| Original research | Peer reviewed |
Cite as: Meyer RE, Whitley JT, Morrow WEM, et al. Effect of physical and inhaled euthanasia methods on hormonal measures of stress in pigs. J Swine Health Prod. 2013;21(5):261–269.
Also available as a PDF.
SummaryObjective: To determine the effect of physical and inhaled euthanasia methods on mean plasma levels of three hormonal stress indicators in young pigs. Materials and methods: Plasma concentrations of cortisol, norepinephrine, and lactate were determined immediately before and after two-step electrocution (n = 39; 7.1 ± 0.5 kg), captive bolt (n = 61; 12.3 ± 1.9 kg), 70% N2/30% CO2 at a displacement rate equivalent to 20% of the chamber volume per minute (n = 16; 2.3 ± 0.3 kg), and 100% CO2 at 10% (n = 4; 1.9 ± 0.2 kg) and 20% (n = 12; 1.9 ± 0.1 kg) chamber volume displacement rate per minute. Results: Mean cortisol concentrations did not differ following captive bolt, electrocution, and 70% N2/30% CO2 or 100% CO2 at 20% of the chamber volume per minute (P > .05). The decrease in cortisol concentrations with 100% CO2 at 10% of the chamber volume per minute was different (P < .05) than the increase observed with 100% CO2 at 20% of the chamber volume per minute and different (P < .05) than the increase observed with captive bolt; however, differences were small. All methods increased mean lactate and norepinephrine concentrations post euthanasia, with no observed differences between methods. Times to loss of consciousness and loss of heartbeat were shorter with CO2 than with 70% N2/30% CO2 (P < .05). Implications: Gradual displacement administration of CO2 and 70% N2/30% CO2 produce similar plasma concentrations of stress indicators as physical euthanasia methods in young pigs. | ResumenObjetivo: Determinar el efecto de los métodos de eutanasia inhalada y física en las concentraciones de plasma promedio de tres indicadores de estrés hormonales en cerdos jóvenes. Materiales y métodos: Se determinaron las concentraciones de plasma de cortisol, norepinefrina, y lactato justo antes y después de la electrocución de dos pasos (n = 39; 7.1 ± 0.5 kg), pistola de bolos (n = 61; 12.3 ± 1.9 kg), 70% N2/30% CO2 a un índice de desplazamiento equivalente a 20% del volumen de cámara por minuto (n = 16; 2.3 ± 0.3 kg), y 100% CO2 a 10% (n = 4; 1.9 ± 0.2 kg) y 20% (n = 12; 1.9 ± 0.1 kg) índice de desplazamiento de volumen de cámara por minuto. Resultados: Las concentraciones de cortisol promedio no difieren post eutanasia en la pistola de bolos, la electrocución, y 70% N2/30% CO2 o 100% CO2 a 20% del volumen de la cámara por minuto (P > .05). La disminución en las concentraciones de cortisol con 100% CO2 a 10% del volumen de la cámara por minuto fue diferente (P < .05) al aumento observado con 100% CO2 a 20% del volumen de la cámara por minuto y diferente (P < .05) al aumento observado con la pistola de bolos; sin embargo, las diferencias fueron pequeñas. Todos los métodos aumentaron las concentraciones de norepinefrina y lactato promedio post eutanasia sin diferencias entre métodos. Los tiempos para la pérdida de conciencia y latidos fueron más cortos con CO2 que con 70% N2/30% CO2 (P < .05). Implicaciones: La administración gradual de desplazamiento de CO2 y 70% N2/30% CO2 produce concentraciones de plasma de indicadores de estrés similares a los métodos de eutanasia en cerdos jóvenes.
| ResuméObjectif: Déterminer l’effet de méthodes physiques et par inhalation d’euthanasie sur les concentrations plasmatiques moyennes de trois indicateurs hormonaux du stress chez les jeunes porcs. Matériels et méthodes: Les concentrations plasmatiques de cortisol, de norépinephrine, et de lactate ont été déterminées avant et après une électrocution en deux étapes (n = 39; 7,1 ± 0,5 kg), avec un percuteur (n = 61; 12,3 ± 1,9 kg), 70% N2/30% CO2 à un taux de déplacement équivalent à 20% du volume de la chambre par minute (n = 16; 2.3 ± 0,3 kg), et 100% CO2 à 10% (n = 4; 1,9 ± 0,2 kg) et 20% (n = 12; 1,9 ± 0,1 kg) de taux de remplacement du volume de la chambre par minute. Résultats: Les concentrations moyennes de cortisol n’ont pas différé post-euthanasie pour le percuteur, pour l’électrocution, pour 70% N2/30% CO2 ou 100% CO2 à 20% du volume de la chambre par minute (P > .05). La diminution des concentrations de cortisol avec 100% CO2 à 10% du volume de la chambre par minute était différente (P < .05) de l’augmentation observée avec 100% CO2 à 20% du volume de la chambre et différente (P < .05) de l’augmentation observée avec le percuteur; toutefois, les différences étaient minimes. Toutes les méthodes causèrent une augmentation post-euthanasie des concentrations moyennes de lactate et de norépinephrine, sans que de différences ne furent observées entre les méthodes. Les délais pour la perte conscience et de battement cardiaque étaient plus courts avec 100% de CO2 qu’avec 70% N2/30% CO2 (P< .05). Implications: L’administration de CO2 et de 70% N2/30% CO2 par déplacement graduel a entrainé chez des jeunes porcs des concentrations plasmatiques d’indicateurs de stress similaires à celles observées lors d’euthanasie par des méthodes physiques. |
Keywords: swine, inhaled gases, captive bolt, electrocution, euthanasia
Search the AASV web site
for pages with similar keywords.
Received: February 12, 2013
Accepted: April 9, 2013
Rapid methods for on-farm swine depopulation are required in response to emergencies such as control of catastrophic infectious diseases or exigent situations caused by natural or man-made disasters. Systems currently used to euthanize swine, which rely on handling and restraint of individual animals (as occurs daily on most farms) will likely prove much too slow to stem the spread of disease. Arguably, the worst-case scenario for the United States is an outbreak of foot-and-mouth disease (FMD) in an area with a high population of animals with cloven hooves (even-toed ungulates of the mammalian order Artiodactyla). Swine depopulation is important: swine are known amplifiers of the FMD virus, and all animals on infected premises with clinical disease should be killed immediately.1 To date, the US swine industry has been fortunate in that it has not had a foreign animal disease (FAD) epidemic for decades and has successfully eradicated both classical swine fever (CSF; eradicated in 1978) and FMD (last outbreak in 1929). However, since 2000, there have been many major outbreaks around the world of FADs affecting swine.2
It is important from an animal-welfare perspective that depopulation methods be both humane and efficient. Certain hormones, such as cortisol and norepinephrine from the hypothalamic-pituitary-adrenal system, commonly have been used to assess levels of stress in pigs.3 In addition, plasma calcium, magnesium, free fatty acids, glucose, and thyroid hormones have been used to help evaluate stress status.4 In a review, Shaw and Tume4 stated that both electrical and mechanical stunning for slaughter, methods that result in immediate unconsciousness, can dramatically increase catecholamine levels (epinephrine and norepinephrine). While cortisol levels seem to be unaffected by stunning method in abattoirs,4 the number of negative interactions of pigs with people are positively correlated with plasma cortisol concentrations.5 Similarly, lactate, a product of glycogenolysis, also increases after stressors such as non-gentle handling.5,6
The feasibility of using CO2 for emergency on-farm swine depopulation has been previously described.7 Carbon dioxide is denser than air, has been extensively studied as a pre-slaughter, controlled-atmosphere stunning agent in pigs,8 and is approved as an agent for euthanasia of swine by the AVMA9 and the AASV.10 Carbon dioxide inhalation causes acute respiratory acidosis and produces a reversible anesthetic state by rapidly decreasing intracellular pH.11,12 Although Duroc and Large White pigs will tolerate 30% CO2 to gain access to a food reward, hypoxia produced by inhalation of the inert gases nitrogen or argon has been proposed by others as less aversive alternatives to CO2 for pre-slaughter stunning of swine.12-15 However, early removal from argon or nitrogen stunning-gas mixtures results in rapid return to consciousness. Thus, when inert gases are used for euthanasia, O2 levels < 2 volume percent and exposure times > 7 minutes are required to ensure killing.14 In contrast, CO2 can render animals unconscious and kill over a wide range of concentrations, even with O2 levels > 2 volume percent.16 Carbon dioxide is recommended by the World Organization for Animal Health (OIE) for killing neonatal pigs, but not adult pigs, for disease purposes.17 However, a CO2 displacement rate of 10% to 30% of the chamber volume per minute is currently recommended for euthanasia of other species, as this rate results in unconsciousness prior to onset of pain due to carbonic acid activation of ocular and mucous membrane nociceptors.9,18
The purpose of the current research was to determine the effect of physical euthanasia methods resulting in immediate unconsciousness (electrocution, captive bolt) and inhaled euthanasia methods resulting in delayed unconsciousness (70% N2/30% CO2, 100% CO2) on plasma concentrations of three commonly measured indicators of stress: plasma cortisol, norepinephrine, and lactate in young pigs. Our hypothesis was that in young pigs being euthanized, plasma cortisol, norepinephrine, and lactate levels would be no different whether the method of euthanasia was inhaled (CO2 or 70% N2/30% CO2 gas mixture) or physical (electrocution or captive bolting).
Materials and methods
All procedures were approved by the North Carolina State University Institutional Animal Care and Use Committee, performed in accordance with the Public Health Service Policy for Humane Care and Use of Laboratory Animals, and complied with the AVMA Guidelines on Euthanasia.9
Crossbred pigs (Landrace × Yorkshire sows crossed to Duroc-based boars) were obtained from the university swine teaching unit. Two physical methods (captive bolt and electrocution) were studied, as well as three inhaled gas methods (100% CO2, administered at either 10% or 20% of the container volume per minute and 70% N2/30% CO2, administered at 20% of the container volume per minute). To reduce the number of pigs being killed, pigs undergoing physical methods included animals that could not enter the food chain at the end of two unrelated nutritional experiments. Pigs used for inhaled-methods studies were obtained specifically for these studies. All pigs were healthy at the time of the study and came from a healthy herd with no respiratory diseases.
In the physical methods electrocution group, 39 pigs (mean body weight [BW] 7.1 ± 0.5 kg) were euthanized using a two-step sequential application (across the brain and across the heart).9 In the physical methods captive-bolt group, 62 pigs (mean BW 12.3 ± 1.9 kg) were euthanized by means of a Schermer captive bolt (QC Supply, Schuyler, Nebraska) loaded with a #17 blank cartridge applied above eye level on the mid-line of the forehead and aiming towards the tail of the pig.10
In the three inhaled-methods groups, 16 pigs (mean BW 2.3 ± 0.3 kg) were euthanized with a 70% N2/30% CO2 mix (Airgas, Inc, Radnor, Pennsylvania) administered at a displacement rate equal to 20% of the chamber volume per minute. For the two CO2 groups, pigs were euthanized with 100% CO2 (Airgas, Inc) administered at 10% of the chamber volume per minute for 10 minutes (n = 4; mean BW 1.9 ± 0.2 kg), or at 20% of the chamber volume per minute for 5 minutes (n = 12; mean BW 1.9 ± 0.1 kg). Pigs were convenience sampled from the available litters on the day of testing. All CO2 tests were performed on the same day and all 70% N2/30% CO2 tests were performed on the same day. As only four pigs could be placed inside the test chamber at one time, each test comprised four pigs with the specified gas treatment applied.
With gradual displacement application of inhaled gases, rate of rise of gas concentration is an exponential process dependent on chamber volume and gas displacement rates such that time to loss of consciousness, and eventually death, is a function of the time constant for that container.7 Assuming the starting concentration of the gas being introduced into a container (washed-in) is near zero, one time constant is required to raise the introduced gas concentration within the container to (0.632 × wash-in gas concentration), two time constants are required to raise the introduced gas concentration to (0.865 × wash-in gas concentration), and three time constants are required to raise the introduced gas concentration to (0.95 × wash-in gas concentration).7 The time constant for a container can be calculated as the container volume divided by the gas displacement rate.7 Thus, a 10% per minute volume displacement rate represents a time constant of 10 minutes (1 ÷ 0.1), while a 20% displacement rate represents a time constant of 5 minutes (1 ÷ 0.2). When using an inert gas mix such as 70% N2/30% CO2, three time constants (15 minutes at a 20% displacement rate) are required to produce a hypoxic killing atmosphere (defined as residual O2 levels < 2 volume percent).
Gases were administered in a purpose-built 114-L Plexiglas chamber (762 mm × 457 mm × 311 mm) with gloved portholes through which operators could manage the pigs within the sealed chamber (Figure 1). Two other ports allowed for the wash-in and wash-out of tested gases. Gases were supplied through a precision flow meter (65-mm correlated flow meter, P/N Y21-B755, brass with a Carboloy float; Airgas, Inc) and regulator (Compressed Gas Regulator, model GPT270–125–580-BV; National Specialty Gases, Durham, North Carolina) calibrated to the density of the individual gases. Time to start of open-mouth breathing and time to loss of righting reflex, as a proxy for loss of consciousness,19-22 were determined from videotape. Time to loss of heart beat, as estimated time to death, was determined by physical palpation of the thorax through the gloved portholes. Gases were cleared from the chamber following each test using a leaf blower (Husqvarna 125BT Power Blower; Husqvarna, Charlotte, North Carolina).
Figure 1: Schematic of the purpose-built 114-L Plexiglas chamber designed for euthanasia of small pigs and accommodating four pigs concurrently. Sixteen pigs, mean body weight (BW) 2.3 ± 0.3 kg, were euthanized with 70% N2/30% CO2 administered at a displacement rate equal to 20% of the chamber volume/minute; four pigs, mean BW 1.9 ± 0.2 kg, and 12 pigs, mean BW 1.9 ± 0.1 kg, were euthanized with 100% CO2 administered at 10% of the chamber volume/minute for 10 minutes or at 20% of the chamber volume/minute for 5 minutes, respectively. All diameter measurements are in mm. 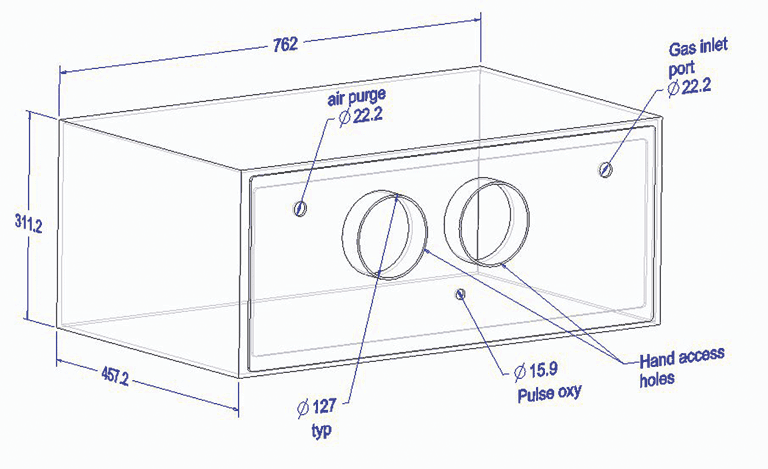 |
Gas levels within the Plexiglas chamber were measured using a CO2 sensor (Edinburgh Instruments, Ltd, Livingston, West Lothian, United Kingdom) and an oxygen sensor (Model UFO-130–2G; Teledyne Analytical Instruments, City of Industry, California). The gas sampling system was controlled through the LabVIEW program (National Instruments, Austin, Texas) running on a laptop personal computer (PC). Using vacuum pumps, samples were drawn through vinyl tubing (Klearon K010 3.5 mm ID, 6.4 mm OD; Kuriyama of America, Schaumburg, Illinois) into the gas sensors at the sensor manufacturer’s recommended flow rate of 0.722 L per minute. Samples were captured, drawn into two proportioned flow paths, and simultaneously analyzed in the CO2 and oxygen sensors. Data was logged in LabVIEW on the laptop PC.
For computational fluid dynamic (CFD) modeling of gas levels, a three-dimensional solid model representing the Plexiglas chamber volume and shape was created in PTC Creo 1.0 (http://creo.ptc.com,). This was basically a thin-walled solid model having inside dimensions matching the inside chamber dimensions. The CFD software used was FloEFD for FEP11.3 (Mentor Graphics, Wilsonville, Oregon) which runs inside Creo. Once the solid model of a given setup was created, the boundary conditions and initial conditions were specified in FloEFD by setting the pressures and flow rates and gas compositions at all openings. A more detailed description of CFD methods can be found in Stikeleather et al.23
Sample handling
Blood samples were collected immediately before and after euthanasia in all treatment groups. Pre-euthanasia, blood was collected by experienced personnel from the anterior vena cava by venipuncture with a 21-gauge needle and a 7-mL EDTA Vacutainer tube (Becton, Dickinson and Company, Franklin Lakes, New Jersey). Pigs undergoing physical methods were housed individually. Each pig was removed from its pen by the caretakers, a blood sample was collected, and the pig was then euthanized. For the pre-euthanasia sample, the larger pigs undergoing physical euthanasia methods were held in a supine position in a V-shaped trough by the project caretakers; one person restrained the pig manually while a second person took the blood sample. The smaller pigs undergoing inhalation methods were sampled within minutes of being removed from the sow, and then immediately placed into the testing chamber. Pigs undergoing inhalation methods were held in a supine position, without the V-shaped trough, on a flat tabletop with one person restraining the pig manually (REM) while a second person took the blood sample (WEMM). The post-euthanasia sample was collected directly into EDTA Vacutainer tubes from the severed vessels in the area of the brachial-plexus immediately after application of physical methods or after loss of heartbeat with the inhalation methods. After 10 minutes, blood samples were placed above, but not in ice to avoid hemolysis. After transport to the laboratory, samples were centrifuged at 4°C and plasma was stored at -20°C until assayed.
Laboratory assays
Plasma cortisol concentrations were determined using a commercially available radioimmunoassay kit (Siemens Medical, Los Angeles, California) in accordance with the manufacturer’s protocol and as previously conducted.24 The inter- and intra-assay coefficients of variation were 6.8% and 4.1%, respectively. Plasma concentrations of norepinephrine were measured using a commercially available assay (ALPCO Diagnostics, Salem, New Hampshire) validated for porcine plasma in our laboratory.24 As previously reported, 300-µL samples were assayed according to the manufacturer’s instructions.24 Sensitivity of the norepinephrine assay was 37.5 pg per mL. Standards and pooled aliquots of porcine plasma were parallel over a serum volume ranging from 100 to 400 µL. The inter- and intra-assay coefficients of variation for norepinephrine were 9.2% and 7.3%, respectively. Lactate concentrations were determined in plasma from pre-euthanasia and postmortem samples using the colorimetric D-lactate assay kit (Eton Bioscience, San Diego, California) according to the manufacturer’s instructions. Inter- and intra-assay coefficients of variation were 4.6% and 3.2%, respectively. All samples were performed in duplicate.
Statistical analysis
To help avoid confounding associated with the difference in the weights of the pigs, each pig served as its own control, with the unit of analysis the individual pig and the method of euthanasia the treatment being evaluated. Differences in concentrations between pre- and post-euthanasia hormone samples, onset of open-mouth breathing, loss of righting reflex, and time to loss of heart beat were compared using an ANOVA and the procedure PROC GLM LSMEANS (SAS/STAT 9.2; SAS Institute, Cary, North Carolina). Duncan’s multiple-range test was used for multiple preplanned comparisons. A probability value of < .05 was considered statistically significant. All values are reported as the mean ± standard error (SE).
Results
The decrease in cortisol with a CO2 displacement rate of 10% of the chamber volume per minute was greater (P < .05) than the increase in cortisol with a CO2 displacement rate of 20% of the chamber volume per minute and greater (P < .05) than the increase with captive bolt (Table 1). Lactate and norepinephrine were increased post euthanasia using all five methods, with no differences among the methods (Table 1).
Table 1: Differences (least squares means and standard error) in plasma hormone concentrations pre-euthanasia and post euthanasia in pigs euthanized by physical or inhalation methods*
* Pigs euthanized by inhalation methods were confined as described in Figure 1. Mean body weights (± SD) of pigs euthanized: two-step electrocution, 7.1 ± 0.5 kg; captive bolt, 12.3 ± 1.9 kg; 100% CO2 at 10% and 20% volume (vol) displacement rates, 1.9 ± 0.2 and 1.9 ± 0.1 kg, respectively; and 70% N2/30% CO2 at 20% vol displacement rate, 2.3 ± 0.3 kg. † n = total number of samples diminished by the reported number of unusable samples. Unusable cortisol samples: two from pigs euthanized by electrocution, one from a pig euthanized by the 70% N2/30% CO2 gas method, and one from a pig euthanized by the CO2 20% vol/min method. Unusable norepinephrine samples: one from a pig euthanized by electrocution. Unusable lactate samples: four from pigs euthanized by the captive bolt method. ab Within a row, values with no common superscript are significantly different (P < .05; generalized linear model with ANOVA). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
All gases and mixtures induced open-mouthed breathing in pigs prior to loss of righting reflex, but mean latency to onset of open-mouth breathing was longest (P < .05) with 70% N2/30% CO2 administered at 20% the chamber volume per minute (Table 2). Similarly, mean time to loss of righting reflex was longest (P < .05) with 70% N2/30% CO2 administered at 20% of the chamber volume per minute (Table 2). There were no difference in mean time to loss of righting reflex between the two CO2 inhalation groups (Table 2). Mean estimated time to loss of heart beat was longest with 70% N2/30% CO2 administered at 20% of the chamber volume per minute (P < .05), while estimated time to loss of heart beat with CO2 administered at 10% of the chamber volume per minute was longer (P < .05) than when administered at 20% of the chamber volume per minute, but shorter (P < .05) than with 70% N2/30% CO2 administered at 20% of the chamber volume per minute (Table 2).
Table 2: Time to onset of open-mouth breathing, loss of righting reflex, and loss of heart beat following administration of 100% CO2 gas and 70% N2/30% CO2 gas for euthanasia of pigs*
* Pigs were confined as described in Figure 1. Mean body weights (± SD) of pigs euthanized using gas inhalation: 100% CO2 at 10% and 20% volume (vol) displacement rates, 1.9 ± 0.2 and 1.9 ± 0.1 kg, respectively; and 70% N2/30% CO2 at 20% vol displacement rate, 2.3 ± 0.3 kg. abc Within a row, values with no common superscript are significantly different (P < .05; generalized linear model with ANOVA). |
|||||||||||||||||||||||||||||||||||||||||||
Computational fluid dynamic simulation and measured gas levels within the Plexiglas chamber for CO2 administered at a displacement rate of 10% of the chamber volume per minute are presented in Figure 2. As predicted by the time constant for this container, maximum CO2 was approximately 66 volume percent after 10 minutes. Similarly, CFD simulation and measured maximum CO2 were approximately 63 volume percent after 5 minutes when CO2 was administered at 20% of the chamber volume per minute (Figure 3). With 70% N2/30% CO2 administered at 20% of the chamber volume per minute, CFD simulation and measured gas CO2 were approximately 27.5 volume percent, and O2 was 1.8 volume percent after 15 minutes (Figure 4). Note that nitrogen levels decreased over time, as 70% N2/30% CO2 is more dilute than the existing N2 normally present in air.
Figure 2: Predicted CO2 levels using computational fluid dynamics (CFD) simulation and measured CO2 levels (Test 1) within a 114-L Plexiglas test chamber. Pigs (n = 4; mean body weight 1.9 ± 0.2 kg) were euthanized with 100% CO2 (Airgas, Inc, Radnor, Pennsylvania) administered at 10% of the chamber volume per minute for 10 minutes (one time constant for this chamber). All CO2 tests were performed on the same day. Procedurally, as only four pigs could be accommodated inside the test chamber at one time, each test represents the measured gas levels within the chamber for four individual pigs. Following loss of heart beat, pigs were removed for postmortem blood sampling. Gases were cleared from the chamber using a leaf blower (Husqvarna 125BT Power Blower; Husqvarna , Charlotte, North Carolina). As predicted by the time constant for this container, maximum CO2 levels were approximately 66 volume percent after 10 minutes. 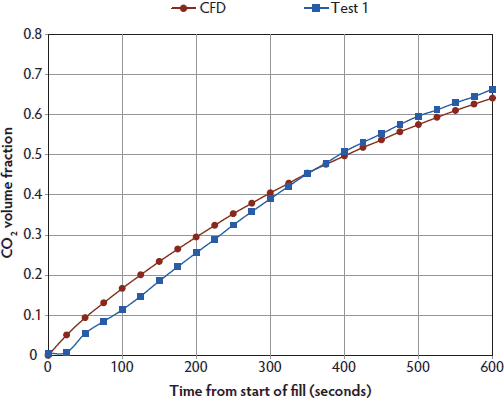 |
Figure 3: Predicted CO2 levels using computational fluid dynamics (CFD) simulation and measured CO2 levels (Test 3, Test 4) within a 114-L Plexiglas test chamber. Pigs (n = 12; mean body weight 1.9 ± 0.1 kg) were euthanized with 100% CO2 (Airgas, Inc, Radnor, Pennsylvania) administered at 20% of the chamber volume per minute for 5 minutes (one time constant for this chamber). All CO2 tests were performed on the same day. As only four pigs could be accommodated inside the test chamber at one time, each test represents measured gas levels within the chamber for four individual pigs. Following loss of heart beat, pigs were removed for postmortem blood sampling. Gases were cleared from the chamber following each test using a leaf blower (Husqvarna 125BT Power Blower; Husqvarna , Charlotte, North Carolina). Measured maximum CO2 levels were approximately 63 volume percent after 5 minutes when CO2 was administered at 20% of the chamber volume per minute. Measured gas data from Test 2 not available. 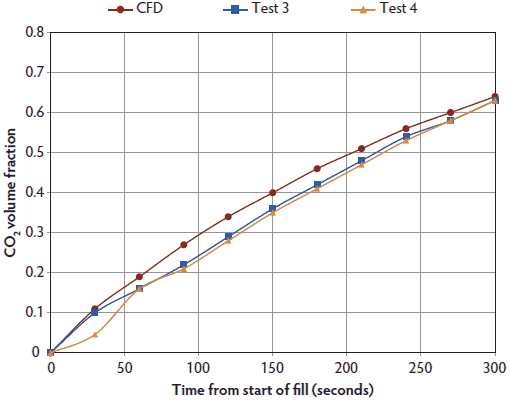 |
Figure 4: Predicted O2, CO2, and N2 levels using computational fluid dynamics (CFD) simulation (panel A), and CFD O2 and measured CO2 levels (panel B; Test 6, Test 7, Test 8) within a 114-L Plexiglas test chamber for 70% N2/30% CO2 supplied at a 20% displacement rate for 15 minutes (three time constants). All 70% N2/30% CO2 tests were performed on the same day. Pigs (n = 16; mean body weight 2.3 ± 0.3 kg) were euthanized with a 70% N2/30% CO2 mix (Airgas Inc, Radnor, Pennsylvania) administered at a displacement rate equal to 20% of the chamber volume per minute. As only four pigs could be accommodated inside the test chamber at one time, each test represents measured gas levels within the chamber for four individual pigs. Gases were cleared from the chamber following each test using a leaf blower (Husqvarna 125BT Power Blower; Husqvarna , Charlotte, North Carolina). CFD simulation and measured gas CO2 concentration were approximately 27.5 volume percent and O2 concentration was 1.8 volume percent after 15 minutes (panels A and B). Nitrogen levels decrease due to dilution of atmospheric nitrogen (78 volume percent) over time with the 70% N2/30% CO2 mixture. Three time constants are required to produce killing O2 levels < 2 volume percent with this gas mix. Nitrogen levels are not shown in panel B, as they were higher than the scaling used. Measured gas data from Test 5 not available. 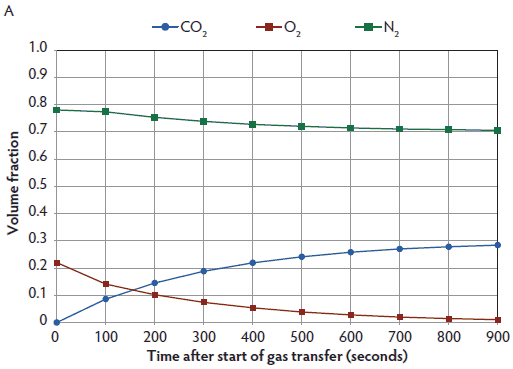 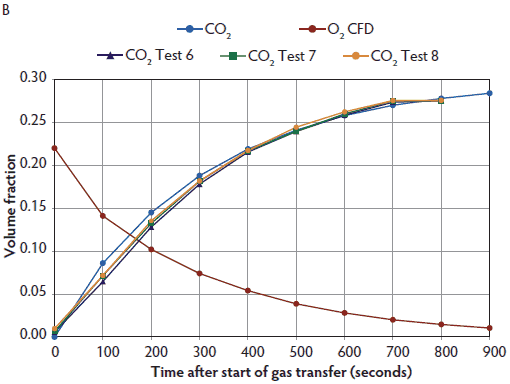 |
Discussion
All inhaled methods of euthanasia have the potential to adversely affect animal welfare because onset of unconsciousness is not immediate; further, gradual-fill CO2 exposure is currently recommended as less likely to cause pain caused by nociceptor activation by carbonic acid prior to onset of unconsciousness.9 Our findings support the use of 100% CO2 at either 10% displacement over 10 minutes (one time constant) or 20% displacement over 5 minutes (one time constant), as well as a 70% N2/30% CO2 mixture applied at a displacement rate of 20% of the volume per minute over 15 minutes (three time constants) for euthanasia of young pigs. Carbon dioxide is readily available in bulk in all areas of the United States with high pig populations, and 70% N2/30% CO2 gas mix is just one of several so called “beer gases” used commercially to push specific brews through draft systems.
As a general rule, a gentle death that takes longer is preferable to a rapid, but more distressing death. However, in some species and under some circumstances, the most humane and pragmatic option may be exposure to an aversive agent or condition that results in rapid unconsciousness with few or no outward signs of distress.9 In our study, all gases and mixtures induced open-mouthed breathing prior to loss of righting reflex; however, the mean latency to onset of open-mouth breathing and subsequent loss of righting reflex was substantially longer with 70% N2/30% CO2 than with CO2. This is similar to findings in laboratory rodents18,25 and in poultry,26 where gradual displacement administration of Ar or N2 gas mixtures to create hypoxic conditions produces overt and extended signs of behavioral distress (eg, open-mouth breathing) prior to loss of righting reflex. For comparison, time to loss of righting reflex with CO2 in our study (between 80 and 124 seconds) was similar to induction time reported for pigs of similar ages anesthetized prior to castration with 5 volume percent isoflurane (90 seconds),27 and 5 volume percent halothane (120 seconds),28 both administered by face mask. In laboratory rodents, 20% per minute gradual displacement administration of 100% CO2 produces unconsciousness at 106 seconds at a CO2 concentration of 30 volume percent.18,29-32 Although we did not attempt to document or evaluate behaviors occurring prior to loss of righting reflex, other than latency to onset of open-mouth breathing, gradual displacement administration of CO2 may be a more humane and pragmatic option for young pigs than 70% N2/30% CO2, as time to loss of consciousness is shorter. Once loss of consciousness occurs, subsequently observed activities, such as convulsions, vocalization, reflex struggling, breath holding, and defecation, can be attributed to either the second stage of anesthesia with inhaled methods, which by definition lasts from loss of consciousness to the onset of a regular breathing pattern, or release of cortical inhibition of motor activity with physical methods.9
Previous research has compared the use of CO2 with electric methods of stunning pigs for meat production. Hembrecht et al33 reported no difference in cortisol concentrations, but reported that catecholamine concentrations were higher in postmortem samples of pigs stunned with CO2 than in pigs electrically stunned. However, the procedures were performed at different abattoirs. There were no differences in plasma concentrations of cortisol, norepinephrine, or lactate post mortem in lambs electrically stunned or stunned with CO2 when the concentration of CO2 produced effective loss of consciousness.34 No direct comparison between CO2 and captive bolt stunning could be found in the literature.
Both physical and inhaled methods of euthanasia increased mean lactate and norepinephrine levels post euthanasia, with no observed differences between methods. This finding could be a consequence of the handling and restraint necessary to collect the pre-euthanasia sample,4,5 as well as factors occurring following loss of consciousness. Normal pre-slaughter handling increases serum lactate concentrations,35 while plasma lactate concentrations do not return to baseline for 2 hours after stress in pigs.6 Increased lactate levels may be due to a rise in norepinephrine, as catecholamine release causes an increase in cardiac rate, lowers pH, and produces an accumulation of lactate.33 Lactate concentrations also increase more quickly after stress than do cortisol concentrations.36 In our study, we attempted to standardize the handling required to collect the pre-euthanasia blood samples.
It has been suggested that autonomic nervous system responses to systemic stressors associated with immediate survival, such as hypoxia and hypercapnia, are likely directly relayed from brainstem nuclei and are not solely associated with higher-order CNS processing and conscious experience.37 Marked increases in circulating catecholamines, glucagon, insulin, lactate, and free fatty acids are reported in porcine experimental models where brain death is induced after induction of general anesthesia.38-40 Forslid and Augustinsson36 reported that concentrations of norepinephrine and lactate increased 1 minute after exposure of pigs to CO2. Borovsky and coworkers41 reported increased norepinephrine in rats following 30 seconds of exposure to 100% CO2. Similarly, Reed and coworkers42 observed tenfold increases in vasopressin and oxytocin concentrations in rats exposed to CO2 for 20 to 25 seconds, which was sufficient to render them recumbent, unconscious, and unresponsive. Kc and coworkers43 found that hypothalamic vasopressin-containing neurons are similarly activated in response to CO2 exposure in both awake and anesthetized rats. While both inhaled and physical methods produced similar concentrations of catecholamines and lactate in young pigs under the conditions of our study, it must be noted that our methods did not allow for assessment of concentrations increased relative to onset of unconsciousness and time to loss of heartbeat.
Our hypothesis was that inhaled CO2 or 70% N2/30% CO2 gas mixture would result in plasma cortisol, norepinephrine, and lactate concentrations in pigs being euthanized that were no different than concentrations produced by electrocution and captive bolting. Although the decrease in cortisol with a CO2 displacement rate of 10% of the chamber volume per minute was different than the increase in cortisol with captive bolt or a CO2 displacement rate of 20%, this difference was small, less than 5 µg per dL, and may not be biologically relevant. In a pig weaning-stress study, postweaning plasma cortisol levels differed by approximately 20 µg per dL.44 Similarly, stressors such as the resident intruder test usually report cortisol differences of 10 to 15 µg per dL.45 Further studies using CO2 applied at the 10% displacement rate will be required to determine whether this is a real and important effect.
Implications
• Under the conditions of this study, gradual displacement administration of CO2 or 70% N2/30% CO2 produces similar plasma concentrations of stress indicators as do physical euthanasia methods in young pigs.
• Under the study conditions, time to onset of open-mouth breathing, as well as times to loss of consciousness and heartbeat, are shorter with gradual displacement of CO2 than of 70% N2/30% CO2.
• Further study will be required to determine whether CO2 administered at a 10% displacement rate per minute is less stressful than CO2 administered at a 20% displacement rate per minute.
Acknowledgements
This study was supported by a United States Department of Agriculture grant. We also thank Drs Chad Stahl and Jack Odle, Department of Animal Science, North Carolina State University, for collecting blood samples from their pigs.
Conflict of interest
None reported.
References
1. Sutmoller P, Barteling SS, Olascoaga RC, Sumption KJ. Control and eradication of foot-and- mouth disease. Virus Res. 2003;91:101–144.
2. World Organisation for Animal Health. Foot and mouth disease portal. Available at: http://www.oie.int/animal-health-in-the-world/fmd-portal/. Accessed 14 April 2013.
3. Minton JE. Function of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system in models of acute stress in domestic farm animals. J Anim Sci. 1994;72:1891–1898.
4. Shaw FD, Tume RK. The assessment of pre-slaughter and slaughter treatment of livestock by measurement of plasma constituents – A review of recent work. Meat Sci. 1992;32:311–329.
5. Hemsworth PH, Barnett JL, Coleman GJ, Dowling S, Boyce J. The effects of fear of humans and pre-slaughter handling on the meat quality of pigs. Aust J Agric Res. 2002;53:493–501.
6. Hamilton DN, Ellis M, Bertol TM, Miller KD. Effects of handling intensity and live weight on blood acid-base balance in finishing pigs. J Anim Sci. 2004;82:2405–2409.
7. Meyer RE, Morrow WEM. Carbon dioxide for emergency on-farm euthanasia of swine. J Swine Health Prod. 2005;13:210–217.
8. Grandin T. Improving Animal Welfare – A Practical Approach. Cambridge: CAB International; 2010:168.
9. AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. March 2013. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf. Accessed 27 June 2013.
10. On Farm Euthanasia: Recommendations for the Producer. 2008. Booklet # 04259–01/09. Des Moines, Iowa: American Association of Swine Veterinarians and the National Pork Board. Available at: http://www.aasv.org/aasv/documents/SwineEuthanasia.pdf. Accessed 26 June 2013.
11. Martoft L, Lomholt L, Kolthoff C, Rodriguez BE, Jensen EW, Jørgensen PF, Pedersen HD, Forslid A. Effects of CO2 anaesthesia on central nervous system activity in swine. Lab Anim. 2002;36:115–126.
12. Raj ABM, Gregory NG. Welfare implications of the gas stunning of pigs 1. Determination of aversion to the initial inhalation of carbon dioxide or argon. Anim Welfare. 1995;4:273–280.
13. Raj ABM, Gregory NG. Welfare implications of the gas stunning of pigs 2. Stress of induction of anaesthesia. Anim Welfare. 1996;5:71–78.
14. Raj ABM. Behaviour of pigs exposed to mixtures of gases and the time required to stun and kill them: welfare implications. Vet Rec. 1999;144:165–168.
15. Dalmau A, Rodriguez P, Llonch P, Velarde A. Stunning pigs with different gas mixtures: aversion in pigs. Anim Welfare. 2010;19:325–333.
16. Raj M. Humane killing of nonhuman animals for disease control purposes. J Appl Anim Welfare Sci. 2008;11:112–124.
17. World Organization for Animal Health (OIE). Chapter 7.6. Killing of animals for disease control purposes. In: Terrestrial Animal Health Code. 20th ed. Paris, France: OIE; 2011. Available at: www.oie.int/index.php?id=169&L=0&htmfile=chapitre_1.7.6.htm. Accessed 11 June 2013.
18. Burkholder TH, Niel L, Weed JL, Brinster LR, Bacher JD, Foltz CJ. Comparison of carbon dioxide and argon euthanasia: effects on behavior, heart rate, and respiratory lesions in rats. J Amer Assoc Lab Anim Sci. 2010;49:448–453.
19. Hendrickx JF, Eger EI II, Sonner JM, Shafer SL. Is synergy the rule? A review of anesthetic interactions producing hypnosis and immobility. Anesth Analg. 2008;107:494–506.
20. Antognini JF, Barter L, Carstens E. Overview: movement as an index of anesthetic depth in humans and experimental animals. Comp Med. 2005;55:413–418.
21. Zeller W, Mettler D, Schatzmann U. Untersuchungen zur Betäubung des Schlachtgeflügels mit Kohlendioxid. Fleischwirtschaft. 1988;68:1308–1312. As cited in Raj ABM, Gregory NG. Effect of rate of induction of carbon dioxide anaesthesia on the time of onset of unconsciousness and convulsions. Res Vet Sci. 1990;49:360–363.
22. Benson ER, Alphin RL, Rankin MK, Caputo MP, Kinney CA, Johnson AL. Evaluation of EEG based determination of unconsciousness vs. loss of posture in broilers. Res Vet Sci. 2012;93:960–964.
*23. Stikeleather LF, Morrow WEM, Meyer RE, Baird CL, Halbert BV. CFD simulation of gas mixing for evaluation of design alternatives for on-farm mass-depopulation of swine in a disease emergency. Proc Am Soc Ag Biol Eng. Dallas, Texas. 2012; Technical Presentation 121338237. Available at: http://elibrary.asabe.org/azdez.asp?JID=5&AID=41889&CID=dall2012&T=1. Accessed 11 June 2013.
24. Velie BD, Cassady JP, Whisnant CS. Endocrine response to acute stress in pigs with differing backtest score. Livest Sci. 2012;145:140–144.
25. Sharp J, Azar T, Lawson D. Comparison of carbon dioxide, argon, and nitrogen for inducing unconsciousness or euthanasia of rats. J Am Assoc Lab Anim Sci. 2006;45:21–25.
26. Webster AB, Collett SR. A mobile modified-atmosphere killing system for small-flock depopulation. J Appl Poult Res. 2012;21:131–144.
27. Walker B, Jäggin N, Doherr M, Schatzmann U. Inhalation anaesthesia for castration of newborn piglets: experiences with isoflurane and isoflurane/N2O. J Vet Med A Physiol Pathol Clin Med. 2004;51:150–154.
28. Jäggin N, Kohler I, Blum J, Schatzmann U. Die Kastration von neugeborenen Ferkeln unter Halothananästhesie [The castration of newborn piglets under halothane anesthesia]. Der praktische Tierarzt. 2001;12:1054–1061.
29. Danneman PJ, Stein S, Walshaw SO. Humane and practical implications of using carbon dioxide mixed with oxygen for anaesthesia or euthanasia of rats. Lab Anim Sci. 1997;47:376–385.
30. Niel L, Weary DM. Behavioural responses of rats to gradual-fill carbon dioxide euthanasia and reduced oxygen concentrations. Appl Anim Behav Sci. 2006;100:295–308.
31. Hewett TA, Kovacs MS, Artwohl JT, Bennett BT. A comparison of euthanasia methods in rats, using carbon dioxide in prefilled and fixed flow-rate filled chambers. Lab Anim Sci. 1993;43:579–582.
32. Hornett TD, Haynes AR. Comparison of carbon dioxide/air mixture and nitrogen/air mixture for the euthanasia of rodents. Design of a system for inhalation euthanasia. Anim Technol. 1984;35:93–99.
33. Hembrecht E, Eissen JJ, Nooijen RIJ, Smits CHM, den Hartog LA, Verstegen MWA. Pre-slaughter stress and muscle energy largely determine pork quality at two commercial processing plants. J Anim Sci. 2004;82:1401–1409.
34. Bornez RM, Linares B, Vergara H. Systems of stunning with CO2 gas on Manchego light lambs: Physiologic responses and stunning effectiveness. Meat Sci. 2009;82:135–138.
35. Salajpal K, Dikic M, Karolyi D, Sinerji Z, Liker B, Kostelic A, Juric I. Blood serum metabolites and meat quality in crossbred pigs experiencing different lairage times. Ital J Anim Sci. 2005;4(suppl 3):119–121.
36. Forslid A, Augustinsson O. Acidosis, hypoxia and stress hormone release in response to one minute inhalation of 80% CO2 in swine. Acta Physiol Scand. 1988;132:222–231.
37. Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84.
38. Barklin A, Larsson A, Vestergaard C, Koefoed-Nielsen J, Bach A, Nyboe R, Wogensen L, Tønnesen E. Does brain death induce a pro-inflammatory response at the organ level in a porcine model? Acta Anaesthesiol Scand. 2008;52:621–627.
39. Chiari P, Hadour G, Michel P, Piriou V, Rodriguez C, Budat C, Ovize M, Jegaden O, Lehot JJ, Ferrera R. Biphasic response after brain death induction: prominent part of catecholamines release in this phenomenon. J Heart Lung Transplant. 2000;19:675–682.
40. Licker M, Schweizer A, Hohn L, Morel DR. Haemodynamic and metabolic changes induced by repeated episodes of hypoxia in pigs. Acta Anaesthesiol Scand. 1998;42:957–965.
41. Borovsky V, Herman M, Dunphy G, Caplea A, Ely D. CO2 asphyxia increases plasma norepinephrine in rats via sympathetic nerves. Am J Physiol. 1998;274:R19–R22.
42. Reed B, Varon J, Chait BT, Kreek MJ. Carbon dioxide-induced anesthesia results in a rapid increase in plasma levels of vasopressin. Endocrinology. 2009;150:2934–2939.
43. Kc P, Haxhiu MA, Trouth CO, Balan KV, Anderson WA, Mack SO. CO2-induced c-Fos expression in hypothalamic vasopressin containing neurons. Resp Physiol. 2002;129:289–296.
44. Kick AR, Flowers WL, Whisnant CS, Almond G. Effects of stress associated with weaning on the adaptive immune system in pigs. J Anim Sci. 2012;90:649–656.
45. Koopmans SJ, Ruis M, Dekkers R, van Diepen H, Kortes M, Mroz Z. Surplus dietary tryptophan reduces plasma cortisol and noradrenaline concentrations and enhances recovery after social stress in pigs. Physiol Behav. 2005;21:469–478.
* Non-refereed reference.
