| Original research | Peer reviewed |
Cite as: Strutzberg-Minder K, Boehmer J, Fischer S, et al. Monitoring influenza A virus infection in pigs by using a competitive enzyme-linked immunosorbent assay to detect virus antibodies in pen-based oral-fluid specimens. J Swine Health Prod. 2015;23(3):126–131.
Also available as a PDF.
SummaryObjective: To investigate monitoring an influenza A virus (IAV) infection in a finishing pig herd by testing pen-based oral fluids for antibodies against the virus using a commercial enzyme-linked immunosorbent assay (ELISA) kit. Materials and methods: Oral fluids were collected weekly from pigs 12 to 24 or 22 weeks of age in four pens (approximately 25 pigs per pen) in two consecutive batches. Serum samples were also collected from two randomly selected pigs in each pen at 12, 16, and 20 weeks of age in both batches and at 24 weeks in Batch 1 only. Oral-fluid and serum samples were tested for antibodies against IAV by a commercial competitive ELISA test kit. Oral fluids were also tested for IAV by reverse transcription-polymerase chain reaction (RT-PCR). Results: One week after initial detection of IAV in oral-fluid samples by RT-PCR, antibodies against the virus were detected in oral fluids as well as in serum samples. Oral fluids continued to test positive for antibodies 4 to 7 weeks after initial detection of virus, but with a decreasing trend in the amounts of virus antibodies detected by ELISA. All samples in Batch 1 tested negative after 9 weeks. Implications: The longitudinal profile of antibodies against IAV detected in oral fluids promises to be a useful tool for monitoring IAV infection in a pig population. A commercial competitive ELISA test kit could easily be adapted for oral fluids by modifying dilution of the specimen. | ResumenObjetivo: Investigar monitoreando una infección del virus A de influenza (IAV por sus siglas en inglés) en cerdos de finalización por medio del análisis de fluidos orales en corral en busca de anticuerpos contra el virus utilizando una unidad de análisis comercial del ensayo por inmunoabsorción ligado a enzimas (ELISA por sus siglas en inglés). Materiales y métodos: Semanalmente se recolectaron fluidos orales de cerdos de 12 a 24 o 22 semanas de edad en cuatro corrales (aproximadamente 25 cerdos por corral) en dos grupos consecutivos. También se recolectaron muestras de suero de dos cerdos seleccionados al azar en cada corral a las 12, 16, y 20 semanas de edad en ambos grupos y a las 24 semanas de edad solamente del Grupo 1. Se analizaron las muestras de suero y fluidos orales en busca de anticuerpos contra el IAV por medio de una unidad comercial de ELISA competitiva. También se analizaron fluidos orales en busca de IAV por medio de la reacción en cadena de la polimerasa de transcriptasa inversa (RT-PCR por sus siglas en inglés). Resultados: Una semana después de la detección inicial de IAV en las muestras de fluidos orales por medio de la RT-PCR, se detectaron anticuerpos contra el virus en fluidos orales y en las muestras de suero. Los fluidos orales continuaron dando resultados positivos a los anticuerpos 4 a 7 semanas después de la detección inicial del virus, pero con una tendencia descendente en la cantidad de anticuerpos virales detectados por ELISA. Todas las muestras en el Grupo 1 resultaron negativas después de 9 semanas. Implicaciones: El perfil longitudinal de anticuerpos contra el IAV detectados en fluidos orales aparenta ser una herramienta útil para el monitoreo de la infección de IAV en una población porcina. Una unidad de prueba comercial de ELISA competitiva podría ser adaptada fácilmente para fluidos orales, modificando la dilución del espécimen. | ResuméObjectif: Étudier la surveillance d’une infection par le virus influenza A (VIA) dans un troupeau de porcs en finition en testant des fluides oraux prélevés au niveau des enclos pour des anticorps dirigés contre le virus à l’aide d’une épreuve immunoenzymatique (ELISA) vendue commercialement. Matériels et méthodes: Des fluides oraux furent prélevés sur une base hebdomadaire chez des porcs âgés de 12 à 24 ou de 22 semaines dans quatre enclos (environ 25 porcs par enclos) lors de deux lots consécutifs. Des échantillons de sérums furent également prélevés de deux porcs choisis de manière aléatoire dans chacun des enclos à 12, 16, et 20 semaines d’âge dans les deux lots, et à 24 semaines dans le Lot 1 uniquement. Les échantillons de fluides oraux et de sérum furent testés pour la présence d’anticorps contre le VIA au moyen d’une trousse commerciale d’un test ELISA compétitif. Les fluides oraux furent également testés pour le VIA au moyen d’une réaction d’amplification en chaîne en utilisant la transcriptase réverse (RT-PCR). Résultats: Une semaine après la détection initiale par RT-PCR de VIA dans des échantillons de fluides oraux, des anticorps dirigés contre le virus furent détectés dans les fluides oraux ainsi que dans les échantillons de sérum. Les fluides oraux étaient toujours positifs pour la présence d’anticorps 4 à 7 semaines après la détection initiale du virus, mais avec tendance à la baisse dans les quantités d’anticorps contre le virus détectées par ELISA. Tous les échantillons du Lot 1 étaient négatifs après 9 semaines. Implications: Le profil longitudinal de détection d’anticorps contre VIA dans des fluides oraux promet de s’avérer un outil utile pour surveiller l’infection par le VIA dans une population de porcs. Une trousse commerciale d’un test ELISA compétitif a pu facilement être adaptée pour des fluides oraux en modifiant la dilution du spécimen. |
Keywords: swine, oral fluid, antibody, influenza A virus, monitoring
Search the AASV web site
for pages with similar keywords.
Received: June 2, 2014
Accepted: November 18, 2014
Monitoring and surveillance of infectious diseases in swine populations is a key component in prevention or control of clinical losses, but is often limited by the cost and inconvenience of collecting individual samples. Analysis of pen-based oral-fluid samples has proven to be an efficient method for monitoring and surveillance of various infectious diseases in swine populations.1 It has been shown that oral fluid is an appropriate specimen for direct detection of important viruses in pig production, eg, porcine reproductive and respiratory syndrome virus (PRRSV),2 porcine circovirus type 2 (PCV2),3 and influenza A virus (IAV).4,5 However, detection of an infectious agent is not diagnostic proof of either an infection or an infectious disease. For this reason, detection of antibodies against an infectious agent as an immunological response to the agent is of additional usefulness in monitoring and surveillance. Antibodies against various viral agents, eg, PRRSV,6 PCV2,3 and IAV,7 have been detected in oral fluids.
Influenza is an important component of the porcine respiratory disease complex (PRDC) and a pathogen with a major economic impact in commercial swine populations. It has been shown that a commercial blocking enzyme-linked immunosorbent assay (ELISA) designed to detect influenza A nucleoprotein (NP) antibodies in avian serum accurately detects NP antibodies in swine serum.8 Furthermore, oral-fluid samples from pigs infected with influenza virus have been shown to contain detectable concentrations of antibodies against the NP of the virus from 7 to 42 days post inoculation, and detection of these antibodies is possible using a commercial blocking ELISA.7 In a longitudinal study in two pig farms,9 the dynamics of IAV infection determined by polymerase chain reaction (PCR) analysis of nasal swabs differed from those determined by testing serum using a commercial ELISA and by a hemagglutination inhibition (HI) test. Therefore, the objective of this study was to investigate the practical feasibility of monitoring IAV infection dynamics in a commercial pig herd by testing for anti-NP antibodies in pen-based oral-fluid samples. For that purpose, results of testing serum samples, still routinely used for antibody PRDC monitoring in Germany,10 were compared to results of testing oral fluids.
Materials and methods
The trial involved no intervention in the animals or medications requiring notification or exemption. The farm complies with German production standards and animal welfare regulations.
Experimental design
The suitability of pen-based oral fluids for monitoring IAV infection in a conventional finishing pig herd was compared to the usual monitoring method, ie, testing serum samples. Status of influenza infection was monitored by testing oral fluids for IAV by a conventional reverse transcription PCR (RT-PCR).
Pig herd
The trial was conducted from June 2013 until January 2014 in two successive batches of finishers in a commercial herd in northwest Germany. Each batch consisted of approximately 100 pigs housed in one room with four pens, with approximately 25 pigs per pen. The herd had a history of circulating PRRSV, IAV, and Mycoplasma hyopneumoniae. This was a closed system in which the sow herd had been vaccinated with Ingelvac PRRSV MLV (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri) every 3 months. The sows were clinically healthy when the piglets used in the study were produced. Piglets were weaned at day 27 or 28 of life and moved to the finishing barn at 10 to 12 weeks of age.
Porcine samples
Oral fluids were collected once a week from each of the four pens, starting when the pigs were 12 weeks old and continuing until they were 24 weeks old for Batch 1 and 22 weeks old for Batch 2. For collection of samples from each pen, one rope was used, which remained in the pen 10 to 15 minutes. Samples were collected in the morning prior to feeding, then chilled and shipped on ice within 24 hours to the IVD GmbH laboratory, Hannover, Germany. Samples were collected using the Swine Oral Fluids Collection Kit (ITL Animal Healthcare, Reston, Virginia). For comparison, serum samples were also collected from two pigs from each pen at 12, 16, and 20 weeks for both batches, and at 24 weeks for Batch 1 only. Pigs for serum collection were randomly selected by drawing two animal identification numbers from a container. Samples were tested on the day they were received by the laboratory and stored at 4°C until all data had been entered in the laboratory information system (Ticono-LC [LabControl]; Ticono GmbH, Hannover, Germany).
Influenza A virus ELISA
All oral-fluid and serum samples were tested for antibodies against the NP of IAV using a commercially available competitive IAV ELISA for serum samples from poultry and swine (ID Screen Influenza A Antibody Competition; IDvet, Grabels, France; officially registered in Germany). Oral-fluid samples were diluted 1:2 using the dilution buffer provided with the test kit, and serum samples were diluted 1:40 as described in the test-kit instructions. For all other procedures, the manufacturer’s instructions were followed exactly. The ELISA results were reported as a ratio percentage (S:N%) of the optical density (OD) of the sample (S) to the OD of the negative control (N). For porcine serum, S:N% ≤ 45% are classified positive; values > 45% and < 50% are classified as questionable; and values ≥ 50% are classified as negative.
Influenza A virus RT-PCR
Nucleic acid was extracted from oral-fluid samples using an RNA-DNA isolation kit (MagMAX Pathogen RNA/DNA Kit; Life Technologies GmbH, Darmstadt, Germany) and an automated nucleic acid isolation processor (MagMAX Express-96 Magnetic Particle Processor; Life Technologies GmbH) that used magnetic bead technology. A total of 450 µL of lysis buffer from the kit was added to 300 µL of each oral-fluid sample, suspensions were vortexed for 3 minutes, and cell debris was removed by centrifugation for 2 minutes at 16,000g. Then, 600 µL of each supernatant was transferred on a 96-well plate into the processor, and nucleic acid isolation was performed according the manufacturer’s protocol and instructions. Nucleic acid extracts were analyzed by RT-PCR as previously described, with the following modifications.11 The assay was performed as a two-step RT-PCR using M-MLV Reverse Transcriptase, RNase H Minus, Point Mutant, and random hexamers (Promega GmbH, Mannheim, Germany) for first-strand cDNA synthesis, 4 µL of first-strand cDNA reaction for second-strand cDNA synthesis, and PCR amplification according to the manufacturer’s recommendations. The PCR was performed in a Mastercycler (Eppendorf AG, Hamburg, Germany) with the following cycling conditions: 95°C for 2 minutes, followed by 40 cycles of 95°C for 30 seconds, 57°C for 30 seconds, and 72°C for 30 seconds, followed by 72°C for 2 minutes, and 15°C until each reaction was analyzed by agarose gel electrophoresis and ethidium bromide staining. Every sample was monitored for inhibition by dividing the PCR mix and adding IAV cDNA to one of the halves; each run of RT and PCR was monitored by a no-template control and an IAV-positive control (RNA prepared from supernatant of an IAV cell culture). The limit of detection in oral fluids spiked with IAV strains of each of the subtypes H3N2, H1N1, and H1N2 was at least 104 median tissue culture infective doses per mL.
Results
Influenza A virus RT-PCR
The IAV was detected in one pen-based oral-fluid sample from each batch when the pigs were 15 weeks of age (Tables 1 and 2). All other samples collected (51 of 52 from Batch 1 and 43 of 44 for Batch 2) were negative by RT-PCR.
Table 1: Results of a conventional reverse transcriptase-polymerase chain reaction (PCR) test to detect influenza A virus in oral fluids collected from pigs in a commercial finishing facility in Germany (Batch 1)*
Oral fluids (pen) | Age of pigs (weeks) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
12 |
13 |
14 |
15 |
16 |
17 |
18 |
19 |
20 |
21 |
22 |
23 |
24 |
|
60A |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
ND |
ND |
60B |
Neg |
Neg |
Neg |
Pos |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
ND |
ND |
61A |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
ND |
ND |
61B |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
Neg |
ND |
ND |
* Oral fluids were collected once weekly from two consecutive batches of pigs in four pens (60A, 60B, 61A, and 61B; approximately 25 pigs and one rope per pen) from 12 weeks to 24 weeks of age. Blood samples were collected from two randomly selected pigs per pen. The oral-fluid samples were tested for influenza A virus by PCR and for virus NP antibodies using a commercial competitive ELISA for porcine serum samples, conducted to confirm the results of virus NP antibody detection in those oral fluids and serum samples from the same pens. All testing was conducted to investigate the usefulness of oral fluids in comparison to serum for influenza A virus monitoring in a finishing pig herd. Positive (Pos): a clearly visible band after ethidium bromide staining of the agarose gel. The lack of a visible band was interpreted as negative (Neg). A weakly colored band, not observed in this study, would have been interpreted as a weakly positive result.
NP = nucleoprotein; ELISA = enzyme-linked immunosorbent assay.
Table 2: Results of a conventional reverse transcriptase-polymerase chain reaction (PCR) test to detect influenza A virus in oral fluids collected from pigs in a commercial finishing facility in Germany (Batch 2)*
| Oral fluids (pen) | Age of pigs (weeks) | ||||||||||||
| 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
| 60A | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| 60B | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| 61A | Neg | Neg | Neg | Pos | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| 61B | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
* Study described in Table 1.
Neg = negative; Pos = positive; ND = not determined; PCR testing not performed for pigs 23 and 24 weeks of age.
Influenza A virus ELISA
Antibodies against IAV were detected both in oral fluids and serum (Figures 1 and 2) for both batches starting at 16 weeks of age. This was 1 week after the first detection of IAV RNA by RT-PCR in the oral fluids. Antibodies were no longer detectable in oral fluids in some pens starting at 20 weeks for Batch 1 and 22 weeks for Batch 2, whereas serum samples stayed positive until the last sampling date, at 24 weeks for Batch 1 and 20 weeks for Batch 2. At 12 weeks, four of eight serum samples from individual pigs of Batch 1 and six of eight serum samples from Batch 2 were ELISA-positive, but oral-fluid samples were all negative.
Figure 1: Enzyme-linked immunosorbent assay (ELISA) results for detection of antibodies against the nucleoprotein of influenza A virus (A) in oral fluids (described in Table 1) and (B) in serum samples collected monthly from two randomly selected pigs of Batch 1 in the same pens (described in Table 1). ELISA results were reported as a ratio percentage (S:N%) of the optical density (OD) of the sample (S) to the OD of the negative control (N). For porcine serum, values of S:N% ≤ 45% are classified positive (red line indicates this cut-off point); values > 45% and < 50% are classified as questionable; and values ≥ 50% are classified as negative.
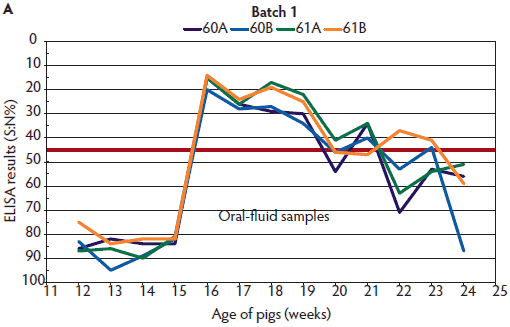

Figure 2: Enzyme-linked immunosorbent assay (ELISA) results for detection of antibodies against the nucleoprotein of influenza A virus (A) in oral fluids (described in Table 1) and (B) in serum samples of Batch 2 in the same pens (described in Table 1). ELISA results were reported as a ratio percentage (S:N%) of the optical density (OD) of the sample (S) to the OD of the negative control (N). For porcine serum, values of S:N% ≤ 45% are classified as positive (red line indicates this cut-off point); values > 45% and < 50% are classified as questionable; and values ≥ 50 are classified as negative.

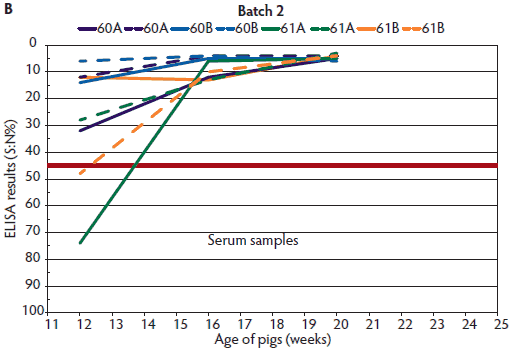
Two or 3 weeks after detection of IAV in the herd by oral-fluid RT-PCR (Figures 1 and 2), there was a decreasing trend in the oral-fluid ELISA results from all four pens in both batches, as demonstrated by the increasing S:N% values of the competitive ELISA. By comparison, S:N% values of the serum samples from both batches were consistently positive (mean of ELISA S:N% 4.5 to 8.4 with a maximum standard deviation of 4.0).
Discussion
While serum samples are still widely used for routine monitoring and surveillance programs in Germany, in most cases the quality of oral-fluid specimens is appropriate for direct and indirect detection of infectious agents1-7 as long as the guidelines for collection are adhered to strictly. A pen-based oral-fluid sample is an aggregate sample from multiple animals, just as a sample from a bulk milk tank is an aggregate sample. In this case, infected animals are more likely to contribute to the sample. It has been shown for PRRSV that testing pen-based oral-fluid samples greatly improved detection over single-animal testing.12 Furthermore, this is a very convenient procedure for collecting a specimen from an animal-welfare perspective. Because of the convenience of this sampling procedure for both humans and animals, it can be used weekly to provide very informative, infection-correlated monitoring. While bi-weekly sampling may be sufficient in terms of the cost-benefit ratio for PRDC monitoring, and therefore is recommended by multiple experts,13 our results indicate that the sampling interval should not be longer than 2 weeks. Otherwise, there is a loss of information about the dynamics of an infection and those of the herd immunity. With bi-weekly sampling, an IAV infection is detected at the latest 2 weeks after infection, whereas it takes 4 weeks, or twice as long, with monthly sampling. Furthermore, the phase of infection can be determined within the shorter time period of 14 days instead of a month, which is helpful for implementing strategies for prevention at the right time. These aspects should be weighed against each other when conducting a monitoring program.
Simply by adapting the dilution of oral fluids to account for the lower concentration of antibodies in oral fluids than in serum, we were able to obtain meaningful and useful antibody profiles in two consecutive finishing batches using a commercial competitive ELISA to monitor the dynamics of naturally occurring IAV infection. While other investigators have already evaluated an NP-blocking ELISA for detection of NP antibodies in oral fluids from pigs infected under experimental conditions,7 further studies are still needed to determine whether a commercial NP-blocking ELISA can also be used for monitoring naturally occurring IAV infections in herds. Nonetheless, the results presented here are very promising. A particular advantage of modifying an NP-blocking or a competitive ELISA for a different sample matrix is the fact that this ELISA format can in principle detect and bind the antibodies of all test antigen-specific classes of immunoglobulin (Ig) to the NP protein. Differences in the Ig classes of different sample matrices therefore, in principle, play no role in the test results. On the other hand, with an indirect ELISA, only Ig classes can be detected for which the conjugates used in the ELISA are specific. In most cases, enzyme-conjugated secondary antibodies against IgG are used in conventional indirect ELISAs. Depending on their specificity, these primarily detect only the IgG that binds the test antigen. Other classes of NP protein-binding Igs, such as IgA and IgM, will not be detected by an indirect ELISA to an extent depending on the specificity of the conjugate used. Differences in the composition of NP-specific Ig classes in different sample matrices therefore have a considerable effect on the test results of an indirect, conjugate-dependent ELISA, because the NP-binding IgG can be detected, but in principle other NP-binding Ig classes, such as IgM and IgA, cannot be detected.14 It would be very practical and cost-effective if the same ELISA kit could be used for different sample matrices simply by appropriate modifications, as has been shown for other ELISA tests. For example, swine Salmonella antibody ELISA kits have been used to detect Salmonella antibodies in porcine serum and meat juice in European monitoring programs.15 Furthermore, ELISA tests are used for detecting antibodies to Mycobacterium paratuberculosis in bovine serum or milk (Idexx MAP Antibody Test Kit; Idexx Laboratories, Westbrook, Maine; available in the United States and Canada).16
In our longitudinal study we also detected NP antibodies in both oral fluids and serum 1 week after detection of IAV in oral fluids by PCR, as did researchers in another study.7 But in contrast to that study, the NP antibodies we detected in oral fluids after PCR detection of IAV showed a decreasing trend within 2 to 3 weeks after detection of virus, and were no longer detectable in oral fluids in some of the pens starting at 5 weeks after exposure to IAV. One reason for these differences may be that those researchers performed an experimental infection study,7 whereas we monitored a naturally occurring IAV infection in a conventional finishing herd.
In our study, at least half of the individual serum samples tested positive for NP antibodies 3 weeks before IAV was detected in oral fluids by PCR, while all oral fluids tested negative until IAV was detected. However, as the objective of our study was to compare the usefulness of oral fluids and serum for antibody monitoring purposes, no nasal swabs were collected, and the infectious state of the pigs for IAV at the age of 12 weeks remains ambiguous. Since the herd had a history of circulating IAV, positive serum samples at the age of 12 weeks may have been induced by an infection earlier than the beginning of our study, whereas any NP antibodies in oral fluids may have already disappeared at this time. Ultimately, this can only be clarified by further analyses.
As known from other studies,17 maternally derived antibodies can be detected using the HI test for up to approximately 70 days. Although we did not perform the HI test for all samples in this study, it is improbable that the NP antibodies detected in serum by ELISA at 12 weeks of age (up to 84 days) were maternally derived, but this possibility cannot be excluded with certainty.
A shorter persistence of NP antibodies in oral fluids would be advantageous for monitoring, because detection of longer-lasting antibodies in serum might mask short periods of circulating IAV.9 The longitudinal profiles of antibodies against IAV detected in pen-based oral fluids in this study were very useful for monitoring the circulation of IAV in this finishing pig herd. The efficiency and possible advantage of monitoring for IAV by antibody detection in pen-based oral fluids should be evaluated further.
Implications
• Under the conditions of this study, IAV infection may be detected 1 week after virus presence is demonstrated in the herd by PCR using a commercial competitive ELISA to detect antibodies against the NP of IAV in serum and pen-based oral fluids.
• A commercial competitive IAV ELISA designed for porcine serum samples can be used for pen-based oral fluids simply by adapting the dilution of oral fluids.
• Monitoring IAV infection dynamics in a commercial pig population is possible by detecting antibodies against the virus in pen-based oral fluids.
Acknowledgements
We thank the pig farmer for providing the batches of pigs for testing. We are grateful to the entire IVD GmbH (Hannover, Germany) laboratory team for performing the ELISA and PCR testing. Many thanks also to Professor Lothar Kreienbrock and Dr Martin Beyerbach of the Department of Biometry, Epidemiology and Information Processing, WHO Collaborating Centre for Research and Training in Veterinary Public Health at the University of Veterinary Medicine, Hannover, Germany, for statistical consultation; to Judith McAlister-Hermann, PhD, for English editorial support; and to Dr Ralf Dürrwald, IDT Biologika GmbH, Dessau-Roßlau, Germany, for providing IAV suspensions for determining the detection limit of IAV RNA by RT-PCR.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Ramirez A, Wang C, Prickett JR, Pogranichniy R, Yoon KJ, Main R, Johnson JK, Rademacher C, Hoogland M, Hoffmann P, Kurtz A, Kurtz E, Zimmerman J. Efficient surveillance of pig populations using oral fluids. Prev Vet Med. 2012;104:292–300.
2. Prickett J, Simer R, Christopher-Hennings J, Yoon KJ, Evans RB, Zimmerman JJ. Detection of Porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: a longitudinal study under experimental conditions. J Vet Diagn Invest. 2008;20:156–163.
3. Prickett JR, Johnson J, Murtaugh MP, Puvanendiran S, Wang C, Zimmerman J, Opriessnig T. Prolonged detection of PCV2 and anti-PCV2 antibody in oral fluids following experimental inoculation. Transbound Emerg Dis. 2011;58:121–127. doi:10.1111/j.1865-1682.2010.01189.x.
4. Detmer SE, Patnayak DP, Jiang Y, Gramer MR, Sagar M, Goyal M. Detection of influenza A virus in porcine oral fluid samples. J Vet Diagn Invest. 2011;23:241–247.
5. Goodell CK, Prickett J, Kittawornrat A, Zhou F, Rauh R, Nelson W, O’Connell C, Burrell A, Wang C, Yoon KJ, Zimmerman J. Probability of detecting influenza A virus subtypes H1N1 and H3N2 in individual pig nasal swabs and pen-based oral fluid specimens over time. Vet Microbiol. 2013;166:450–460.
6. Kittawornrat A, Prickett J, Wang C, Olsen C, Irwin C, Panyasing Y, Ballagi A, Rice A, Main R, Johnson J, Rademacher C, Hoogland M, Rowland R, Zimmerman J. Detection of Porcine reproductive and respiratory syndrome virus (PRRSV) antibodies in oral fluid specimens using a commercial PRRSV serum antibody enzyme-linked immunosorbent assay. J Vet Diagn Invest. 2012;24:262–269.
7. Panyasing Y, Goodell CK, Wang C, Kittawornrat A, Prickett JR, Schwartz KJ, Ballagi A, Lizano S, Zimmerman JJ. Detection of influenza A virus nucleoprotein antibodies in oral fluid specimens from pigs infected under experimental conditions using a blocking ELISA. Transbound Emerg Dis. 2014;61:177–184. doi:10.1111/tbed.12019.
8. Ciacci-Zanella JR, Vincent AL, Prickett JR, Zimmerman SM, Zimmerman J. Detection of anti-influenza A nucleoprotein antibodies in pigs using a commercial influenza epitope-blocking enzyme-linked immunosorbent assay developed for avian species. J Vet Diagn Invest. 2010;22:3–9.
9. Simon-Grife M, Martin-Valls GE, Vilar MJ, Busquets N, Mora-Salvatierra M, Bestebroer TM, Fouchier RAM, Martin M, Mateu E, Casal J. Swine influenza virus infection dynamics in two pig farms; results of a longitudinal assessment. Vet Res. 2012;43:24–34.
10. Nathues H, Nienhoff H, grosse Beilage E, Blaha T, Ritzmann M, Reiner G, Lahrmann KH, Kauffold J, Waberski D, Hennig-Pauka I, Wendt M, Waldmann KH. Monitoring-Systeme in Zuchtschweinebeständen aus Sicht der Wissenschaft. Deutsches Tierärzteblatt. 2011;10:1324–1334.
11. Fouchier RA, Bestebroer TM, Herfst S, van der Kemp L, Rimmelzwaan GF, Osterhaus ADME. Detection of influenza A viruses from different species by PCR amplification of conserved sequences in the matrix gene. J Clin Microbiol. 2000;38:4096–4101.
12. Olsen C, Wang C, Christopher-Hennings J, Doolittle K, Harmon KM, Abate S, Kittawornrat A, Lizano S, Main R, Nelson EA, Otterson T, Panyasing Y, Rademacher C, Rauh R, Shah R, Zimmerman J. Probability of detecting Porcine reproductive and respiratory syndrome virus infection using pen-based swine oral fluid specimens as a function of within-pen prevalence. J Vet Diagn Invest. 2013. doi:10.1177/1040638713481471.
13. Iowa State College of Veterinary Medicine. Veterinary Diagnostic and Production Animal Medicine. Oral Fluids. 2014. Available at: http://vetmed.iastate.edu/vdpam/disease-topics/oral-fluids. Accessed 9 March 2015.
14. Escribano D, Gutiérrez AM, Subiela SM, Tecles F, Cerón JJ. Validation of three commercially available immunoassays for quantification of IgA, IgG, and IgM in porcine saliva samples. Res Vet Sci. 2012;90:682–687.
15. Idexx Laboratories Inc. Idexx Salmonella Ab test. Available at: https://www.idexx.com/livestock-poultry/swine/swine-salmonella.html. Accessed 5 March 2015.
16. Idexx Laboratories. Paratuberculosis (Johne’s disease)/Mycobacterium avium subsp. paratuberculosis (MAP). Idexx MAP Ab test. Available at: https://www.idexx.com/livestock-poultry/ruminant/map.html. Accessed 5 March 2015.
17. Rose N, Herve S, Eveno E, Barbier N, Eono F, Dorenlor V, Andraud M, Camsusou C, Madec F, Simon G. Dynamics of influenza A virus infection in permanently infected pig farms: evidence of recurrent infections, circulation of several swine influenza viruses and reassortment events. Vet Res. 2013;44:72–85.
