| Original research | Peer reviewed |
Cite as: Nguyen K, Cassar G, Friendship RM, et al. An investigation of the impacts of induced parturition, birth weight, birth order, litter size, and sow parity on piglet serum concentrations of immunoglobulin G. J Swine Health Prod. 2013;21(3):139-143.
Also available as a PDF.
SummaryObjective: To determine the impacts of induced parturition, birth weight, birth order, litter size, and sow parity on piglet serum concentrations of immunoglobulin G (IgG) . Materials and methods: In Experiment 1, sows were either induced to farrow (n = 56) or allowed to farrow naturally (n = 84). Litters of induced sows were placed immediately into a warm crèche until farrowing was complete, then all piglets were weighed and placed with the sow at the same time. Blood samples were collected at 3 days of age from one or two of the smallest pigs, one medium pig, and the largest pig in each litter for measurement of serum IgG. In Experiment 2, the firstborn and last-born piglets in 78 litters were blood sampled at 3 days of age and sera were assayed for total IgG. Results: Experiment 1. Mean serum IgG concentration was higher in piglets from induced litters than in piglets from control sows (P < .001). Serum IgG concentrations increased with increased piglet weight (P < .001). Piglets from larger litters had lower serum IgG (P < .001). Serum IgG concentrations in piglets were not affected by sow parity (Experiment 1) or birth order (Experiment 2). Implications: Supervision of farrowing may allow for improved colostrum intake with benefits to passive immunity. However, first-born pigs do not appear to get a disproportionate share of available immunoglobulins. | ResumenObjetivo: Determinar el impactos del parto inducido, peso al nacimiento, orden de nacimiento, tamaño de camada, y paridad de la hembra en las concentraciones de inmunoglobulina G (IgG por sus siglas en inglés) del suero del lechón. Materiales y métodos: En el Experimento 1, se indujo a las hembras a parir (n = 56) o se les permitió parir naturalmente (n = 84). Las camadas de las hembras inducidas se colocaron inmediatamente en una lechonera atemperada hasta que el parto terminó, entonces se pesaron todos los lechones y se colocaron con la hembra al mismo tiempo. Se recolectaron muestras de sangre a los 3 días de edad de uno o dos de los lechones más pequeños, un lechón mediano, y el lechón más grande en cada camada para medir el suero IgG. En el Experimento 2, en 78 camadas se tomaron muestras de sangre del primero y del último lechón en nacer a los 3 días de edad y se midió el total de IgG en el suero. Resultados: Experimento 1. La media de la concentración IgG del suero fue más alta en los lechones de las camadas inducidas que en los lechones de las hembras control (P < .001). Las concentraciones de IgG del suero aumentaron con el aumento de peso del lechón (P < .001). Los lechones de camadas más grandes tuvieron menos IgG en el suero (P < .001). Las concentraciones de IgG del suero en los lechones no se afectaron con relación a la paridad de la hembra (Experimento 1) o el orden de nacimiento (Experimento 2). Implicaciones: La supervisión del parto puede permitir un mejor consumo de calostro con beneficios para la inmunidad pasiva. Sin embargo, los cerdos que nacieron primero no parecen obtener una porción desproporcionada de las inmunoglobulinas disponibles.
| ResuméObjectif: Déterminer les impacts de la parturition induite, du poids à la naissance, de l’ordre de naissance, de la taille de la portée, et de la parité de la truie sur les concentrations sériques d’immunoglobuline G (Ig G) des porcelets. Matériels et méthodes: Dans l’expérience 1, les truies étaient soit induites à mettre-bas (n = 56) ou laissées à mettre-bas naturellement (n = 84). Les portées des truies induites étaient placées immédiatement dans une crèche chauffée jusqu’à ce que la mise-bas soit complétée, les porcelets étaient ensuite pesés et mis avec la truie tous en même temps. Des échantillons sanguins étaient prélevés à 3 jours d’âge à partir d’un ou deux des porcelets les plus petits, un porcelet de taille moyenne, et le porcelet le plus gros dans chaque portée pour mesurer les IgG sériques. Dans l’expérience 2, à l’âge de 3 jours du sang a été prélevé des premiers et derniers porcelets nés dans 78 portées et le sérum testé pour les IgG totales. Résultats: Expérience 1. La concentration sérique moyenne des IgG était plus élevée chez les porcelets des mises-bas induites comparativement aux porcelets des truies témoins (P < .001). Les concentrations sériques d’IgG augmentaient avec le poids des porcelets (P < .001). Les porcelets provenant des portées les plus nombreuses avaient les concentrations sériques d’IgG les plus faibles (P < .001). Les concentrations sériques d’IgG des porcelets n’étaient pas influencées par la parité des truies (Expérience 1) ou l’ordre de la naissance (Expérience 2). Implications: La supervision des mises-bas pourrait permettre une meilleure prise de colostrum avec des bénéfices pour l’immunité passive. Toutefois, les porcelets nés en premier ne semblent pas obtenir une portion disproportionnée d’immunoglobulines disponibles. |
Keywords: swine, farrowing supervision, piglets, immunoglobulin G
Search the AASV web site
for pages with similar keywords.
Received: August 23, 2012
Accepted: January 21, 2013
Pigs are born with virtually no circulating immunoglobulins and a relatively immature immune system.1 Therefore, it is vital that neonates ingest adequate amounts of colostrum to establish a robust passive immunity. At parturition, colostrum is high in total solids and protein but low in sugar and fat content. Mammary secretions gradually change from colostrum to milk within 4 days postpartum.2 Colostral immunoglobulins are predominantly immunoglobulin G (Ig G) (80%), but this decreases rapidly relative to IgA, which constitutes 70% of the immunoglobulin in mature milk. Sow colostrum contains approximately 30 to 70 mg per mL of IgG,3 although concentrations differ among sows. Newborn piglets have serum Ig concentrations < 1 mg per mL, but after colostrum uptake, concentrations should increase to 25 to 30 mg per mL.4
Due to variation among sows in colostral quality and the many factors influencing ingestion of colostrum and absorption of colostral IgG by piglets, there is a high degree of variation within and among litters in the quantity of circulating IgG in piglets.5 Absorption of colostral IgG is crucial for protection against infectious diseases and for development of the immune system,6-8 but may be problematic in large litters and for small pigs because of the difficulty in obtaining a teat suckling position.9 Weak pigs with poor immunity are more likely to be infected with pathogens, which may cause problems with infectious disease after weaning, when passive immunity has waned.
Supervision and assistance at farrowing has been shown to reduce losses due to stillbirth,10 but little attention has been paid to other potential benefits, such as ensuring that all piglets receive colostrum within the first few hours of birth. The purposes of this study were to determine if induction of parturition with supervision of colostral intake affects serum IgG level in piglets, compared to piglets born unsupervised, and to determine whether serum IgG level in piglets is affected by birth weight, birth order, litter size, or sow parity.
Material and methods
The protocols in this study were approved by the University of Guelph Animal Care Committee (Experiment 1) and the Michigan State University Institutional Animal Care and Use Committee (Experiment 2).
Experiment 1 was conducted on a 600-sow (Yorkshire × Landrace) farrow-to-feeder-pig farm located near Guelph, Ontario, Canada. Each sow was randomly assigned to one of two treatment groups, with similar parity distribution between the two groups. Sows farrowed over a 1-month period. The average gestation length for this farm was 115 days. Sows were either induced to farrow by intravulval injection of the prostaglandin F2α analogue cloprostenol (1 mL Planate; Schering-Plough, Kirkland, Quebec) at 8:00 am and 2:00 pm on day 114 of gestation (Induced; n = 56) or were allowed to farrow naturally (Control; n = 84). It was intended that an approximately equal number of sows would be included in each treatment group; however, 19 Induced sows began farrowing before researchers arrived the morning after induction, and these sows were excluded from the trial. Details of assistance and handling of newborn piglets are provided in a previously published paper.10 Briefly, Induced sows farrowing during working hours the day after induction were provided with a high level of supervision: piglets were dried and kept warm, and workers ensured that each piglet received colostrum through supplemental colostrum feedings or by placing each piglet at a teat. Control sows received supervision twice daily during feeding as per the farm’s usual practice. The Control piglets were not dried and were left on their own to find the heated creep area and to access the udder. The only intervention was to euthanize weak or injured piglets.
At farrowing, piglets from Induced sows were ear notched for birth order and litter number, and they were weighed. Additional weights were obtained at 3 days and 21 days. Control piglets were weighed only at 3 and 21 days of age. Piglet 3-day weight was used to arbitrarily categorize piglets as “small” (< 1.1 kg), “medium” (≥ 1.1 kg and < 2.0 kg), and “large” (≥ 2.0 kg). At the day-3 weighing, castration was performed and iron dextran injections were administered. Preweaning mortality was recorded, but cause of death was not determined. At 3 days of age, a blood sample from the orbital venous sinus was collected from one or two of the smallest piglets, a medium piglet, and the largest piglet of each litter. Blood samples were centrifuged and sera separated and stored at -70°C until analyzed for IgG concentrations using a commercial pig IgG ELISA kit (Bethyl Laboratories, Montgomery, Texas).
Experiment 2 was conducted on a 2500-sow (Yorkshire) farrow-to-feeder-pig operation in Michigan. Gestating sows were housed in outdoor paddocks, but were moved indoors prior to the expected farrowing day. Farrowing induction was not performed. Sows were observed continuously (24 hours per day). At farrowing, the first and last pigs born were ear notched for identification. At 3 days of age, a blood sample from the orbital venous sinus was collected from the firstborn and last-born piglets of each sampled litter (n = 78 litters). Blood samples were centrifuged and sera stored at -70°C until analyzed for IgG concentrations as described above.
Statistical analysis
All data were entered into an Excel spreadsheet and imported into Stata 10 Intercooled for Windows XP (StataCorp LP, College Station, Texas) for data analysis: P < .05 was considered statistically significant.
In Experiment 1, the median IgG concentration for the 466 piglets in the study (Induced and Control groups combined) was arbitrarily chosen as a cutoff point between piglets with “low” and “high” concentrations of IgG. Univariable analysis of the independent variables “treatment group” and “birth order” and their impact on serum IgG concentration was performed by Wilcoxon rank-sum test. In addition, a mixed multivariable linear regression model with sow as a random effect was used to investigate the impact of induction and supervision on the concentration of IgG in piglet sera. It is possible that the level of IgG is affected by other parameters, including parity, size of piglet (small, medium, or large), and number of pigs nursing. Therefore, these parameters were included in the analysis to control for possible confounding and to evaluate their impact on serum IgG concentrations in piglets.
For Experiment 2, a linear regression model with sow as a random effect was applied to determine the association between birth order (firstborn and last-born) and serum IgG of the piglets. Serum IgG values in both experiments were transformed to square roots to meet the normal distribution assumption in linear regression analysis.
Results
For Experiment 1, samples from 198 piglets from Induced sows and 268 samples from Control sows were analyzed. The mean concentrations of IgG for Induced and Control piglets combined were 75.4 mg per mL and 57.3 mg per mL, respectively. The distribution of IgG concentrations for the two treatment groups are presented in Figure 1. Samples having concentrations of IgG below 63 mg per mL (the median value for the 466 piglets in the study) were classified as having low concentrations of serum IgG; 59% of Control and 37% of Induced piglets had low serum IgG concentrations (Table 1). Of the 466 piglets, 212 (45.5%), 132 (28.3%), and 122 (26.2%) were classified as small, medium, and large size, respectively. The mean IgG concentrations for small, medium, and large piglets were 51.2 ± 36.1 mg per mL, 65.9 ± 31.7 mg per mL, and 87.8 ± 45.5 mg per mL, respectively. Preweaning mortality did not differ between Induced and Control litters, as reported previously.10
Figure 1: Experiment 1. Histogram showing distribution of serum IgG concentrations at 3 days of age for 466 piglets born by Induced farrowings (red bars; n = 56 litters) or Control farrowings (blue bars; n = 84 litters). Induced sows were induced on day 114 of gestation; farrowing was constantly supervised and piglets were provided with a high level of care, including provision of colostrum. Control piglets were born to sows that farrowed without extra supervision; sows were observed twice a day and piglets were not provided with supplemental colostrum. Blood was collected from one or two of the smallest piglets, a medium piglet, and the largest piglet of each litter. 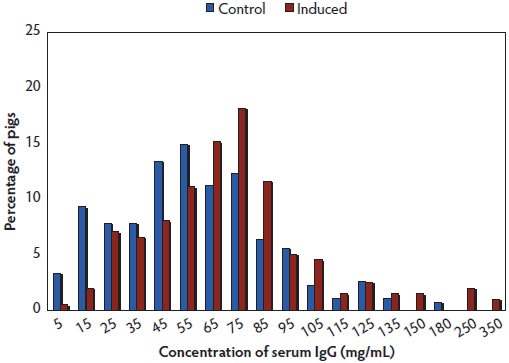 |
Table 1: Distribution of piglets with low serum IgG by treatment (Control or Induced farrowings) and by piglet birth weight (Experiment 1) *
* Induced and control farrowings described in Figure 1. Low serum IgG concentration was defined as < 63 mg/mL, the median for all 466 piglets in the experiment. |
|||||||||||||||||||||||||||||||||||||||
Induced piglets had higher serum IgG than did Control piglets (P < .001). Also, size of piglets and number of nursing piglets were correlated with serum IgG in univariable analysis (P < .001), but parity was not.
In multivariable analysis, inducing and supervising farrowing, as well as the size of piglets at birth and the number of nursing pigs, affected the level of IgG in sera (Table 2). When controlling for other independent variables, the serum IgG concentration in Induced piglets was higher than that in Control piglets (P < .001). Larger piglets had higher IgG concentrations than smaller piglets, and piglets from larger litters had lower serum IgG concentrations than piglets from smaller litters (P < .001). However, sow parity had no effect on serum IgG concentration. No confounding or interaction was evident.
Table 2: Multivariable linear regression analysis of the effect of inducing parturition and providing farrowing assistance and neonatal care on serum IgG concentration in 466 piglets (Experiment 1)*
* Treatments described in Figure 1. Piglet size categories described in Table 1. Values represent the square roots of IgG concentrations, which were transformed to square roots to meet normal distribution assumption in linear regression analysis. Ig = immunoglobin; NA = not applicable.
|
||||||||||||||||||||||||||||||||||||||||||
In Experiment 2, IgG was low in 67.9% of firstborn and 66.7% of last-born piglets. The mean IgG concentrations (± SD) for firstborn and last-born pigs were 68.2 ± 73.2 mg per mL and 61.1 ± 59.2 mg per mL, respectively. In linear regression analysis, there was no effect of birth order on serum IgG concentrations at P < .05.
Discussion
Consuming sufficient colostrum is vital for development of neonatal passive immunity. The results of Experiment 1 show that inducing parturition to facilitate supervision of farrowing and neonatal care significantly affects the serum IgG concentration by reducing the number of piglets having low serum IgG concentrations. The implication of this is that supervision of piglet delivery allows for more uniform consumption of colostrum by the piglets within the litter. However, although supervised piglets did show higher serum IgG concentrations at 3 days of age than did Control piglets, there was no evidence in this trial that there was an economic benefit associated with higher IgG levels, in that preweaning mortality did not differ between the two groups. The majority of piglets with low serum IgG concentrations were small (< 1.1 kg), presumably less competitive, and so struggled to obtain sufficient colostrum. The potentially less adequate passive immunity of these smaller piglets may increase their risk of infection. Feeding supplemental colostrum to small piglets in the supervised litters resulted in a lower proportion of small piglets in the low IgG category and supports the suggestion that timely supplemental colostrum will improve passive immunity of small piglets. Further, supplemental colostrum will also improve the energy status of small pigs, with further potential benefits to their growth and survival.
We reasoned that firstborn piglets can nurse without competition and that they are in a better position when it comes to establishing a teat suckling position. However, contrary to expectation, these studies demonstrated that firstborn piglets did not have higher serum IgG than last-born piglets, and birth order did not have a significant effect on the degree of passive immunity attained. Similarly, Devillers et al11 reported that birth order is not associated with the volume of colostrum ingested. However, Klobasa et al12 reported a significant effect of birth order on serum IgG concentrations in piglets in the first day of life. They attributed the effect to changes in colostrum composition that occur between the births of the first and last piglets in a litter. These researchers12 measured serum IgG concentrations in German Landrace piglets between 12 and 24 hours of age using radioimmunodiffusion, whereas the present study evaluated serum IgG by ELISA in 3-day-old Yorkshire pigs. It is unclear whether these differences in methodology contributed to the differences in findings. It is possible that other management parameters might affect serum IgG in piglets. In Experiment 2, factors such as parity of the sow, litter size, and other possibly contributing factors were not measured, and this may explain in part the lack of observed association between birth order and serum IgG in the piglets in this study.
In the present study, piglet weight did have an influence on the amount of IgG present in the serum, presumably due to an improved competitive position. Larger piglets have been noted to ingest larger volumes of colostrum.11 Intervention by assisting small piglets to suckle and providing additional sources of nutrition may improve their chances for survival.
The IgG concentration of colostrum is several-fold greater than the concentration present in sow serum.13 Devillers et al11 documented up to 2.8-fold differences among sows in total colostral volume. In the present study, the serum IgG concentrations in piglets demonstrated that serum IgG levels are variable even in circumstances where piglets are provided with adequate colostrum in the first few hours of life. There are few published data for IgG concentrations in 3-day-old piglets for comparison with the present study. Nielsen et al4 reported average Ig levels of 25 to 30 mg per mL, which is lower than the present results. Ariza-Nieto et al,14 using the same commercial ELISA assay used in the present study, reported a mean concentration of serum IgG of 56.8 mg per mL in 1-day-old piglets, comparable to that of piglets born to Control sows in the present study. Since the half-life of serum IgG in neonatal pigs is approximately 14 days,15 a difference of 2 days in age at sampling is unlikely to affect mean IgG concentrations substantially. Pausenberger et al,16 comparing three ELISA assays for porcine serum IgG, reported considerable differences in standard deviations among assays.
The swine industry has adopted all-in, all-out management and off-site nursery facilities in order to prevent transmission of disease from the sow herd to the young growing pigs. However, this strategy is defeated if passive immunity fails to protect piglets until they are old enough to be weaned. Porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 are examples of pathogens that are endemic on many farms, and their control depends on minimizing the leakage of viremic piglets from the farrowing room to the nursery.
Implications
• Supervision of farrowing, including ensuring adequate colostrum intake by neonatal piglets, can increase serum immunoglobulin levels, particularly in small piglets.
• First-born pigs do not appear to get a disproportionate share of available immunoglobulins.
Conflict of interest
None reported.
References
1. Wagstrom ES, Yoon K, Zimmerman JJ. Immune components in mammary secretions. Viral Immunol. 2000;13:383–397.
2. Hartmann PE, Hughes PE. Lactation. In: Dunkin AC, Taverner MR, eds. Pig Production. Amsterdam, The Netherlands: Elsevier; 1996:89.
3. Tizard IR. Veterinary Immunology: An Introduction. 8th ed. St Louis, Missouri: Saunders Elsevier; 2009:229.
*4. Nielsen JP, Kjaersgaard HD, Kristensen CS. Colostrum uptake – effect on health and daily gain until slaughter. Proc IPVS. Hamburg, Germany. 2004;2:272.
5. Simensen E, Karlberg K. A survey of preweaning mortality in pigs. Nord Vet Med. 1980;32:194–200.
6. Sangild PT. Uptake of colostral immunoglobulins by the compromised newborn farm animal. Acta Vet Scand. 2003;98(suppl):105–122.
7. Brambell FWR. The passive immunity of the young mammal. Biol Rev. 1958;33:488–531.
8. Chaniago TD, Watson DL, Owen RA, Johnson RH. Immunoglobulins in blood serum of foetal pigs. Aust Vet J. 1978;54:30–33.
9. Baxter EM, Jarvis S, D’Eath RB, Ross DW, Robson SK, Farish M, Nevison IM, Lawrence AB, Edwards SA. Investigating the behavioural and physiological indicators of neonatal survival in pigs. Theriogenology. 2008;69:773–783.
10. Nguyen K, Cassar G, Friendship RM, Dewey C, Farzan A, Kirkwood RN. Stillbirth and preweaning mortality in litters of sows induced to farrow with supervision compared to litters of naturally farrowing sows with minimal supervision. J Swine Health Prod. 2011;19:214–217.
11. Devillers N, Farmer C, Le Dividich J, Prunier A. Variability of colostrum yield and colostrum intake in pigs. Animal. 2007;1:1033–1041.
12. Klobasa F, Schröder C, Stroot C, Henning M. Passive immunization in neonatal piglets in natural rearing – effects of birth order, birth weight, litter size and parity. Berliner und Munchener Tierarztliche Wochenschrift (Berlin). 2004;117:19–23.
13. Le Dividich J, Rooke JA, Herpin P. Nutritional and immunological importance of colostrum for the newborn pig. J Agr Sci. 2005;143:469–485.
14. Ariza-Nieto C, Bandrick M, Baidoo SK, Anil L, Molitor TW, Hathaway MR. Effect of dietary supplementation of oregano essential oils to sows on colostrum and milk composition, growth pattern and immune status of suckling pigs. J Anim Sci. 2011;89:1079–1089.
15. Curtis J, Bourne FJ. Half-lives of immunoglobulins IgG, IgA and IgM in the serum of new-born pigs. Immunology. 1973;24:147–155.
16. Pausenberger A, Schuster K, Erhard MH. Investigation on the immunoglobulin G (IgG) levels in newborn piglets by use of three different ELISA tests. Tierärztliche Praxis. Ausgabe G, Grosstiere/Nutztiere. 2012;40:77–84.
*Non-refereed reference.
