| Case report | Peer reviewed |
Cite as: Corzo CA, Gramer M, Kuhn M, et al. Observations regarding influenza A virus shedding in a swine breeding farm after mass vaccination. J Swine Health Prod. 2012;20(6):283–289.
Also available as a PDF.
SummaryAn outbreak of respiratory disease in suckling piglets started in December 2010 in a 1200-sow farrow-to-wean facility. Swine influenza virus H1N2 was isolated from nasal swabs of affected piglets and determined to be the cause of the respiratory disease. After 2 months of continuous respiratory disease in the suckling-piglet and nursery populations, a change in the influenza vaccination strategy was adopted. Administration of swine influenza autogenous vaccine at 85 to 91 days of gestation was discontinued, and mass vaccination of the breeding herd was performed with two doses of a commercial multivalent vaccine. Prevalence of virus shedding was monitored by real-time reverse transcriptase polymerase chain reaction assay in nasal swabs and oral fluids from sows and suckling piglets before and after mass vaccination. After vaccination, there was a significant increase (P < .001) in hemagglutination inhibition serum-antibody titers in breeding females. Prevalence of shedding in sows and suckling piglets decreased through the 13 weeks of monitoring until no influenza-positive samples were detected in suckling and recently weaned pigs. This case report provides insights into a potential control strategy for swine influenza in breeding herds through mass vaccination. | ResumenEn Diciembre del 2010 inició un brote de enfermedad respiratoria en los lechones de la maternidad en una granja de 1200 hembras (nacimiento y destete). Se aisló el virus H1N2 de influenza porcina en muestras de hisopos nasales de lechones afectados y se determinó como la causa de la enfermedad respiratoria. Después de 2 meses de enfermedad respiratoria continua en la población de lechones de maternidad y destete, se adoptó un cambió en la estrategia de vacunación contra influenza. Se descontinuó la administración de una vacuna autógena de influenza porcina los días 85 a 91 de gestación, y se realizó la vacunación general del hato de cría con dos dosis de una vacuna comercial multivalente. Antes y después de la vacunación en masa, se monitoreó la prevalencia de la excreción de virus mediante la prueba de reacción en cadena de la polimerasa de transcriptasa reversa en tiempo real en muestras nasales y fluidos orales de las hembras y lechones en lactancia. Después de la vacunación, en las hembras, hubo un incremento significativo (P < .001) de los títulos de anticuerpos séricos detectados mediante la prueba de la inhibición de la hemaglutinación. La prevalencia de la excreción en hembras y lechones de lactancia se redujo a lo largo de las 13 semanas de monitoreo hasta que se no detectaron muestras positivas a influenza en lechones lactantes y recién destetados. Este reporte de caso ofrece ideas sobre una potencial estrategia de control contra la influenza porcina en hembras mediante la vacunación generalizada. | ResuméUne éclosion de maladies respiratoires chez des porcelets à la mamelle a débuté en décembre 2010 dans une unité de maternité de 1200 truies. Le virus de l’influenza porcin H1N2 a été isolé d’écouvillons nasaux de porcelets affectés et il fut identifié comme étant la cause des maladies respiratoires. Après 2 mois de maladies respiratoires en continu chez les porcelets à la mamelle et dans la pouponnière, un changement dans la stratégie de vaccination contre l’influenza a été adopté. L’administration à 85 à 91 jours de gestation d’un vaccin autogène de l’influenza porcin a été discontinuée, et une vaccination en masse du troupeau reproducteur a été faite avec deux doses d’un vaccin multivalent commercial. La prévalence de l’excrétion du virus a été suivie par réaction d’amplification en chaîne par la polymérase en temps réel à l’aide de la transcriptase réverse à partir d’écouvillons nasaux et de fluides oraux provenant de truies et de porcelets à la mamelle avant et après la vaccination massive. Après la vaccination, une augmentation significative (P < .001) des titres sériques fut détectée chez les femelles reproductrices par épreuve d’inhibition de l’hémagglutination. La prévalence d’excrétion chez les truies et les porcelets à la mamelle a diminué durant les 13 semaines de suivi jusqu’à ce qu’aucun échantillon positif pour l’influenza ne fut détecté chez les porcelets à la mamelle et ceux récemment sevrés. Ce cas donne un aperçu d’une stratégie potentielle pour la maîtrise de l’influenza porcin dans des troupeaux de reproducteurs via une vaccination de masse. |
Keywords: swine, influenza, vaccination, prevalence, shedding
Search the AASV web site
for pages with similar keywords.
Received: December 13, 2011
Accepted: May 9, 2012
Influenza virus in swine causes respiratory disease with clinical signs such as coughing, nasal discharge, and sneezing. Morbidity can reach up to 100% in the at-risk population, but mortality is generally low.1 Pigs frequently are pyrexic and anorexic, which results in reduced growth performance.1 Influenza has also been known to induce abortions2 as a clinical manifestation of pyrexia in pregnant females. In the North American pig population, three subtypes of influenza A, H1N1, H1N2, and H3N2, circulate continuously throughout the year.3 Transmission of influenza occurs horizontally through nose-to-nose contact and can also be transmitted through droplets.4
Control and prevention of influenza A in swine has become a priority.5 Biosecurity measures, changes in pig flow, medications, and vaccinations have been tools used in the industry for disease control. Recently, Torremorell et al5 reported elimination of H3N2 influenza A virus from a multi-site system through a combination of herd closure and partial depopulation. Vaccines have been the first and perhaps the only tool for prevention of influenza; however, the degree of protection may be variable due to mismatch of the antigen in the vaccine and the wild-type virus circulating in the pig population,6 unique host immune responses, and vaccination timing. Experiences in Italy7 with avian influenza have highlighted the key role vaccination plays when used as an emergency tool for disease control, containment, and prevention. Emergency vaccination programs at the flock level in a defined area were able to stop disease spread to neighboring farms by generating a minimal yet uniform level of immunity, which decreased viral shedding and transmission rate in Italian poultry.7
Presently, to the authors’ knowledge, there are no data regarding the efficacy of using a killed vaccine administered through a vaccination protocol in swine breeding herds. In this case, gilts and sows in a breeding herd were mass vaccinated using a commercial swine influenza vaccine, and the prevalence of virus shedding in these females, suckling pigs, and recently weaned pigs was monitored for 13 weeks by real-time reverse-transcriptase polymerase chain reaction (RRT-PCR).
Case description
The case farm was a commercial 1200-sow farrow-to-wean unit located in southern Minnesota. This farm was part of a two-site production system weaning 21-day-old pigs into an off-site, continuous-flow, 4000-head nursery located 1.98 km south of the sow farm. The sow farm historically tested seropositive for porcine reproductive and respiratory syndrome virus (PRRSV), Mycoplasma hyopneumoniae, and porcine circovirus type 2 (PCV2). One dose of an autogenous influenza vaccine (Newport Laboratories, Worthington, Minnesota) containing previously isolated A/Swine/MN/02011/2008/H1N1 and A/Swine/MN/02588/2009/H3N2 viruses, plus the A/Swine/MN/ 003252/2010/H1N2 virus isolated from the outbreak described in this report, was administered to breeding females at 85 to 91 days of gestation. Gilts originating from an off-site gilt-developer unit were vaccinated twice after arrival at the breeding herd, at 100 and 110 kg of body weight. Piglets received one dose of PCV2 vaccine (Circumvent PCV M; Intervet/Schering-Plough Animal Health, Omaha, Nebraska) at weaning.
In early December 2010, the breeding herd experienced an outbreak of acute respiratory disease in suckling piglets. Respiratory disease was characterized by cough ranging from light to severe, which was evident in 10- to 21-day-old piglets and lasted approximately 2 weeks. Shortly after the initial respiratory episode in the farrowing unit, pigs with similar respiratory signs were seen in the nursery.
The herd veterinarian suspected influenza and collected 10 nasal swabs from suckling piglets in different litters for molecular diagnostic testing by an influenza A virus matrix gene RRT-PCR8,9 and by virus isolation at the University of Minnesota Veterinary Diagnostic Laboratory. Four of the 10 nasal swabs were positive for influenza A viral RNA, and virus was isolated from two of the four RRT-PCR-positive swabs. The virus was successfully subtyped and genetically characterized as an H1N2 virus grouping with the delta 1 cluster human-swine double reassortant viruses.10 During the first weeks of February 2011, as part of the routinely scheduled herd-health visits, the herd veterinarian again collected nasal swabs from 21-day-old piglets. Twelve of 30 (40%) tested positive for influenza, indicating that suckling pigs were still becoming infected during the lactation phase.
On the basis of the RRT-PCR results and the persistence of clinical signs of respiratory disease in both the breeding and nursery farms, the veterinarian and owner concluded that the influenza vaccination strategy was not working as expected. Thus, they decided to change the vaccination strategy from vaccination during late gestation to a two-dose vaccination of the entire breeding herd, which, for the purposes of this report, included lactating, gestating, and open sows and gilts at the site. The first vaccination was administered to the entire breeding herd in 1 day. The second dose was administered 5 weeks later. All lactating, gestating, and open sows were vaccinated on the same day, and all gilts 3 days later. The autogenous vaccine was replaced by a commercial vaccine (FluSure XP; Pfizer Animal Health, Kalamazoo, Michigan), a killed multivalent vaccine that uses Amphigen as the adjuvant and contains four distinct inactivated influenza isolates: A/Swine/North Carolina/031/05 (H1N1), A/Swine/Missouri/069/05 (H3N2), A/Swine/Iowa/726H/2005 (H1N2), and A/Swine/Iowa/110600/00 (H1N1). The two H1N1 vaccine strains belong to the delta 1 and delta 2 groups, respectively. The H1N2 strain belongs to the delta 1 group, and the H3N2 vaccine strain is a cluster 4H3. The hemagglutinin (HA) gene of virus isolated from the farm samples shared 98.9% nucleotide similarity with the HA gene in the delta 1 H1N2 virus in FluSure XP (Figure 1).
Figure 1: Phylogenetic tree comparing the H1N2 virus isolated from a 1200-sow farm undergoing an outbreak of respiratory disease in suckling piglets and the virus strains included in the commercial vaccine (FluSure XP; Pfizer Animal Health, Kalamazoo, Michigan) used in a vaccination protocol introduced to control the outbreak. 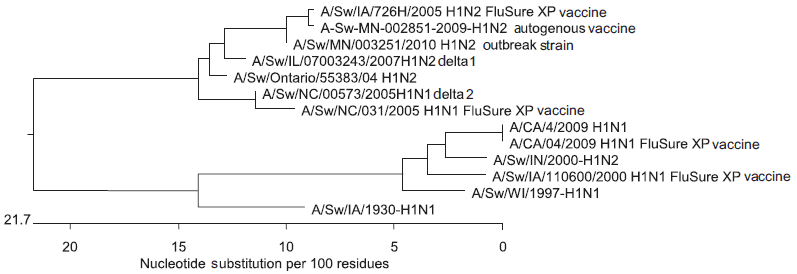 |
Monitoring
The change in vaccination composition and timing was accompanied by a protocol (Figure 2) which included monitoring for influenza virus A by RRT-PCR testing in the breeding herd and in both suckling piglets and nursery pigs.
Figure 2: Summary of the sampling protocol and vaccination timeline in a 1200-sow farm undergoing a respiratory disease outbreak in suckling piglets. An inactivated commercial vaccine was administered to the entire breeding herd (lactating, gestating, and open sows, and gilts) in 1 day during week 6 of 2011 (red arrow). A second dose was administered 5 weeks later: lactating, gestating, and open sows were vaccinated on the same day and gilts 3 days later (blue arrow). Nasal swabs and blood samples were collected from lactating and gestating females 1 week before the first vaccination and 3 weeks after the second vaccination. Suckling-piglet samples were collected 3 weeks after the first vaccination and then every other week (seven occasions total). Samples were collected from one nursery cohort on the sixth week of the year and then every other week during the nursery phase. A second nursery cohort was monitored in the same manner starting on week 16 of the year. 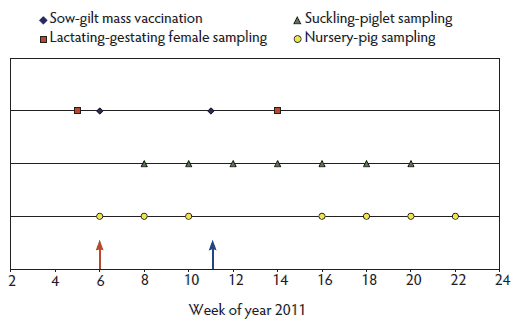 |
Breeding-herd nasal-swab testing protocol
A total of 120 nasal swabs (95% confident of detecting at least one positive sample when the prevalence of virus shedding is ≥ 2.5%) were collected 1 week before the first vaccination and 3 weeks after the second vaccination. The first set of 120 swabs originated from lactating sows, and the second set from sows in the breeding-gestation area. Sixty of the 120 were paired nasal swabs.
Breeding-herd serological testing protocol
Sixty paired blood samples were collected from sows 1 week before the first vaccination and 3 weeks after the second vaccination. Samples were tested for influenza A antibodies by hemagglutination inhibition (HI)11 against all strains in the FluSure XP vaccine and against the H1N2 outbreak virus previously isolated from suckling pigs.
Suckling-piglet nasal-swab collection protocol
Suckling pigs were monitored by collecting 30 nasal swabs (95% confident of detecting at least one positive sample when shedding prevalence is ≥ 10%) from 14- and 21-day-old piglets for a total of 60 nasal swabs. Sampling targeted parity one and two litters; however, when there were few parity one and two litters, litters showing signs of respiratory disease were sampled. The first samples were collected 2 weeks after the first breeding-herd vaccination, and subsequent samples were collected every other week for a total of seven samples. Sampling schemes included nasal swabs from pigs born prior to the vaccination event (eg, the 21-day-old pigs), pigs born to sows receiving only the first vaccine dose, and pigs born to sows that received both doses.
Nursery-cohort longitudinal monitoring
Two cohorts of approximately 650 pigs, one pre-vaccination and one postvaccination, were monitored throughout their time in the nursery by collecting 30 individual nasal swabs and pen oral-fluid samples (16 oral-fluid samples total) at 4, 6, 8, and 10 weeks of age. Each pen-based oral-fluid sample represented 30 to 40 pigs. The cohorts were conveniently selected so that the pre-vaccination cohort was moved out of the nursery prior to the arrival of the postvaccination cohort. The objective of longitudinal monitoring was to determine the prevalence over time of influenza virus in the nursery pre- and post vaccination.
Nursery cross-sectional monitoring
On the day when samples for the postvaccination cohort were being collected, 30 nasal swabs and 16 oral-fluid samples per age group at 4, 6, 8, and 10 weeks of age were also collected. The objective of the cross-sectional monitoring was to determine the prevalence by age group of influenza virus in the nursery at each time point post vaccination. In both pre-vaccination and postvaccination groups of pigs monitored, oral fluids collected at different time points originated from the same rooms and pens.
Statistical analyses
Comparison of pre-vaccination and postvaccination HI titers was performed by a paired t test using log2-transformed values. Titers below 10 were assigned a value of 5. Univariate and multivariate logistic regression was used to determine associations between piglets with influenza A virus detected in nasal swabs and age of the piglet (14 versus 21 days of age), weeks after vaccination protocol was initiated, parity of the sow, and whether piglets were born to a sow that had completed the vaccination protocol 2 weeks before farrowing (yes versus no). Litter was included in the model as a random effect to account for clustering within litter. Statistical analyses were performed using SAS 9.2 (SAS Institute Inc, Cary, North Carolina).
Results of RRT-PCR and serological testing
Breeding-female nasal swabs and serology
Of 120 nasal swabs collected 1 week before vaccination, two (1.6%) tested positive for influenza RNA. Eleven additional nasal swabs were classified as suspect (cycle threshold 36 to 40). None of the 120 nasal swabs collected from females that had been vaccinated twice tested positive for influenza RNA. A significant increase (P < .001) between pre- and postvaccination antibody titers was detected (Table 1).
Table 1: Paired hemagglutination inhibition (HI) reciprocal geometric mean antibody titers* of breeding females against the four influenza strains in a commercial killed vaccine (FluSure XP; Pfizer Animal Health, Kalamazoo, Michigan) and the outbreak H1N2 strain
* Blood samples were collected from 60 females 1 week before and 56 females 3 weeks after a two-dose mass vaccination protocol for influenza virus in a 1200-sow breeding herd undergoing an outbreak of respiratory disease in suckling pigs. Four sows had been culled by the time the second blood sample was obtained. † Paired t test. |
Suckling-piglet nasal swabs
The prevalence of influenza A virus in the suckling-pig population, as determined by PCR detection of influenza RNA in nasal swabs, decreased after vaccination (Table 2). A notable decrease in prevalence of influenza A virus RNA-positive nasal swabs occurred after the second vaccination, with 14-day-old piglets having no detectable influenza virus in nasal swabs as early as 3 weeks post vaccination. There were no influenza RNA-positive nasal swabs from suckling pigs at 5, 7, and 9 weeks post vaccination. One swab from a 21-day-old pig was classified as suspect (cycle threshold value 37) nine weeks after vaccination (Table 2).
Table 2: Results of testing nasal swabs from suckling piglets for swine influenza virus by RRT-PCR during and after vaccination of the entire breeding herd with a commercial influenza vaccine*
* All breeding females in a 1200-sow farrow-to-wean herd infected with influenza H1N2 were vaccinated twice with a commercial vaccine (FluSure XP; Pfizer Animal Health, Kalamazoo, Michigan). Suckling piglets were monitored beginning 2 weeks after the first vaccination was administered and then every other week for seven sampling events. During each sampling event, nasal swabs were collected from 14- and 21-day-old piglets (n = 30 per age group) and tested for influenza virus by RRT-PCR with cycle threshold (Ct) cut-off positive Ct < 35, suspect = Ct 35-40, and negative = Ct > 40. RRT-PCR = real-time reverse-transcriptase polymerase chain reaction; Pos = positive; Sus = suspect; Neg = negative. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The decrease in prevalence of influenza A virus RNA-positive nasal swabs was affected by piglet age and time post vaccination, as determined by the final statistical model, which retained only these two variables and was described by the log odds of testing positive for influenza virus = 2.017 + 0.138 × (age) - 0.802 × (weeks after vaccination) + αi (random effect of the ith litter). The likelihood of a suckling pig testing positive for influenza virus increased 1.14 times with each increasing day of age (CI, 1.02-1.28) and decreased 0.44 times with each successive week after vaccination (CI, 0.34-0.57).
Nursery cohorts
Table 3 summarizes results of testing nasal swabs and oral-fluid samples for swine influenza virus in the pre- and postvaccination cohorts.
Table 3: Results of testing individual nasal swabs and pen oral fluids by RRT-PCR for swine influenza virus* in nursery pigs from pre- and postvaccination cohorts weaned from a 1200-sow breeding herd that underwent a respiratory disease outbreak in suckling piglets
* Thirty nasal swabs and 16 oral-fluid samples were collected every other week starting at 4 weeks of age. Each oral-fluids sample represented 30-40 pigs. RRT-PCR cycle threshold (Ct) cut-off: positive Ct < 35; suspect = Ct 35-40; negative = Ct > 40. † In the pre-vaccination cohort at 4 weeks of age, only two oral-fluid samples were collected. ‡ No samples were collected from the pre-vaccination cohort at 10 weeks of age, as the pigs had been moved to the off-site finisher barn. RRT-PCR = real-time reverse-transcriptase polymerase chain reaction; Pos = positive; Sus = suspect; Neg = negative; NT = not tested. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Nursery cross-sectional monitoring
Nursery cross-sectional monitoring results are summarized in Table 4. In the last two 4-week-old groups of pigs tested during the study, no nasal swabs or oral fluids tested positive.
Table 4: Results of cross-sectional monitoring of nursery pigs by RRT-PCR for swine influenza virus*
* Cross-sectional sampling performed at weeks 10, 12, 14, and 16 of 2011 during the 2 months post vaccination of the sows weaning pigs into this off-site nursery; nasal swabs, n = 30 per age group; pen oral-fluid samples, n = 16 per age group. Each oral-fluid sample represented 30-40 pigs. Vaccination and sampling protocols described in Figure 2. RRT-PCR cycle threshold (Ct) cut-off: positive Ct < 35; suspect = Ct 35-40; negative = Ct > 40. Pos = positive; Sus = suspect; Neg = negative; NS = nasal swab; OF = oral fluids. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Discussion
Mass vaccination approaches for viral disease control in swine, together with other management interventions, have been reported for Aujeszky’s disease,12 foot-and-mouth disease,13 and porcine reproductive and respiratory syndrome virus (PRRSV)14-16 in both experimental and field scenarios. In some cases,12,13 mass vaccination was part of an elimination campaign for a specific pathogen. To the authors’ knowledge, there have been no scientific reports regarding mass vaccination as a control measure for swine influenza. In many US herds, gestating sows are vaccinated before farrowing, and in some herds, growing pigs are vaccinated17 with either commercial or autogenous vaccines. Perhaps logistics or lack of resources explain why mass vaccination is not a common practice. Nevertheless, results of this case show that it is apparently worth the effort in endemically or clinically affected herds.
Prevalence of influenza A virus in adult females in the case herd was minimal, which is in agreement with an earlier study18 in which 60 nasal swabs from breeding females were collected in two endemically infected breeding herds without finding a positive result. An explanation for this could be that breeding females have some immunity due to previous infection with field viruses. Van Reeth et al19 demonstrated that after an H1N2 challenge, pigs previously infected with both H1N1 and H3N2 had lower viral titers in both lung and nasal secretions than did pigs that had been previously infected with either H1N1 or H3N2 alone. On the basis of this finding, it is assumed that sows have a diverse anti-influenza antibody repertoire which may play a role in decreasing the severity of clinical signs during a respiratory outbreak, as well as in virus shedding dynamics and transmission to piglets. In this herd, some sows may not have been infected, and if infected, may have shown no detectable clinical signs. In adult populations in general, duration and magnitude of nasal shedding can be quite limited, thereby decreasing the probability of detecting virus through a sow-sampling protocol.
A two-dose regimen of a killed commercial vaccine administered to the entire herd significantly increased HI antibody titers when sera were tested against five different strains. Increase in HI titers after vaccination has been reported.20 In experimental settings, pigs that were vaccinated and challenged had fewer lung lesions, shorter shedding periods, and lower clinical-signs scores than did nonvaccinated challenged pigs.21-23 Experimental vaccine studies suggest that vaccines confer protection by increasing anti-influenza antibodies that can theoretically be transferred to newborn piglets after colostrum intake.24-26 One study24 reported that piglets born to sows vaccinated before farrowing, either with an autogenous or commercial bivalent vaccine, had significantly higher HI titers than did piglets born to nonvaccinated sows. Besides protecting the sow, vaccination before farrowing can have an additional benefit in that maternally derived antibodies may provide some protection for the piglet for up to 8 to 10 weeks after birth.25 In our study, since sows had a significant increase in HI, we can hypothesize that piglets may have received a high concentration of antibodies in colostrum (high piglet HI), which is believed26 to be correlated with protection, and that may have played a role in reducing transmission. However, in this case, antibodies were not measured in piglets.
Viral shedding in suckling piglets 14 and 21 days of age was detected in the first three sampling points, consisting entirely of pigs born to sows that had not been vaccinated or had received only the first dose. Three weeks after the second dose, virus was found only in 21-day-old piglets. That point marked the transition between pigs born to sows receiving one and two doses. During the next 6 weeks of sampling, all nasal swabs tested negative except for one suspect result in a 21-day-old piglet. The increase in HI titers in sows induced by vaccination may have played a role in the decreased prevalence of nasal shedding in suckling piglets, associated with an increase in the level of maternally derived antibodies as reported earlier.24 This may have improved herd immunity, thus decreasing transmission of the virus. In two earlier studies,27,28 pigs with maternally derived antibodies did become infected; however, pigs in those studies were experimentally challenged.
Our multivariate logistic regression model detected an association between the age of the pig and the presence of the virus. The odds of detecting influenza-positive piglets increased as pig age increased. This finding agrees with previous reports16 that suggested the likelihood of finding influenza-positive piglets in the suckling phase increased as the piglets got older. In this case, the odds of detecting influenza-positive piglets decreased as weeks after vaccination program increased.
Results from the cross-sectional sampling supported the observation that nasal shedding in suckling piglets decreased over time. However, by the sixth week of age (third week in the nursery), pigs became infected, which could be the result of the combination of maternally derived antibodies decreasing and horizontal transmission occurring in this continuous-flow population.
Changes in shedding prevalence in this herd may have been associated with vaccination. However, it is difficult to conclude that the decrease in shedding prevalence was solely the result of vaccination. The role of natural immunity, which occurs in convalescing animals, could not be accurately assessed in the case herd. Natural immunity is arguably more robust and protective than vaccine immunity.19
Overall, this case provides important insights into the ecology of influenza A virus in breeding farms by highlighting the role of suckling piglets as an important population in which the virus circulates and reaches endemicity. Additionally, prevalence of shedding in suckling piglets increased with age, suggesting that by the time piglets are weaned and sent to another site, the virus will be present at detectable levels. These shedding weanlings become a potential source for on-site horizontal and regional transmission. The data presented here are intriguing and suggest that implementation protocols for commercial swine influenza vaccine and their impact on the ecology of the virus in the breeding herd should be further investigated. Such protocols may have benefits beyond protecting the sow from clinical episodes.
Implications
• Vaccination of breeding females with two doses of a multivalent commercial killed swine influenza vaccine can reduce viral shedding detected in nasal swabs from their offspring.
• Suckling piglets may be a source of influenza virus infection for littermates and younger piglets, maintaining circulation of the virus.
• Mass vaccination of the breeding herd for swine influenza may play an important role in transmission dynamics.
Acknowledgements
Antigen testing and sample collection costs in this case herd were supported in whole or in part with federal funds from the NIH, National Institute of Allergy and Infectious Diseases, and Department of Health and Human Services under the contract No.HHSN266200700007C. Vaccine and antibody-testing costs were supported in part by Pfizer Animal Health, Madison, New Jersey.
References
1. Olsen CW, Brown IH, Easterday BC, Van Reeth K. Swine influenza. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, eds. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Publishing; 2006:469–482.
2. Karasin AI, Anderson GA, Olsen CW. Genetic characterization of an H1N2 influenza virus isolated from a pig in Indiana. J Clin Microbiol. 2000;38:2453–2456.
3. Webby RJ, Rossow K, Erickson G, Sims T, Webster R. Multiple lineages of antigenically and genetically diverse influenza A virus co-circulate in the United States swine population. Virus Res. 2004;103:67–73.
4. Brown IH. The epidemiology and evolution of influenza viruses in pigs. Vet Microbiol. 2000;74:29–46.
5. Torremorell M, Juarez A, Chavez E, Yescas J, Doporto JM, Gramer M. Procedures to eliminate H3N2 swine influenza virus from a pig herd. Vet Rec. 2009;165:74–77.
6. Thacker E, Janke B. Swine influenza virus: zoonotic potential and vaccination strategies for the control of avian and swine influenzas. J Infect Dis. 2008;15:19–24.
7. Capua I, Marangon S. The use of vaccination to combat multiple introductions of Notifiable Avian Influenza viruses of the H5 and H7 subtypes between 2000 and 2006 in Italy. Vaccine. 2007;25:4987–4995.
8. Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002;40:3256–3260.
9. Spackman E, Suarez DL. Type A influenza virus detection and quantitation by real-time RT-PCR. Methods Mol Biol. 2008;436:19–26.
10. Vincent AL, Ma W, Lager KM, Gramer MR, Richt JA, Janke BH. Characterization of a newly emerged genetic cluster of H1N1 and H1N2 swine influenza virus in the United States. Virus Genes. 2009;39:176–185.
11. Pedersen JC. Hemagglutination-inhibition test for avian influenza virus subtype identification and the detection and quantitation of serum antibodies to the avian influenza virus. Methods Mol Biol. 2008;436:53–66.
12. Willeberg P, Leontides L, Ewald C, Mortensen S, McInerney JP, Howe KS, Kooij D. Effect of vaccination against Aujeszky’s disease compared with test and slaughter programme: epidemiological and economical evaluations. Acta Vet Scand. 1996;90:25–51.
13. Poulin MC, Christianson WT. On-farm eradication of foot-and-mouth disease as an alternative to mass culling. Vet Rec. 2006;154:467–472.
14. Cano JP, Dee SA, Murtaugh MP, Pijoan C. Impact of a modified-live porcine reproductive and respiratory syndrome virus vaccine intervention on a population of pigs infected with a heterologous isolate. Vaccine. 2007;25:4382–4391.
15. Cano JP, Dee SA, Murtaugh MP, Trincado CA, Pijoan CB. Effect of vaccination with a modified-live porcine reproductive and respiratory syndrome virus vaccine on dynamics of homologous viral infection in pigs. Am J Vet Res. 2007;68:565–571.
16. Gillespie TG, Carroll AL. Methods of control and elimination of porcine reproductive and respiratory syndrome virus using modified live vaccine in a two-site production system. J Swine Health Prod. 2003;11:291–295.
17. USDA. Swine 2006, Part II. Reference of Swine Health and Health Management Practices in the United States, 2006. USDA:APHIS:VS, CEAH. Fort Collins, Colorado. #N479.1207. 2007. Available at: http://www.aphis.usda.gov/animal_health/nahms/swine/downloads/swine2006/Swine2006_dr_PartII.pdf. Accessed 1 September 2012.
*18. Allerson M, Gramer M, Torremorell M. The disease ecology of influenza virus in swine breeding farms. Proc AASV. Phoenix, Arizona. 2011;37.
19. Van Reeth K, Gregory V, Hay A, Pensaert M. Protection against a European H1N2 swine influenza virus in pigs previously infected with H1N1 and/or H3N2 subtypes. Vaccine. 2003;21:1375–1381.
*20. Schaefer N, Allerson M, Gramer M, Wayne S, Nerem JL, Bradford JR, Newberry JR. Evaluation of swine influenza virus serum antibody levels following vaccination of sows with FluSure and Biomune autogenous vaccines. Proc AASV. San Diego, California. 2008;289–291.
21. Van Reeth K, Labarque G, De Clercq S, Pensaert M. Efficacy of vaccination of pigs with different H1N1 swine influenza viruses using a recent challenge strain and different parameters of protection. Vaccine. 2001;19:4479–4486.
22. Lee JH, Gramer MR, Joo HS. Efficacy of swine influenza A virus vaccines against an H3N2 virus variant. Can J Vet Res. 2007;71:207–212.
23. Vincent AL, Ciacci-Zanella JR, Lorusso A, Gauger PC, Zanella EL, Kehrli ME Jr, Janke BH, Lager KM. Efficacy of inactivated swine influenza virus vaccines against the 2009 A/H1N1 influenza virus in pigs. Vaccine. 2010;28:2782–2787.
*24. Allerson M, Schaefer N, Gramer M, Wayne S, Nerem JL, Bradford JR, Newberry JR. Maternally derived antibody transfer to piglets following SIV vaccination. Proc AASV. San Diego, California. 2008;41–44.
25. Loeffen WLA, Nodelijk G, Heinen PP, van Leengoed LAMG, Hunneman WA, Verheijden JHM. Estimating the incidence of influenza-virus infections in Dutch weaned piglets using blood samples from a cross-sectional study. Vet Microbiol. 2003;91:295–308.
26. Vincent AL, Ma W, Lager K, Janke BH, Rich JA. Swine influenza viruses a North American perspective. Adv Virus Res. 2008;72:127–154.
27. Loeffen WLA, Heinen PP, Bianchi ATJ, Hunneman WA, Verheijden JHM. Effect of maternally derived antibodies on the clinical signs and immune response in pigs after primary and secondary infection with an influenza H1N1 virus. Vet Immunol Immunopathol. 2003;92:23–35.
28. Kitikoon P, Nilubol D, Erickson BJ, Janke BH, Hoover TC, Sornsen SA, Thacker EL. The immune response and maternal antibody interference to a heterologous H1N1 swine influenza virus infection following vaccination. Vet Immunol Immunopathol. 2006;112:117–128.
*Non-refereed references.
