| Original research | Peer reviewed |
SummaryObjectives: To compare the effects of porcine circovirus type 2 (PCV2) vaccination on growth rate, backfat depth, and loin depth of pigs in a high-health herd which contained different genetic lines. Materials and methods: A total of 454 pigs (20.6 ± 1.98 days of age; 6.1 ± 1.27 kg body weight) were used in a 130-day randomized controlled field trial. Genetic designations were A×A (Duroc line), B×B (synthetic White Pietrain line), A×B, and B×A. Pigs were randomly assigned to vaccination treatments (Vaccinated or nonvaccinated Control) within litter by gender (boar or gilt). Vaccinated pigs received two doses of a PCV2 vaccine at 3 and 5 weeks of age. Serum samples were collected and pigs were individually weighed on Days 0 (weaning), 40, and 130 to determine PCV2 viral load, antibody levels, and average daily gain (ADG). Data were analyzed from 417 pigs with complete growth records. Results: The greater ADG from Day 0 to Day 130 for Vaccinated pigs depended on the genetic line (genetic line-by-vaccination interaction; P < .05). The mean weight difference between Vaccinated and Control pigs was almost four times greater in the A×A pigs than in the B×B pigs on Day 130. On average, compared with Control pigs, Vaccinated pigs had lower serum PCV2 viral load levels on Days 40 and 130 (P < .001). Implications: Pig genetic line affects growth rate response to PCV2 vaccination and should be considered a risk factor for circoviral disease expression. | ResumenObjetivos: Comparar los efectos de la vacunación contra circovirus porcino tipo 2 (PCV2 por sus siglas en inglés) en el índice de crecimiento, profundidad de grasa dorsal, y la profundidad del lomo de cerdos en una piara de alta salud que contenía diferentes líneas genéticas. Materiales y métodos: Un total de 454 cerdos (20.6 ± 1.98 días de edad; 6.1 ± 1.27 kg de peso corporal) se utilizaron en una prueba de campo de 130 días, controlada al azar. Las elecciones genéticas fueron A×A (línea Duroc), B×B (línea sintética Pietrain Blanco), A×B, y B×A. Los cerdos se asignaron a tratamientos de vacunación al azar (Vacunados ó Control no vacunados) dentro de la camada por género (macho ó hembra). Los cerdos vacunados recibieron dos dosis de una vacuna de PCV2 a las 3 y 5 semanas de edad. Se recolectaron muestras de suero y se pesaron los cerdos individualmente los Días 0 (destete), 40, y 130 para determinar la carga viral de PCV2, los niveles de anticuerpos, y la ganancia diaria promedio (ADG por sus siglas en inglés). Se analizó la información de 417 cerdos con registros de crecimiento completos. Resultados: Los días de mayor ADG, 0 a 130, en los cerdos Vacunados dependió de la línea genética (línea genética por interacción de vacunación; P < .05). La diferencia de peso promedio entre cerdos Vacunados, y Control fue casi cuatro veces mayor en los cerdos A×A que en los B×B en el Día 130. En promedio, comparados con los cerdos Control, los cerdos Vacunados tuvieron niveles más bajos de carga viral de PCV2 en suero los Días 40 y 130 ( P < .001). Implicaciones: La línea genética del cerdo afecta la respuesta del índice de crecimiento a la vacunación contra PCV2 y debería ser considerada como un factor de riesgo para la expresión de la enfermedad circoviral. | ResuméObjectifs: Comparer les effets de la vaccination contre le circovirus porcin de type 2 (PCV2) sur le taux de croissance, l’épaisseur du gras dorsal, et la surface de la longe dans un troupeau porcin à statut sanitaire élevé constitué de différentes lignées génétiques. Matériels et méthodes: Un total de 454 porcs (20.6 ± 1.98 jours d’âge; 6.1 ± 1.27 kg de poids corporel) ont été inclus dans un essai clinique aléatoire d’une durée de 130 jours. Les appellations génétiques étaient A×A (lignée Duroc), B×B (lignée Piétrain Blanc synthétique), A×B, et B×A. Les porcs ont été répartis de manière aléatoire aux groupes de traitement (Vacciné et Témoin non-vacciné) à l’intérieur des portées par genre (verrat ou cochette). Les porcs vaccinés ont reçu deux doses d’un vaccin PCV2 à 3 et 5 semaines d’âge. Des échantillons de sérum ont été obtenus et les porcs pesés individuellement aux Jours 0 (sevrage), 40, et 130 afin de déterminer la charge virale de PCV2, les titres d’anticorps, et le gain moyen quotidien (GMQ). Les résultats provenant de 417 porcs avec des données complètes de production ont été analysées. Résultats: Le GMQ le plus élevé pour les Jours 0 à 130 pour les porcs Vaccinés montrait une dépendance en fonction de la lignée génétique (interaction de la lignée génétique par la vaccination; P < .05). La différence de poids moyenne entre les porcs Vaccinés et Témoins était presque quatre fois plus grande chez les porcs A×A comparativement aux porcs B×B au jour 130. Comparativement aux porcs Témoins, les porcs Vaccinés avaient en moyenne des charges virales sériques de PCV2 inférieures aux Jours 40 et 130 ( P < .001). Implications: La lignée génétique des porcs affecte le taux de croissance suite à une vaccination contre PCV2 et devrait être considérée comme un facteur de risque dans l’expression de la maladie à circovirus. |
Keywords: swine, genetic line, growth, porcine circovirus type 2, vaccine
Search the AASV web site
for pages with similar keywords.
Received: December 3, 2010
Accepted: May 20, 2011
The etiologic agent of porcine circovirus associated disease (PCVAD)1 is porcine circovirus type 2 (PCV2). Risk factors for the development of PCVAD include concurrent viral or bacterial infections, management factors,2 and gender, as well as genetic background. In one study, Landrace pigs were at increased risk for developing clinical PCVAD when compared with Duroc or Large White pigs.3 In another study, there were differences in postweaning mortality between pigs from Pietrain, Large White × Pietrain, and Large White × Duroc lines, with many of the pigs that died having lesions consistent with PCVAD.4 In contrast, yet another study failed to detect differences in PCVAD-attributed mortality when comparing offspring sired by either Pietrain boars or boars less than 50% Pietrain.5 The results of these studies support speculation that varying degrees of genetic susceptibility to PCV2 infection or expression of PCVAD may exist. Widespread availability of circovirus vaccines, documented to be effective in reducing mortality and increasing pig growth rate,6 has promoted research efforts to determine the consistency and the magnitude of the effect of circovirus immunization. The limited reports of interaction between genetic background and PCVAD expression provoke questions regarding the impact of response to circovirus immunization between differing genetic lines. The focus of this study was to compare the effects of PCV2 vaccination on growth rate, backfat depth, and loin depth of pigs in a high-health herd which contained different genetic lines. Secondary measurements included serum PCV2 viral load and PCV2 antibody titers.
Materials and methods
Procedures used in this field trial were approved by the Kansas State University Institutional Animal Care and Use Committee.
This 130-day randomized controlled field trial was performed in a 1700-sow genetic multiplication farm in Kansas. Boars and gilts in this farm were routinely placed on growth tests and the data collected were used to add information to the genetic selection indexes. This farm was of a high-health status, being negative for porcine reproductive and respiratory syndrome virus and without clinical or diagnostic evidence of Mycoplasma hyopneumoniae infection since stocking in 2000. In 2006, an increase in morbidity characterized by ill-thrift was observed. Histopathology lesions and gross clinical lesions consistent with PCVAD were documented, and immunohistochemistry staining for PCV2 antigen and polymerase chain reaction (PCR) differentiation of the PCV2 genotype confirmed the presence of PCV2b as a result of natural exposure and infection.
Prior to the start of the trial, the farm had not been vaccinating pigs against PCV2. As vaccines were novel at the time of the study, limited data were available on the magnitude of the effects of vaccination. Therefore, the sample size for this study was determined to be the largest sample size that could be tested with available resources.
In addition, the primary objective was to characterize the effects of PCV2 vaccination on growth rate and carcass composition. As the trial was performed in a genetic multiplication farm, with the expectation that genetic line affects these responses, the various genetic lines were also accounted for in the trial design.
This study utilized pigs born over a 7-day period. Each pig was weighed at birth and an individual ear tag with a unique number was applied by farm staff. Allotment to vaccination treatments was performed using the birth weight data.
Pigs were assigned within litter by gender to PCV2-vaccinated (Vaccinated) and nonvaccinated (Control) treatments by one investigator (SSD). Prior to weaning, pigs were ranked by birth weight within litter by gender (gilt or boar). The numbers of Vaccinated and Control pigs per litter were sequentially allocated for litters within a genetic line that had an odd number of pigs per gender by randomly selecting the first litter to have one more pig assigned to either the Control or Vaccinated treatment. The remaining odd numbers per litter and gender were alternately assigned to Control or Vaccinated treatment. A list of the appropriate numbers of Control and Vaccinated pigs for each gender within litter was generated and each treatment was assigned a random number using Excel (Microsoft Corporation, Redmond, Washington). The treatments were then sorted within gender and litter on the basis of the random number. This random order was then assigned to the ranking of birth weight by litter within gender.
A total of 454 pigs (20.6 ± 1.98 days of age; 6.1 ± 1.27 kg body weight) representing 55 litters from the four genetic populations were entered into the study at weaning and allocated to the previously assigned vaccination treatment (Vaccinated or Control). Genetic designations were pure lines of A×A (Duroc line) and B×B (synthetic White Pietrain line) and crossbreds A×B (Duroc sire × synthetic White Pietrain dam) and B×A (synthetic White Pietrain sire × Duroc dam). The PCV2 vaccine administered to pigs assigned to the Vaccinated treatment was a killed, 2-dose vaccine (Circumvent PCV; Intervet/Schering-Plough Animal Health, Millsboro, Delaware).
At weaning (Day 0 of the trial) and again 2 weeks later, all pigs were individually picked up and identified by ear-tag number, and pigs allotted to the Vaccinated treatment were injected with the PCV2 vaccine according to label dose and route of administration (2 mL per dose; intramuscular injection). Control pigs were not injected at either time point. Vaccination and blood collection procedures were performed by or directly supervised by two investigators (MLP and LMT). Therefore, during application of the treatments, individuals involved in the study were not blinded to treatments. However, farm staff responsible for day-to-day pig care and individuals involved with collecting the blood samples and data subsequent to application of vaccination were unaware of treatment designations. In this farm, farrowing and finishing facilities were managed all-in, all-out by room; nursery rooms were filled over 2 weeks and each room was emptied at a single time point (all-out). All pigs entered in the study were moved from a total of two nursery rooms on Day 40, temporarily housed in a single grower facility for 4 weeks, and then moved to a single finishing barn for the remainder of the trial. Vaccinated and Control pigs of the various genetic lines were comingled in single-sex pens throughout every phase of the trial. As the number of pigs per pen varied between the nursery pens and the finishing pens, pen integrity was not maintained and Control and Vaccinated pigs were comingled within each pen at the beginning of each growing period. Pigs from the weaning groups that were not entered into the study were penned with the trial pigs, but were not vaccinated against PCV2.
Whole blood samples were collected from all pigs on Days 0 (weaning), 40 (end of the nursery period), and 130 (approximately 150 days of age or the time-point that final data were collected on the farm for routine genetic-selection testing) to determine PCV2 viral load in serum and PCV2 antibody titers resulting from natural PCV2 exposure and infection. Pigs were individually identified and weighed at these time points to measure ADG. Due to time constraints as pigs were weighed at the end of the study, 17% of the pigs (40 Control pigs and 31 Vaccinated pigs) with representation from all genetic lines were weighed on Day 131. For these 71 pigs, one additional day was used in calculating ADG for Days 40 to 130 and Days 0 to 130. Backfat and loin depths, measured when pigs were weighed for the final time for the trial, were determined by real-time ultrasound at the 10th rib P2 location to determine whether these carcass-composition measurements differed between treatments.
Removals and deaths were recorded throughout the trial. Statistical analyses were performed on individual records from 417 pigs that had complete growth records at the end of the trial. Of the 454 pigs entered into the study at weaning, data from 37 pigs were excluded from the analysis. There were six deaths between Day 0 and Day 39 (two deaths prior to Day 14) and 25 deaths between Day 40 and Day 130. Other animals removed included one late-castrated cryptorchid, two pigs with missing final weights, one with a final data-entry error, one with a severe tail bite, and one downer pig (three Control and three Vaccinated pigs). The pigs not included in the final analysis were categorized according to the following categories (total and deaths within each category): A×A Control gilt, 1 (0); A×A Vaccinated gilt, 3 (2); A×A Control boar, 1 (0); A×A Vaccinated boar, 3 (3); A×B Control gilt, 6 (6); A×B Vaccinated gilt, 2 (2); A×B Control boar, 7 (7); A×B Vaccinated boar, 5 (5); B×A Control gilt, 1 (1), B×A Vaccinated gilt, 0 (0); B×A Control boar, 0 (0); B×A Vaccinated boar, 0 (0); B×B Control gilt, 3 (2); B×B Vaccinated gilt, 3 (2); B×B Control boar, 0 (0); and B×B Vaccinated boar, 2 (1).
Diagnostic testing
Serum was stored at -80ºC prior to indirect fluorescent antibody (IFA) and PCR testing. Diagnostic testing was performed at the Kansas State Veterinary Diagnostic Laboratory (KSVDL) after all samples had been collected. Diagnostic testing methods were accepted and validated in accordance with the American Association of Veterinary Laboratory Diagnosticians’ standard requirements necessary for diagnostic laboratory accreditation (available at http://www.aavld.org/accreditation). Serum samples were assayed for PCV2 antibodies using the 96-well format KSVDL PCV2 IFA assay with serial 1:2 dilutions in phosphate-buffered saline, beginning with a 1:20 dilution. Sample sets from the same 417 pigs used in the growth analysis were assayed simultaneously, and all samples from an individual pig were tested on the same IFA plate. Serum samples from Days 40 and 130 were individually tested for PCV2 nucleic acid using the KSVDL PCV2 quantitative PCR assay. Extraction of PCV2 DNA and PCR testing were performed under similar laboratory conditions for individual Control and Vaccinated pig serum samples.
Statistical analysis
The effect of PCV2 vaccination on growth rate, backfat depth, and loin depth of pigs was determined by analysis of variance using the GLIMMIX procedure in SAS version 9.1.3 (SAS Institute Inc, Cary, North Carolina) to obtain least squares means and standard errors for all response variables. The fixed and random effects included in the statistical model were planned for during initial trial design. The model included the fixed effects of vaccination treatment, genetic line, gender, and all interactions. Litter of origin was included as a random effect. Response variables evaluated were days to weaning and to the end of the study, birth weight, Day 0 (weaning) weight, and Day 130 weight. Individual pig growth rate (ADG) was calculated by dividing the period weight gain by the number of days in the period. The ADG period designations were Days 0 to 39 (nursery period), Days 40 to 130 (grow-finish period), and Days 0 to 130 (wean-to-finish period). Backfat and loin depths were analyzed as unadjusted and adjusted values. For the adjusted analysis, backfat and loin depths were adjusted to a common average Day 130 body weight.
Significance tests were performed for comparisons of the least squares adjusted means of fixed effect combinations and their interactions for all response criteria. Individual least squares mean comparisons within treatment or interactive terms were evaluated only if there was a significant F-test value for the overall effect of treatment or for the interactions. Values of P < .05 were considered significant.
Prior to analysis, IFA titers were log2 transformed to approximate a normal distribution. For the IFA analysis, the log2 of 10 was used when PCV2 antibody was not detected in a sample at the most concentrated dilution (1:20). The log2 of 5120 was used when samples were strongly positive at the least concentrated dilution (1:2560). These methods allowed results for samples of low antibody concentration (< 1:20) or high antibody concentration (> 1:2560) to be weighted differently than samples with normal-intensity fluorescence detected at dilutions of 1:20 and 1:2560. The main and interactive effects of genetic line, vaccination treatment, gender, and day of the study (time) on IFA antibody responses were tested by repeated measures analysis using the MIXED procedure in SAS. The statistical model included the fixed effects of vaccination treatment, genetic line, gender, time, and all interactions. The resulting means were transformed back to the original scale for presentation as geometric mean titers.
Viral template quantities were log10 transformed before analysis to achieve normality for the PCR data. Serum samples with any detectable PCV2 nucleic acid were considered to be PCV2-positive samples. Positive quantitative values were included in the analysis for these PCV2-positive samples along with zero values from samples with no PCV2 DNA detected. The main and interactive effects of genetic line, vaccination treatment, and gender on Day 40 and Day 130 serum PCV2 nucleic acid load were determined using the GLIMMIX procedure in SAS to obtain means and standard errors. Litter was included as a random effect for the analysis of PCR data. The resulting least squares means were transformed back to the original scale for presentation as geometric means.
The effect of Day 40 PCV2 DNA template quantity on ADG was determined as a post hoc test using the GLIMMIX procedure in SAS. Fixed effects in the model included genetic line, vaccination treatment, Day 40 log10 transformed PCV2 template copies, and all interactions. Litter was included as a random effect. The solutions statement in SAS was used to determine intercepts and coefficients for the regression model.
Results
Vaccine reactions and mortality
After the initial vaccination, 15 pigs in the Vaccinated treatment group had swellings on their necks at the site of vaccination, and one pig exhibited a transient “fainting” reaction with convulsion-like activity. Postweaning mortality for the Control pigs (Days 0 to 130) was 7.0% (16 of 230) while Vaccinated pig mortality was 6.8% (15 of 224). Because the sample size was inadequate to detect a relevant difference in mortality, statistical analysis was not performed on these data. However, there was no discernable difference in mortality between the genetic lines.
Performance analysis
There were no three-way interactions between genetic line, vaccination treatment, and gender detected for any responses with the exception of weight-adjusted backfat depth (P < .05). This three-way interaction was a result of Control A×B boars having greater backfat depth than Vaccinated A×B boars (11.9 ± 0.41 versus 10.9 ± 0.41 mm; P < .05). Within all other genetic line and gender combinations, backfat depth did not differ (P > .05) between Control and Vaccinated pigs (data not shown).
There were no two-way interactions (P > .05) observed between vaccination treatment and gender for any growth or carcass response criteria (data not shown). However, for ADG responses, there were two-way interactions between genetic line and vaccination treatment and between genetic line and gender (Table 1).
Table 1: P values for age, growth performance, and carcass trait response criteria for the interactive effects between genetic line (Genetic) and vaccination treatment (Vaccine), and genetic line and gender, and the main effects of genetic line, vaccination treatment, and gender*
* A total of 454 pigs (boar or gilt) from four genetic designations were assigned to vaccination treatment by ranking them by weight within litter by gender and randomly assigning each pig to either a Vaccinated or nonvaccinated (Control) treatment. Pigs were individually weighed at birth, at weaning (Day 0, 21 days of age), and on Days 40 (end of the nursery period) and 130 (end of the study). Backfat and loin depth were measured when pigs were weighed on Day 130. Analysis of variance was performed using the GLIMMIX procedure in SAS version 9.1.3 (SAS Institute Inc, Cary, North Carolina) on records from 417 pigs with complete growth records at the end of the study. The statistical model included fixed effects of genetic line, vaccination treatment, gender, and all interactions. Litter of origin was included as a random effect. † Genetic designations were A×A (Duroc line), A×B, B×A, and B×B (synthetic White Pietrain line). ‡ A porcine circovirus type 2 vaccine (Circumvent PCV; Intervet/Schering-Plough Animal Health, Millsboro, Delaware) was administered intramuscularly (2 mL per dose) to the Vaccinated pigs at 21 and 35 days of age. § Backfat depth and loin depth were adjusted to a common average Day 130 weight. There was a three-way interaction (P < .05) with genetic line, vaccination treatment, and gender for weight-adjusted backfat depth. This interaction was a result of Control A×B crossbred boars having greater backfat depth than Vaccinated A×B crossbred boars (11.9 ± 0.41 versus 10.9 ± 0.41 mm; P < .05). Within boars or gilts of A×A, B×A, or B×B, weight-adjusted backfat depth did not differ between Control and Vaccinated pigs (P > .05). |
Age
An interaction between genetic line and gender was observed for ages at Day 0 (P < .05) and Day 130 (P < .001) of the trial. Because of an uneven birth pattern for boars and gilts within the A×A genetic line during the week of farrowing, on Day 0, A×A boars were 0.3 days younger than A×A gilts (21.0 ± 0.43 versus 21.3 ± 0.43 days of age; P < .01). There were no differences (P > .05) between ages of boars and gilts within B×B (boars, 19.6 ± 0.49 days; gilts, 19.7 ± 0.49 days), A×B (boars, 20.3 ± 0.47 days; gilts, 20.3 ± 0.47 days), and B×A (boars, 21.2 ± 0.65 days; gilts, 21.3 ± 0.65 days). The interaction at Day 130 of the trial was a result of A×A boars being 0.2 day younger than A×A gilts (151.4 ± 0.46 versus 151.6 ± 0.46 days, P < .01), while B×B boars were 0.6 day younger than B×B gilts. (149.7 ± 0.52 versus 150.3 ± 0.52 days, P < .001). There were no differences (P > .05) between ages of boars and gilts within A×B (boars, 150.6 ± 0.51 days; gilts, 150.6 ± 0.51 days) and B×A (boars, 151.7 ± 0.69 days; gilts, 150.7 ± 0.69 days) at Day 130 of the trial. More importantly for evaluation of vaccination effects, there was no interaction between genetic line and vaccination treatment (P > .05; Table 2) or effect of vaccination (P > .05) observed for age at Day 0 or Day 130 of the trial.
Table 2: Means and standard errors for age, growth performance, and carcass trait response criteria for Control and Vaccinated pigs of different genetic designations*
* Pigs and procedures described in Table 1. Analysis of variance was performed using the GLIMMIX procedure in SAS version 9.1.3 (SAS Institute, Cary, North Carolina) on records from 417 pigs with complete growth records at the end of the study. The statistical model included fixed effects of genetic line, vaccination treatment, gender, and all interactions. Litter of origin was included as a random effect. Results are reported as least squares means ± SEM. † Genetic designations were A×A (Duroc line), A×B, B×A, and B×B (synthetic White Pietrain line). ‡ Vaccination treatments were Vaccinated and nonvaccinated (Control). Vaccination procedures described in Table 1. § Backfat depth not adjusted to a common weight. ¶ Loin depth not adjusted to a common weight. ** Backfat depth adjusted to a common average Day 130 weight. There was a three-way interaction (P < .05) with genetic line, vaccination treatment, and gender for weight-adjusted backfat depth as a result of Control A×B crossbred boars having greater backfat depth (11.9 ± 0.41 versus 10.9 ± 0.41 mm; P < .05) than Vaccinated A×B crossbred boars. Within boars or gilts of A×A, B×A, or B×B, weight-adjusted backfat depth did not differ (P > .05) between Control and Vaccinated pigs. ‡‡ Loin depth was adjusted to a common average Day 130 weight. abcde Within a row, means with no common superscript differ (P < .05). |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Body weight
There were no interactions (P > .05) observed between genetic line and vaccination treatment or genetic line and gender for birth or Day 0 weights. In addition, neither genetic line, vaccination treatment, nor gender affected birth or Day 0 weights (P > .05).
Weight on Day 130 depended upon the two-way interactions between genetic line and vaccination treatment (P < .05) and genetic line and gender (P < .05). Within A×A, gilts weighed less than boars (93.1 ± 2.11 versus 98.1 ± 2.09 kg body weight; P < .05). Within B×B, A×B and B×A, weights on Day 130 did not differ (P > .05) between boars and gilts.
The two-way interaction between genetic line and vaccination treatment was a result of A×A Control pigs being lighter than A×A Vaccinated pigs on Day 130 (P < .001), whereas weights of Control and Vaccinated pigs of B×B, A×B, and B×A did not differ (P > .05). Nevertheless, within all genetic populations, mean weights of Control pigs on Day 130 were numerically less than those of Vaccinated pigs. Vaccination was associated with greater weight, compared with that of Controls, within the A×A population (Vaccinated pig mean body weight 100.1 kg versus Control pig mean body weight 91.1 kg, 9.0 kg heavier with vaccination). This difference was almost four times that of the effect within the B×B population (Vaccinated pig mean body weight 102.4 kg versus Control pig mean body weight 100.1 kg, 2.3 kg heavier with vaccination). The vaccination effect on growth rate in the crossbred pigs was intermediate to that of pure lines (A×A and B×B).
The distribution of Day 130 weights for Control and Vaccinated pigs within A×A and B×B were determined (Figure 1). These distributions demonstrated the right shift in the Day 130 weights of the Vaccinated pig population relative to the Control pigs. Demonstrated by the population shift within both distributions, vaccination affected the entire Vaccinated pig population, though the extent of the effect of vaccination was different within each genetic population.
Figure 1: Distribution of pig weights at Day 130 for Vaccinated versus Control pigs of Duroc (A×A; panel A) and synthetic White Pietrain (B×B) genetic lines (panel B). Pigs were randomly assigned within litter by gender to Vaccinated or nonvaccinated Control treatments prior to weaning. Vaccine treatment described in Table 1. 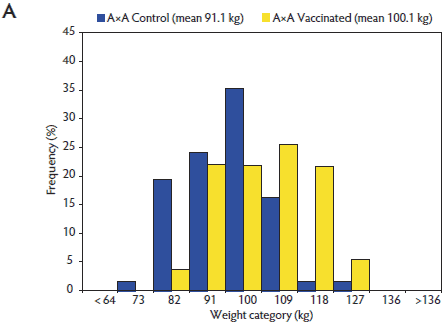 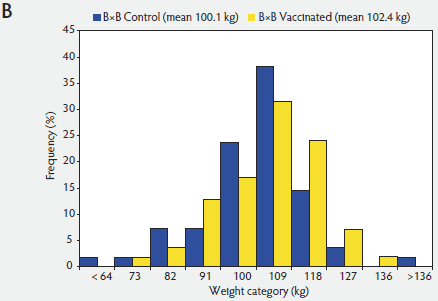 |
Average daily gain
There was no interactive effect (P > .05) between genetic line and gender on ADG for Days 0 to 39; however, gender did affect ADG during this period (P < .001). From Day 0 to Day 39, gilts grew faster than boars (430 ± 9.4 versus 403 ± 9.2 g per day).
A tendency for an interactive effect (P < .10) was detected between genetic line and vaccination treatment for ADG for Days 0 to 39. There was an interaction for ADG of Control and Vaccinated pigs within the A×B and B×A populations. In A×B Control pigs, ADG was greater than in A×B Vaccinated pigs (P < .05), while ADG was numerically greater in B×A Vaccinated pigs than in B×A Controls (P > .05).
There was a two-way interactive effect between both genetic line and gender (P < .05) and a tendency for an interaction between genetic line and vaccination treatment (P < .10) detected for ADG for Days 40 to 130. Boars of A×A grew faster (845 ± 17.6 versus 790 ± 17.8 g per day; P < .01) than A×A gilts, whereas ADG of boars and gilts did not differ (P > .05) within B×B, A×B, and B×A. For Days 40 to 130, ADG was lower (P < .01) for the A×A Controls than for the Control pigs of A×B, B×A, and B×B. For Days 40 to 130, ADG did not differ (P > .05) for Controls from the latter three genetic populations. In contrast, ADG of A×A Vaccinated pigs did not differ (P > .05) from that of Vaccinated pigs from the other genetic lines, with the exception of Vaccinated A×B pigs (P < .05). Thus, the difference in ADG between Vaccinated and Control pigs was greater within A×A than within B×B, A×B, and B×A.
There were interactive effects between genetic line and gender (P < .05) and genetic line and vaccination treatment (P < .05) observed for ADG for Days 0 to 130. Boars of A×A grew faster than gilts of A×A (705 ± 15.2 versus 671 ± 15.3 g per day; P < .05), whereas within B×B, A×B, and B×A, ADG for Days 0 to 130 did not differ (P > .05) between boars and gilts. Growth rate was lower in A×A Control pigs than in Control pigs from the other genetic lines (P < .01). Vaccinated A×A pigs grew more slowly than B×A Vaccinated pigs (P < .05), but ADG did not differ between Vaccinated A×B and B×B pigs (P > .05). Therefore, the magnitude of the difference between Vaccinated and Control pig mean growth rates was greatest in A×A pigs.
Backfat and loin depth
There were no three-way or two-way interactive effects observed for unadjusted backfat depth (P > .05). Genetic line did affect backfat depth (P < .05), as A×A (11.7 ± 0.29 mm) and A×B pigs (12.0 ± 0.30 mm) had greater backfat depth (P < .05) compared with B×B pigs (10.7 ± 0.32 mm). Backfat depth of B×A pigs (11.5 ± 0.42 mm) was intermediate to measurements of A×A and B×B pigs (P > .05).
There were no three-way or two-way interactive effects detected for unadjusted loin depth (P > .05); however, loin depth was affected by genetic line, vaccination treatment, and gender (P < .001). Loin depth of A×A pigs was less (60.7 ± 0.75 versus 66.3 ± 0.77 mm; P < .001) than that of A×B pigs. Loin depth of A×B pigs did not differ (P > .05) from that of B×A pigs (67.7 ± 1.06 mm) and was less (P < .01) than that of B×B pigs (69.2 ± 0.81 mm). Control pigs had less loin depth than Vaccinated pigs (65.0 ± 0.50 versus 66.9 ± 0.51 mm; P < .01), while boars had less loin depth than gilts (63.9 ± 0.50 versus 68.0 ± 0.52 mm; P < .01).
After backfat depth measurements were adjusted to a common average Day 130 weight, there was a three-way interaction (P < .05) observed with genetic line, gender, and vaccination treatment. This interaction was the result of Control A×B boars having greater weight-adjusted backfat depth than Vaccinated A×B boars (11.9 ± 0.41 versus 10.9 ± 0.41 mm; P < .05). Within all other gender-by-genetic line combinations, backfat depth did not differ between Control and Vaccinated pigs (P > .05).
After loin depths were adjusted to a common Day 130 weight, there was no significant three-way interaction observed (P > .05). There was a two-way interaction detected between genetic line and gender (P < .05). Despite loin depths consistently being greater in gilts than in boars, within the A×B population the difference was 2.2 mm, whereas within A×A, B×B, and B×A, the difference was 4.3 mm or greater. Although there was a significant effect of vaccination treatment prior to weight adjustment, after adjustment to a common average Day 130 weight, vaccination treatment did not affect loin depth (P > .05).
Indirect fluorescent antibody test results
The IFA geometric mean titer profiles for Control pigs (Day 0: 233.7, Day 40: 188.5, and Day 130: 3951.0) and Vaccinated pigs (Day 0: 226.0, Day 40: 1928.1, and Day 130: 974.6) indicate the timing of the Vaccinated pig PCV2 antibody rise was due to vaccination with the two-dose PCV2 vaccine when contrasted with timing of the Control pig antibody rise, a response produced from natural PCV2 exposure. There were three-way interactions with genetic line, vaccination treatment, and time (P < .05) as well as with vaccination treatment, gender, and time (P < .01) detected for IFA antibody response. However, on average, Vaccinated pigs demonstrated an increase in PCV2 antibody titer by Day 40, which decreased by Day 130, while a rise in PCV2 antibody titer was not detected in Control pigs until Day 130.
Polymerase chain reaction results
There were no three-way or two-way interactions (P > .05) with genetic line, vaccination treatment, or gender for Day 40 or Day 130 PCV2 viral template copy quantity.
Viral template copy quantity was affected by both genetic line (P < .01; Figure 2) and vaccination treatment (P < .001). On Day 40, PCV2 DNA load was lower in B×B pigs (56.8 template copies per reaction; P < .05) than in A×A, A×B, and B×A pigs (420.1 template copies per reaction or higher). Vaccinated pigs also had lower PCV2 viral template copy quantities than did Control pigs (20.9 viral copies per reaction versus 4582.5 viral copies per reaction; P < .001).
Figure 2: Porcine circovirus type 2 (PCV2) viral template quantity determined by PCV2 polymerase chain reaction (PCR) in serum collected on Day 40 (end of nursery period) from pigs of pure-line Duroc (A×A) and pure-line synthetic White Pietrain (B×B) and their crosses (sire × dam). Pigs were randomly assigned within litter by gender to Vaccinated or nonvaccinated (Control) treatments prior to weaning at Day 0 (21 days of age). Vaccine treatment described in Table 1. Individual pig PCV2 PCR data were log10 transformed and then were analyzed by analysis of variance using the GLIMMIX procedure in SAS version 9.1.3 (SAS Institute Inc, Cary, North Carolina). The model included the fixed effects of vaccination treatment, genetic line, gender, and all interactions. Litter of origin was included as a random effect. Resulting means were transformed back to the original scale for presentation. The main effect of genetic line (P < .01) on Day 40 PCV2 viral template quantity is presented, thus each bar includes both Control and Vaccinated pig data within a genetic line. a,b: means with no common letter differ (P < .05). 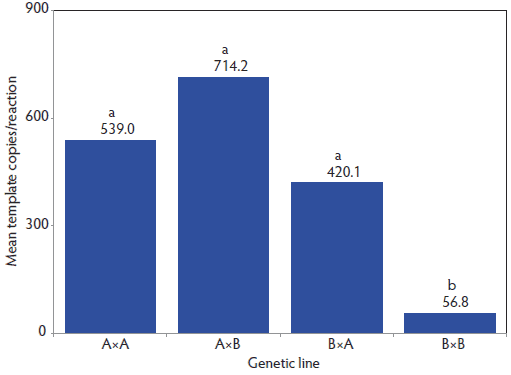 |
Only vaccination treatment affected Day 130 PCV2 viral template copy quantity (P < .001). Viral template quantities were lower in Vaccinated pigs than in Control pigs (1.3 viral template copies per reaction versus 3.8 viral template copies per reaction).
There was a three-way interaction (P < .05) with genetic line, vaccination treatment, and Day 40 PCV2 DNA template quantity observed for Days 40 to 130 ADG. Average daily gain for Days 40 to 130 depended not only on both genetic line and vaccine status, but also on Day 40 viral load. As viral load increased, the ADG response differed depending on genetic line and vaccination status. Average daily gain was modeled with the log10 transformed Day 40 PCR data, and to demonstrate the disparity in ADG, the models for A×A Controls and B×B Controls are included (Figure 3).
Figure 3: Nonvaccinated (Control) pig porcine circovirus type 2 (PCV2) viral template quantity in serum collected on Day 40 (end of the nursery period) as a predictor of average daily gain (ADG) Days 40 to 130 for pure-line Duroc (A×A) and synthetic White Pietrain (B×B) genetic lines. Pigs were randomly assigned within litter by gender to Vaccinated or Control treatments prior to weaning (Day 0; 21 days of age). Vaccine treatments described in Table 1. Individual pig PCR data were log10 transformed before analysis. The effect of Day 40 PCV2 DNA template quantity on ADG was determined using the GLIMMIX procedure in SAS version 9.1.3 (SAS Institute Inc, Cary, North Carolina). The model included the fixed effects of genetic line, gender, and vaccination treatment as categorical variables and Day 40 log10 transformed PCV2 template copies as a continuous variable. Litter was included as a random effect. There was an interaction (P < .05) with genetic line, vaccination treatment, and Day 40 log10 transformed PCV2 template quantity observed for ADG for Days 40 to 130. The solutions statement in SAS was used to determine intercepts and coefficients for the regression model.  |
Discussion
Porcine circovirus disease is a devastating disease affecting multiple organ systems. Infection with PCV2 has become endemic in many swine herds. The virus itself is ubiquitous, present in nearly all herds, yet the expression of disease (PCVAD) pertaining to morbidity and mortality varies. Factors associated with the risk for development of clinical PCVAD or lesions have been identified, including host genetic differences,3 gender,7 litter of origin,8 low birth or weaning weight,7 and management factors.2 Though PCV2 is the necessary etiologic agent of PCVAD, there is evidence that clinical disease is exacerbated when accompanied by additional pathogenic agents, also called cofactors.9,10 In general, circovirus vaccines have been effective in lessening the severity of, or preventing, clinical PCVAD.11,12 In development of vaccination programs as standard practice for many farms, understanding vaccine limitations and expected responses to vaccination has become a focus within the industry.
Results from our study indicate that growth-rate response to PCV2 vaccination varies with genetic line. To our knowledge, this is the first study demonstrating differential responses to PCV2 vaccination based on genetic line under field conditions. Genetic background, however, had been previously implicated by other researchers as a risk factor for expression of PCVAD.3,13 Our study supports the evidence that PCVAD risk is dependent on the genetic line of the pig.
Field reports have suggested pigs from Pietrain background may be less susceptible to PCVAD;4 however, some published study results do not support these observations.5 In our study, the magnitude of the response was greater in pigs of the Duroc genetic line than in pigs of the White Pietrain genetic line. Also, our research adds to the body of literature indicating that pig genetic line affects responses to vaccination or disease expression.14-16
Performing this study in a high-health status herd with few clinical signs of PCVAD other than an increase in morbidity provides unique insight on PCVAD to the current literature. Previous research, evaluating host genetic line as a risk factor, has focused on documenting the effects of genetic line on PCVAD-associated mortality, observed clinical disease with wasting, or differences in severity of PCV2 lesions.3-5,13 Mortality had not increased in the herd used for our study, thus mortality was not a primary response of interest. Nonetheless, pig deaths were recorded and there was only a 0.2% difference in mortality between Control and Vaccinated pigs.
The effect of PCV2 vaccination on ADG from Days 0 to 130 for pigs of A×A was about four times greater (A×A: 66 g per day versus B×B: 16 g per day) than the effect of vaccination on ADG for pigs of B×B. Although the magnitude of the difference between Control and Vaccinated populations was greater in the A×A pigs than in the B×B pigs, the Control and Vaccinated pig response patterns within the genetic lines were similar. Evident from weight distributions for both pure-line populations, Day 130 weights were shifted to the right for the Vaccinated pigs compared with the Control pigs. It was apparent that all pigs in both populations were affected by vaccination, with only the extent of the response being different. These findings support previous reports that PCV2 vaccine affected all pigs, even the fastest-growing pigs.6,8 Detection of the more pronounced right shift for the Vaccinated pigs within the weight distribution of the A×A population than in the B×B population provided new evidence that genetic line affects this PCV2 vaccine response.
Similar to the results from another study,17 vaccination against PCV2 had little or no influence on carcass composition in our study. The loin-depth differences detected initially between pigs of different vaccination treatments resulted from differences in Day 130 weights. After weight adjustment to a common Day 130 weight, no difference was detected between loin depths across vaccination treatment, as the difference in loin depth noted initially was due to bigger pigs having larger muscles and smaller pigs having smaller muscles. Vaccination did not alter the Vaccinated pig carcass composition relative to the nonvaccinated Control pigs; it just resulted in larger and heavier pigs on average.
Presence of PCV2 virus and active infection was confirmed during this trial by IFA and PCR testing. The IFA results indicate a PCV2 antibody rise in the Vaccinated pigs by Day 40. By Day 130, antibody levels in the Vaccinated pigs had decreased, suggesting the increase by Day 40 was primarily due to vaccination and not early natural viral exposure. In contrast, the rise in antibody titer between Days 40 and 130 for the Control pigs indicated that natural PCV2 exposure had occurred.
Active PCV2 infection was documented with detection of PCV2 DNA in serum of both Control and Vaccinated pigs. Viremia in both populations was documented by the end of the 40-day nursery phase, with some pigs still PCV2-viremic on Day 130. Although some nursery pigs were PCV2-viremic, the rise in IFA titer after Day 40 suggests that many Control pigs seroconverted after being moved from the nursery. These results indicate PCV2 circulation and infection during the nursery period, with subsequent transmission or persistence during the finishing period. Nevertheless, Control and Vaccinated pigs were comingled during both the Days 0 to 39 and Days 40 to 130 periods, and at both Days 40 and 130, PCV2 viral load in Vaccinated pigs was markedly less than that of Control pigs.
Under experimental conditions, severity of histopathologic lesions, particularly in liver and lymphoid tissues, worsened with increased PCV2 viral load detected by immunohistochemistry.18 This trial provided data which suggests that ADG is affected by serum viral load. Pure-line (A×A and B×B) pig results indicate that as Day 40 viral load increased, ADG for Days 40 to 130 decreased; however, the rate at which the change occurred was dependent upon genetic line. Average daily gain decreased at a faster rate as viral load increased for Control pigs of A×A than for Controls of the B×B line. Further research is needed to comprehensively explain the effects of serum viral load level as it relates to performance. Our data provided initial information linking serum viral load and growth performance; however, the biological significance of this has yet to be fully characterized. The results of this study confirm previous research findings that circovirus vaccination effectively decreases serum viral load,6,19,20 even when vaccinated pigs are housed with nonvaccinated pigs.
Genetic line also affected Day 40 PCV2 viral loads. Pigs of B×B genetic line had the lowest level of PCV2 DNA detected on Day 40. This genetic population also had the smallest response to PCV2 vaccination as measured by ADG. While these findings support observations that ADG was negatively affected by increasing viral load, they also provide evidence that genetic line may play a role in PCV2-infection susceptibility or resistance.
McIntosh et al21 documented breed differences in PCV2 shedding duration. A survey of boars positive for PCV2 antibodies in a commercial boar stud revealed detectable PCV2 DNA in semen from Duroc and Landrace boars. Throughout the sampling time frame, no PCV2 DNA was detected in semen from Hamline, Large White maternal or paternal lines, or Meishan-synthetic breeds.21 While pig genetic background has been shown to affect expression of other viral diseases,14,22,23 McIntosh et al21 suggested that genetic line may be important for explaining differences in PCV2-infection susceptibility and expression.
Although the responses observed in our study were influenced by PCV2 vaccination, these data may indicate that genetic line affects PCVAD expression. If the severity of PCV2 infection was dependent at some level on host genetic background, this might explain why vaccination affected the genetic lines differently. The B×B population was of a White Pietrain line, and the magnitude of the growth difference between Control and Vaccinated pigs was less than that of the A×A pigs of a Duroc line. These findings resulted in additional questions regarding differences in susceptibility between these genetic lines and whether it might be possible to derive animals with improved resistance to PCVAD. However, the primary objective of this trial was to determine whether pigs of different genetic lines differed in their responses to PCV2 vaccination. With that difference clearly demonstrated, further research is needed to address these additional questions.
Results of this study indicate that pig genetic line affects the growth rate response to PCV2 vaccination under field conditions with natural PCV2 exposure. Thus, pig genetic line must be considered a risk factor relative to expression of PCVAD and when evaluating performance responses to PCV2 vaccination.
Implications
• Different genetic lines respond differently to PCV2 vaccination as measured by growth rate.
• Under the conditions of this field trial, with natural viral exposure, vaccinated pigs have lower PCV2 viral load levels and greater ADG compared with nonvaccinated pigs.
• Genetic line should be considered a risk factor for either PCVAD expression or response to PCV2 vaccine.
References
1. Allan GM, Ellis JA. Porcine circoviruses: a review. J Vet Diagn Invest. 2000;12:3–14.
2. Rose N, Larour G, Le Diguerher G, Eveno E, Jolly JP, Blanchard P, Oger A, Le Dimna M, Jestin A, Madec F. Risk factors for porcine post-weaning multisystemic wasting syndrome (PMWS) in 149 French farrow-to-finish herds. Prev Vet Med. 2003;61:209–225.
3. Opriessnig T, Fenaux M, Thomas P, Hoogland MJ, Rothschild MF, Meng XJ, Halbur PG. Evidence of breed-dependent differences in susceptibility to porcine circovirus type-2-associated disease and lesions. Vet Path. 2006;43:281–293.
*4. López-Soria S, Segalés J, Nofrarias M, Calsamiglia M, Ramírez H, Mínguez A, Serrano IM, Marín O, Callén A. Genetic influence on the expression of PCV disease [letter]. Vet Rec. 2004;155:504.
5. Rose N, Abhervé-Guéguen A, Le Diguerher G, Eveno E, Jolly JP, Blanchard P, Oger A, Jestin A, Madec F. Effect of the Pietrain breed used as terminal boar on Post-weaning Multisystemic Wasting Syndrome (PMWS) in the offspring in four PMWS-affected farms. Livest Prod Sci. 2005;95:177–186.
6. Horlen KP, Dritz SS, Nietfeld JC, Henry SC, Hesse RA, Oberst R, Hays M, Anderson J, Rowland RR. A field evaluation of mortality rate and growth performance in pigs vaccinated against porcine circovirus type 2. JAVMA. 2008;232:906–912.
7. Corrégé I, Pirouelle H, Gaudré D, Le Tiran MH. [A study of the influence of various animal husbandry criteria on the occurrence of postweaning multisystemic wasting syndrome (PMWS) in an experimental pig farm]. Journées de la Recherche Porcine en France. 2001;33:283–290.
8. Madec F, Eveno E, Morvan P, Hamon L, Blanchard P, Cariolet R, Amenna N, Morvan H, Truong C, Mahé D, Albina E, Jestin A. Post-weaning multisystemic wasting syndrome (PMWS) in pigs in France: Clinical observations from follow-up studies on affected farms. Livest Prod Sci. 2000;63:223–233.
9. Dorr PM, Baker RB, Almond GW, Wayne SR, Gebreyes WA. Epidemiologic assessment of porcine circovirus type 2 coinfection with other pathogens in swine. JAVMA. 2007;230:244–250.
10. Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng XJ, Halbur PG. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol Online. 2004;41:624–640. doi: 10.1354/vp.41-6-624.
11. Desrosiers R, Clark E, Tremblay D, Tremblay R, Polson D. Use of a one-dose subunit vaccine to prevent losses associated with porcine circovirus type 2. J Swine Health Prod. 2009;17:148–154.
12. Kixmöller M, Ritzmann M, Eddicks M, Saalmüller A, Elbers K, Fachinger V. Reduction of PMWS-associated clinical signs and co-infections by vaccination against PCV2. Vaccine. 2008;26:3443–3451.
13. Opriessnig T, Patterson AR, Madson DM, Pal N, Rothschild M, Kuhar D, Lunney JK, Juhan NM, Meng XJ, Halbur PG. Difference in severity of porcine circovirus type two-induced pathological lesions between Landrace and Pietrain pigs. J Anim Sci. 2009;87:1582–1590.
14. Reiner G, Melchinger E, Kramarova M, Pfaff E, Büttner M, Saalmüller A, Geldermann H. Detection of quantitative trait loci for resistance/susceptibility to pseudorabies virus in swine. J Gen Virol. 2002;83:167–172.
15. Doeschl-Wilson AB, Kyriazakis I, Vincent A, Rothschild MF, Thacker E, Galina-Pantoja L. Clinical and pathological responses of pigs from two genetically diverse commercial lines to porcine reproductive and respiratory syndrome virus infection. J Anim Sci. 2009;87:1638–1647.
16. Vincent AL, Thacker BJ, Halbur PG, Rothschild MF, Thacker EL. An investigation of susceptibility to porcine reproductive and respiratory syndrome virus between two genetically diverse commercial lines of pigs. J Anim Sci. 2006;84:49–57.
17. Jacela JY, Dritz SS, DeRouchey JM, Tokach MD, Goodband RD, Nelssen JL. Field evaluation of the effects of a porcine circovirus type 2 vaccine on finishing pig growth performance, carcass characteristics, and mortality rate in a herd with a history of porcine circovirus-associated disease. J Swine Health Prod. 2011;19:10–18.
18. Krakowka S, Ellis J, McNeilly F, Waldner C, Allan G. Features of porcine circovirus-2 disease: correlations between lesions, amount and distribution of virus, and clinical outcome. J Vet Diagn Invest. 2005;17:213–222.
19. Fachinger V, Bischoff R, Jedidia SB, Saalmüller A, Elbers K. The effect of vaccination against porcine circovirus type 2 in pigs suffering from porcine respiratory disease complex. Vaccine. 2008;26:1488–1499.
20. Opriessnig T, Patterson AR, Madson DM, Pal N, Halbur PG. Comparison of efficacy of commercial one dose and two dose PCV2 vaccines using a mixed PRRSV-PCV2-SIV clinical infection model 2–3-months post vaccination. Vaccine. 2009;27:1002–1007.
21. McIntosh KA, Harding JCS, Parker S, Ellis JA, Appleyard GD. Nested polymerase chain reaction detection and duration of porcine circovirus type 2 in semen with sperm morphological analysis from naturally infected boars. J Vet Diagn Invest. 2006;18:380–384.
22. Petry DB, Holl JW, Weber JS, Doster AR, Osorio FA, Johnson RK. Biological responses to porcine respiratory and reproductive syndrome virus in pigs of two genetic populations. J Anim Sci. 2005;83:1494–1502.
23. Halbur PG, Rothschild MF, Thacker BJ, Meng XJ, Paul PS, Bruna JD. Differences in susceptibility of Duroc, Hampshire, and Meishan pigs to infection with a high virulence strain (VR2385) of porcine reproductive and respiratory syndrome virus (PRRSV). J Anim Breed Gen. 1998;115:181–189.
*Non-referred reference.
