3, p4, p3, p4) or premolar occlusion (between p3 and p4; between p3, p4, and p4) changed over time in the same population, a mixed model analysis of covariance using the GLIMMIX procedure was employed. Separate analyses were carried out for each of the three age groups (2-, 3- and 4-week weaning) with piglet gender, weaning weight, sow parity, number of live births, and number of stillbirths per litter being used as covariates. Pair-wise differences between years for piglet, sow, and litter variables were assessed using t-tests.
Results are presented as mean ± SE with P < .05 being considered statistically significant.
Results
Study One
During the study, seven piglets were euthanized for traumatic or progressive injuries and 11 were found dead. These animals were removed from all analyses. The mean age and weight for piglets at weaning were 21.50 ± 0.12 days (range, 14-27 days) and 5.25 ± 0.05 kg (range, 1.30-9.91 kg), respectively. Mean piglet weight 3 weeks after weaning was 10.13 ± 0.09 kg (range, 2.80-17.90 kg). Mean sow parity was 6.15 ± 0.14 (range, 1-16), mean number of live births per litter was 11.36 ± 0.12 (range, 3-18), and mean number of stillbirths per litter was 1.80 ± 0.06 (range, 0-7).
Overall, weight gain increased with increasing weaning age (P < .001) and decreased as the number of live-born piglets per litter increased (P < .001). The overall prevalence of abnormal oral and dental conditions, as well as eruption and occlusion status of all deciduous teeth at the time of weaning, are provided in Table 1. The percentage of piglets having one of the three reported abnormal conditions was 33.2%. The majority of piglets that were affected (29.7%) had staining and (or) caries, or had oral lesions, while 4.5% of the population had both. Few individuals within the examined population had broken incisors (prevalence rate, 1.25%). These individuals were often difficult to examine orally and displayed pain-responsive behaviors (eg, high frequency vocalizations) if contact was made with the affected tooth or neighboring teeth and gingiva. When present, fracturing was seen at all levels of the exposed tooth, with inflammation around the gingiva-tooth junction being present in most cases. One piglet had subgingival i1 breakage causing severe abscessing into the jaw that appeared to displace the normal positioning of the neighboring teeth. No piglets had all three abnormal conditions.
Table 1: Prevalence* in a commercial herd of deciduous tooth eruption, premolar occlusion, and abnormal oral and dental conditions at the time of weaning (14-27 days of age) and the effect of each condition on weight gain during the 3 weeks after weaning (Study One)
* Prevalence defined as the percentage of the weaner pig population displaying a particular dental or oral characteristic (N = 717). a Significant effect on weight gain at P < .001 (linear mixed model). b Significant effect on weight gain at P < .05 (linear mixed model). c Effect on weight gain at P < .10 (linear mixed model). |
For the eruption of teeth and premolar occlusion (Table 1), both p3 and p4 were positively associated with weight gain (p3, P < .001; p4, P = .048), with i1 showing a trend in this direction (P < .10). Piglets having the p3 erupted at weaning were 1.90 kg heavier after 3 weeks than piglets not having this premolar. Likewise, individuals with the p4 and ii erupted were 1.51 kg and 1.14 kg heavier, respectively, than their counterparts without eruption at weaning.
For the effect of abnormal dental and oral conditions on weight gain, only dental caries (or staining, or both caries and staining) on the i1 were negatively associated with future weight gain (P < .05). Piglets having one of these conditions at weaning were 0.56 kg lighter after 3 weeks than piglets without them. “Large” gaining piglets were also less likely to display staining compared to either “medium” or “small” gaining piglets (P < .01).
No abnormal condition was positively associated with weight gain.
Study Two
Mean weaning weight, sow parity, and numbers of live births and stillbirths per litter for the weaner pigs examined in 2007 and 2009 are presented in Table 2. The percentage of weaner pigs with various premolars erupted and occluded when weaned at 2, 3, and 4 weeks of age in 2007 and 2009 are presented in Table 3. More piglets in 2009 had eruption of p3 (P < .01) and p4 (P < .05) and occlusion between p3 and p4 (P < .01) in the 2-week age category. This pattern of earlier eruption in 2009 continued on for 3-week-old piglets in the eruption of p3 (P < .01). Though sample sizes were small for the 4-week age category, more piglets in 2009 had occlusion between three premolars (p3, p4, and p4)(P < .01). As only nine piglets (originating from one litter) were weaned at the 4-week age category in 2009, analysis between years for this age category should be viewed with caution.
Table 2: Mean piglet weaning weight, sow parity, and litter parameters* for piglets weaned at 2, 3, and 4 weeks of age in a commercial herd during 2009 and 2007 (Study Two)
* Data presented as mean ± SE. Piglets were within 1 day of 2, 3, or 4 weeks of age at weaning. † All piglets originated from a single litter. abc Values within a row with different superscripts are significantly different (P < .05; t-test). |
|||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 3: Percentages of piglets with various premolars erupted and occluded in a commercial herd during 2009 and 2007* (Study Two)
* Differences between years for tooth eruption and occlusion were analyzed for each age category using a mixed model analysis of covariance (GLIMMIX) with piglet gender, weaning weight, sow parity, number of live births, and number of stillbirths per litter being used as covariates. Piglets were within 1 day of 2, 3, or 4 weeks of age at weaning. Abbreviations for names of deciduous teeth are provided in Figure 1. † No. of piglets examined during 2009 and 2007 = 60 and 60, respectively. ‡ No. of piglets examined during 2009 and 2007 = 139 and 60, respectively. ¶ No. of piglets examined during 2009 and 2007 = 9 and 60, respectively, with all of the 2009 piglets from a single litter. NA = not applicable. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Discussion
Dental eruption and oral health are unique areas of research within the swine industry and are likely to provide novel insights into the growth, health, and development of individuals. Although body weight is the preferred measure for growth, one may argue that teeth provide a more stable marker because their development is more resistant to nutritional disruptions as compared to other tissues (ie, fat, muscle, bone).15,16 Dental eruption provides an interesting area of investigation, particularly when examining the potential growth of an individual early in its development. Possible mechanisms underlying the relationship between growth and dental eruption include enhanced feeding efficiency, improved digestion after a more thorough mechanical breakdown of food, and underlying genetic linkages. Our lack of knowledge in all three of these areas suggests more research should be carried out on the fundamental development of teeth in pigs and their synergistic role in food intake, mastication, and digestion.
One study examining dental eruption from birth through to 5 weeks of age found strong associations between birth weight, weight gain, and deciduous dental eruption.8 However, another study conducted on a noncommercial research farm with Yorkshire piglets weaned at 28 days of age found no associations between dentition at weaning and weight gain over the next 3 and 7 days.6 In contrast, the current study was able to demonstrate a significant difference in weight gain during the first 3 weeks after weaning. One possible explanation for these differing results is the age at which piglets were weaned. As only piglets weaned at 28 days were examined in the previous study, it may be that dental eruption is more important for piglets weaned at younger ages. Alternatively, the effect of dentition on growth may simply take longer to be realized after weaning. It should also be noted that these two studies differed with regard to herd genotypes, feeding management, and herd-health status.
In this study, only the eruption of certain teeth (p3 and p4) was associated with weight gain, and this may reflect the functional role of these teeth for animals at weaning. Alternatively, it may reflect the fact that these teeth are better indicators of growth rate or growth potential. The previous study by Tucker et al6 also found the eruption of these premolars to be influential on feeding behavior, along with the occlusion of p3 and p4, though only during the preweaning period. One reason that occlusion may not have been associated with weight gain in the current study could be that the population was highly skewed towards already having this dental condition (91.77% of piglets had occlusion at weaning). Further investigations to determine why the eruption of specific teeth (p3 and p4) are associated with weight gain and feeding development are warranted.
In addition to determining the tooth eruption status of a typical commercial weaner population, this is the first study to the authors’ knowledge that demonstrates an association between the condition of deciduous teeth and future weight gain in any agricultural species. It is also the first to report on the prevalence of oral lesions, broken incisors, dental caries, and dental staining in weaner pigs. It should be noted that all piglets examined in this study had previously had their teeth clipped, and so the effect of earlier dental trauma on the measured dental conditions are unknown. The possibility that the incisor breakage or oral lesions seen in this study were caused by routine teeth clipping is conceivable, but should be investigated with further comparative studies.
That we found a negative association between the presence of staining or caries and weaner-pig weight gain suggests that these conditions may reflect a reduced capacity for growth. Although not performed in the current study, quantifying the degree of staining or caries could provide a means of estimating future growth differences between individuals.
In humans, dental staining is classified as being intrinsic or extrinsic in nature, depending on its depth of penetration within the tooth.17,18 Intrinsic discoloration involves the incorporation of chromogenic or hematological agents into the enamel or dentine matrix during development, illness, or trauma.19,20 Most forms of intrinsic staining are pre-eruptive in that they occur prior to the eruption of the tooth (eg, fluorosis, enamel hypoplasia, dentinogenesis imperfecta, amelogenesis imperfecta, tetracycline staining). However, hemorrhaging of the pulp chamber and resultant trapping of blood within the dental tubules may occur at any age by way of dental trauma.17,18 Extrinsic stains, which can be caused by chromogenic agents, metal salts, or cationic antiseptics (such as chlorhexidine), reside within either the dental pellicle (protein film covering the enamel surface) or the dental plaque, and therefore develop only after the eruption of the tooth.21-23 As piglets were examined well after birth in this study, with only i1 being formally examined for stains, the etiology cannot be conclusively determined as belonging to one category or the other; however, it was casually noted that nearly all teeth erupting later in sequence to i1 were not stained. As the deciduous dentition of piglets begins developing in utero, with needle teeth (c1, c1, i3, i3) being fully erupted at birth and i1 erupting shortly thereafter,8 it logically follows that differences in these teeth between littermates at birth would reflect differences relating to genotype or environmental conditions in utero. As similar staining within these same teeth have previously been reported in newborn piglets, the possibility that this condition was caused by any chemical agent within the farrowing crate or from blunt trauma appears low, particularly when the physical integrity of the teeth and gingiva appeared normal. It therefore seems likely that the staining seen in this study was intrinsic in nature and reflects developmental differences among littermates.8 To the authors’ knowledge, no study has previously examined the nature and extent of these prenatal variations as they relate to dental condition for any agricultural species.
Dental caries is a chronic and infectious disease caused by cariogenic bacteria that accumulate on dental plaque.12 Microbial diversity as identified by real-time polymerase chain reaction indicate that over 50% of the species present on human teeth with dental caries were Lactobacillus species,24 though Streptococcus mutans strains are also highly prevalent with the disease.12 In addition to the eight serotypes of Streptococcus mutans recognized, recent studies examining the oral microflora of domestic pigs have discovered a novel Streptococcus mutans serotype specific to swine.25,26 Given that sow culling rates remain high within the industry, and these rates are known to be higher if degenerative oral conditions are present,5 research efforts should be made to examine factors associated with oral health across the lifespan of the animal. Interestingly, the passive transfer of immunity against dental caries from mother to offspring has been documented via the ingestion of colostrum and milk in rats,27 suggesting an early nutritional role for disease prevention in mammals. It should also be mentioned that only gross visual examination of the smooth surfaces of the i1 were used in the diagnosis of caries in this study. Carious lesions on neighboring teeth, or other types of caries more difficult to view because of location, such as proximal caries or root caries, may have remained undiagnosed.
In human children, numerous studies have linked dental caries with suboptimal growth.13,28,29 Three possible underlying mechanisms to explain this association include decreased growth from a reduced quality of life from pain and discomfort;30 reduced food intake;13 and chronic inflammation affecting metabolic pathways.31 In the current study, we examined only the i1 for caries, as the prevalence of carries in these teeth was previously reported.8 Interestingly, human infants have a higher prevalence of caries in these teeth than in their remaining deciduous dentition.32 The terms “baby-bottle caries” or “early childhood caries” are often used to describe this phenomenon, as it is believed to develop from children falling asleep with high-glucose-containing substances in their mouths. As piglets often fall asleep while still attached to their dam’s teat, a similar process of cariogenic accumulation may be occurring on the surface of their teeth.
Although it has been previously shown that different weaner populations display variation in eruption times, this is the first example where the same population has been seen to change over time. The reasons for this variation remain unclear. Though the animals examined in 2009 originated from the same genetic pool as those examined in 2007, the same dams were not used to produce the piglets in both years. Additionally, herd-health status did change over that time period with the introduction of a novel strain of porcine reproductive and respiratory syndrome (PRRS) virus. Although the producer reported herd-wide infection and clinical signs in his animals, neither the piglets examined in this study nor their dams were tested for viremia. Consequently, it is difficult to say if this change in health status could have influenced the changes seen in dental eruption. As PRRS is associated with increased secondary bacterial disease in weaned pigs,33 any resultant increases in morbidity within the population would be expected to slow growth and possibly delay tooth eruption.
Regardless of why more piglets achieved earlier eruption and occlusion than had previously been reported, the fact that this shift occurred may present an interesting avenue for geneticists and producers. The possibility of selecting for earlier eruption times may prove beneficial, given that piglets are known to be more interested in feed after premolar eruption has occurred.6 However, the ability to genetically influence eruption times has yet to be established.
Implications
- Piglets with stains or caries on their incisor teeth at weaning grow more slowly than unaffected pigs during the 3 weeks following weaning.
- Piglets with erupted p3 and p4 at weaning have higher growth rates during the 3 weeks following weaning.
- Premolar eruption and occlusion status can change over time on the same commercial farm, providing potential opportunities to select for earlier eruption.
Acknowledgements
We gratefully acknowledge financial support from West Penetone Inc and the University of Guelph-Ontario Ministry of Agriculture Sustainable Production Systems Research Program. We also wish to thank the producer for allowing us access to his pigs, and those individuals who assisted with data collection (Aleksandar Jovanovic, Glen Cassar, and Bryan Bloomfield).
References
1. Samuel JL, Woodall PF. Periodontal disease in feral pigs (Sus scrofa) from Queensland, Australia. J Wildl Dis. 1988;24:201–206.
2. Penny RH, Mullen PA. Atrophic rhinitis of pigs: abattoir studies. Vet Rec. 1975;96:518–521.
3. Davies ZE, Guise HJ, Penny RHC, Sibly RM. Effects of stone chewing by outdoor sows on their teeth and stomachs. Vet Rec. 2001;149:9–11.
4. Taylor IA. Design of the Sow Feeder: A Systems Approach [PhD thesis]. Urbana, Illinois: University of Illinois at Urbana-Champaign; 1990.
*5. Johnson EW. Sow Dentistry 101. National Hog Farmer. Available at: http://nationalhogfarmer.com/mag/farming_sow_dentistry/. Accessed 17 August 2009.
6. Tucker AL, Duncan IJH, Millman ST, Friendship RM, Widowski TM. The effect of dentition on feeding development in piglets and on their growth and behavior after weaning. J Anim Sci. In press.
7. Tucker AL. Dental Ontogeny and Its Relationship to Feeding and Abnormal Behaviour [PhD thesis]. Guelph, Ontario, Canada: University of Guelph; 2009.
8. Tucker AL, Widowski TM. Normal profiles for deciduous dental eruption in domestic piglets: Effect of sow, litter and piglet characteristics. J Anim Sci. 2009;87:2274–2281.
9. Bhat M, Nelson KB. Developmental enamel defects in primary teeth in children with cerebral palsy, mental retardation, or hearing defects: A review. Adv Dent Res. 1989;3:132–142.
10. Hall RK. The prevalence of developmental defects of tooth enamel (DDE) in a pediatric hospital department of dentistry population (Part I). Adv Dent Res. 1989;3:114–119.
11. Ziskin DE, Siegel EH, Loughlin WC. Diabetes in relation to certain oral and systemic problems: Part I: Clinical study of dental caries, tooth eruption, gingival changes, growth phenomena, and related observations in juveniles. J Dent Res. 1944;23:317–331.
12. Alvarez JO, Navia JM. Nutritional status, tooth eruption, and dental caries: a review. Am J Clin Nutr. 1989;49:417–426.
13. Acs G, Shulmann R, Ng MW, Chussid S. The effect of dental rehabilitation on the body weight of children with early childhood caries. Pediatr Dent. 1999;21:109–113.
14. Smith BH. Sequence of emergence of the permanent teeth of Macaca, Pan, Homo, and Australopithecus: Its evolutionary significance. Am J Hum Biol. 1994;6:61–76.
15. Garn SM, Lewis AB, Blizzard RM. Endocrine factors in dental development. J Dent Res. 1965;44:243–258.
16. Garn SM, Lewis AB, Kerewsky RS. Genetic, nutritional, and maturational correlates of dental development. J Dent Res. 1965;44:228–242.
17. Watts A, Addy M. Tooth discolouration and staining: a review of the literature. Br Dent J. 2001;190:309–316.
18. Nathoo SA, Gaffar A. Studies on dental stains induced by antibacterial agents and rational approaches for bleaching dental stains. Adv Dent Res. 1995;9:462–470.
19. Shafer WG, Hine MK, Levy BM. A Textbook of Oral Pathology. 4th ed. Philadelphia, Pennsylvania: W. B. Saunders Co; 1983:581.
20. Feinman RA, Goldstein RE, Garber DA. Bleaching Teeth. Chicago, Illinois: Quintessence Publishing; 1987:18–33.
21. Eriksen HM, Gjermo P. Incidence of stained tooth surfaces in students using chlorhexidine dentifrices. Scand J Dent Res. 1973;81:533–537.
22. Addy M, Moran J, Griffiths AA, Wills-Wood NJ. Extrinsic tooth discoloration by metals and chlorhexidine. 1. Surface protein denaturation or dietary precipitation? Br Dent J. 1985;159:281–285.
23. Harris NO. Dentifrices, mouth rinses and oral irrigators. In: Harris NO, Christen AG, eds. Primary Preventive Dentistry. Norwalk, Connecticut: Appleton and Lange; 1991:141–147.
24. Chhour K-L, Nadkarni MA, Byun R, Martin FE, Jacques NA, Hunter N. Molecular analysis of microbial diversity in advanced caries. J Clin Micro. 2005;42:843–849.
25. Takada K, Igarashi M, Yamaguchi Y, Hirasawa M. New serotype of mutans streptococci isolated from pig oral cavity. Microbiol Immunol. 2008;52:64–68.
26. Takada K, Hirasawa M. Streptococcus orisuis sp. nov. isolated from pig oral cavity. Int J Syst Evol Microbiol. 2007;57:1272–1275.
27. Michalek SM, McGhee JR. Effective immunity to dental caries: Passive transfer to rats of antibodies to Streptococcus mutans elicits protection. Am Soc Micro. 1977;17:644–650.
28. Acs G, Lodolini G, Kaminski S, Cisneros GJ. Effect of nursing caries on body weight in a pediatric population. Pediatr Dent. 1992;14:302–305.
29. Miller J, Vaughan-Williams SE, Furlong R, Harrison L. Dental caries and children’s weights. J Epidemiol Community Health. 1982;36:49–52.
30. Reisine ST. The impact of dental condition on social functioning and quality of life. Annu Rev Public Health. 1988;9:1–19.
31. Sheiham A. Dental caries affects body weight, growth and quality of life in pre-school children. Br Dent J. 2006;201:625–626.
*32. American Dental Association. Early childhood tooth decay (baby bottle tooth decay). Available at: http://www.ada.org/public/topics/decay_childhood_faq.asp. Accessed 1 June 2009.
33. Stephenson GW, VanAlstine WG, Kanitz CL, Keffaber KK. Endemic porcine reproductive and respiratory syndrome virus infection of nursery pigs in two swine herds without concurrent reproductive failure. J Vet Diagn Investig. 1993;5:432–434.
*Non-refereed references.
