| Original research | Peer reviewed |
Cite as: Schneider PT, Zhang J, Ramirez A, et al. Evaluation of disinfection protocols to reduce virus transmission via livestock transport vehicles using model trailers and experimental conditions. J Swine Health Prod. 2015;23(6):306–316.
Also available as a PDF.
SummaryObjective: To determine the efficacy of accelerated hydrogen peroxide disinfectant and combined glutaraldehyde and quaternary ammonium disinfectant after a high-pressure wash against porcine reproductive and respiratory syndrome virus (PRRSV) and transmissible gastroenteritis virus (TGEV) in experimental settings mimicking field conditions commonly experienced on livestock trailers. Materials and methods: Aluminum model livestock trailers (1:61) were contaminated with PRRSV- and TGEV-spiked feces. Each model trailer underwent a simple washing procedure and an assigned disinfectant application. Four environmental swabs were collected per trailer at five time points and tested by PRRSV quantitative polymerase chain reaction (qPCR) and TGEV polymerase chain reaction (PCR). Ten-week old pigs were inoculated orally and intramuscularly with supernatant from environmental samples taken from model trailers at two time points after disinfection. Fecal swabs and blood collected at 7 and 14 days post inoculation were tested by PRRSV qPCR and TGEV PCR to determine if the inoculum had contained live infectious virus. Results: All Positive Control pigs were positive by PRRSV qPCR at 7 and 14 days post inoculation and by PRRSV enzyme-linked immunosorbent assay (ELISA) at day 14. Pigs in the other treatment groups were negative by PRRSV qPCR and PRRSV ELISA at all time points. Results of TGEV testing were inconclusive because the Positive Control group failed to become infected. Implication: Under study conditions, a high-pressure wash with cold water plus application of an accelerated hydrogen peroxide or a combined glutaraldehyde and quaternary ammonium disinfectant is effective at inactivating PRRSV. | ResumenObjetivo: Determinar la eficacia contra el virus del síndrome reproductivo y respiratorio porcino (PRRSV por sus siglas en inglés) y el virus de la gastroenteritis transmisible (TGEV por sus siglas en inglés) del desinfectante peróxido de hidrogeno acelerado y combinado con el desinfectante glutaraldehído y cuaternarios de amonio después de un lavado con alta presión en condiciones experimentales simulando situaciones de campo comúnmente experimentadas en camiones pecuarios. Materiales y métodos: Se contaminaron camiones modelo de aluminio, escala 1:61, con heces contaminadas artificialmente con PRRSV y TGEV. Cada camión fue sometido a un proceso de lavado simple y a una aplicación de desinfectante asignado. Se recolectaron cuatro muestras medioambientales por camión en cinco puntos de tiempo y se probaron por medio de la reacción en cadena de la polimerasa cuantitativa (qPCR por sus siglas en inglés) de PRRSV y la reacción en cadena de polimerasa (PCR por sus siglas en inglés) de TGEV. En dos puntos de tiempo se inocularon oralmente e intramuscularmente cerdos de diez semanas de edad con el sobrenadante de muestras medioambientales de los camiones modelo después de la desinfección. Se analizaron muestras fecales y sangre tomadas a los 7 y 14 días después de la inoculación por medio de qPCR PRRSV y PCR TGEV para determinar si el inoculo contenía virus vivo infeccioso. Resultados: Todos los cerdos Control Positivos resultaron positivos por medio de qPCR PRRSV a los 7 y 14 días después de la inoculación y por medio de la prueba de ensayo de inmunoabsorción ligado a enzimas (ELISA por sus siglas en inglés) de PRRSV en el día 14. Los cerdos en los otros grupos de tratamiento resultaron negativos por medio del qPCR PRRSV y de la ELISA de PRRSV en todos los puntos de tiempo. Los resultados de las pruebas de TGEV no fueron concluyentes porque el Grupo de Control Positivo no logró ser infectado. Implicación: Bajo las condiciones del estudio, un lavado de alta presión con agua fría más la aplicación de peróxido de hidrógeno acelerado o un desinfectante combinado de glutaraldehído y cuaternarios de amonio es efectivo para desactivar el PRRSV. | ResuméObjectif: Déterminer l’efficacité d’un désinfectant de type peroxyde d’hydrogène accéléré et d’une combinaison de glutaraldéhyde et d’ammonium quaternaire envers le virus du syndrome reproducteur et respiratoire porcin (VSRRP) et le virus de la gastro-entérite transmissible (VGET) suite à un lavage à haute-pression dans des conditions expérimentales imitant des conditions de champs rencontrées avec des remorques à bétail. Matériels et méthodes: Des modèles en aluminium de remorques à bétail à l’échelle 1:61 furent contaminés avec des fèces inoculées avec du VSRRP et du VGET. Chaque modèle de remorque fut soumis à une procédure simple de lavage suivi de l’application d’un désinfectant spécifique. Quatre écouvillons environnementaux furent prélevés par remorque à cinq moments différents et testés par réaction d’amplification en chaine par la polymérase quantitative (qPCR) pour le VSRRP et par réaction d’amplification en chaîne par la polymérase (PCR) pour VGET. Des porcelets âgés de 10 semaines furent inoculés par voies orale et intramusculaire avec du surnageant provenant d’échantillons environnementaux de remorques à bétail pris à deux moments dans le temps après la désinfection. Des écouvillons de fèces et de sang prélevés 7 et 14 jours post inoculation (PI) ont été testés par qPCR pour VSRRP et PCR pour VGET afin de déterminer si l’inoculum avait contenu du virus vivant infectieux. Résultats: Tous les porcs témoins étaient positifs par qPCR VSRRP à 7 et 14 jours PI et par épreuve immuno-enzymatique (ELISA) au jour 14. Les porcs dans autres groupes de traitement étaient négatifs par qPCR VSRRP et ELISA VSRRP à tous les autres temps d’échantillonnage. Les résultats des épreuves pour VGET étaient non-concluants étant donné que le groupe témoin positif n’a pas développé l’infection. Implication: Sous les conditions expérimentales de la présente étude, une procédure de lavage à haute pression avec de l’eau froide suivie de l’application de désinfectant de type peroxyde d’hydrogène accéléré ou d’une combinaison de glutaraldéhyde et d’ammonium quaternaire est efficace pour inactiver le VSRRP. |
Keywords: swine, disinfectant, porcine reproductive and respiratory syndrome virus, transport, biosecurity, PRRSV
Search the AASV web site
for pages with similar keywords.
Received: October 3, 2014
Accepted: June 16, 2015
The modern swine industry is structured such that frequent movements of pigs are necessary. Movements occur between production sites and from production sites to terminal markets, resulting in exposure of transport vehicles, personnel, and loading equipment to groups of pigs of varying health status. These factors make transportation events and transport vehicles likely means for transmission of undesirable pathogens to swine.1,2 Swine transport vehicles, sorting panels, load-out areas, and loading chutes are generally not disposable items. Therefore, effective sanitation practices are necessary to mitigate the risk of contaminated items serving as fomites for pathogens that can infect swine.
Porcine reproductive and respiratory syndrome virus (PRRSV), in the family Arteriviridae, and transmissible gastroenteritis virus (TGEV), in the family Coronaviridae, are two viruses for which transportation and transport vehicles serve as transmission fomites. Porcine reproductive and respiratory syndrome (PRRS), caused by the highly infectious PRRSV, is a costly and frustrating challenge to the global swine industry. Ramifications from PRRSV introduction into stable or naive swine herds include late-term abortions, increased preweaning mortality, and other reproductive losses in sows,3 and mortality and slowed growth in growing pigs.4 Productivity losses in the United States swine industry are estimated to be $664 million annually.5 In 2006, the virus decimated China’s pig population and drove up pork prices by 85%.6 PRRS-related clinical signs and lesions in swine herds occur when a previously PRRSV-naive animal is exposed to PRRSV,7 or when a heterologous strain of PRRSV is introduced into a PRRSV-exposed herd.8,9
Transmissible gastroenteritis (TGE) occurs when the highly transmissible TGEV is introduced into a previously TGEV-naive herd. Transmissible gastroenteritis is characterized by vomiting, severe diarrhea, and high mortality in seronegative pigs less than 2 weeks of age.10 Transmissible gastroenteritis is associated with productivity losses, including slower gain and poorer feed conversion in the wean-to-finish stage of production. Clinical signs are commonly non-differentiable from those of porcine epidemic diarrhea (PED).11
Previous research has demonstrated that PRRSV present in swine transport vehicles can infect PRRSV-naive pigs. A study using model trailers showed “seeder pigs” experimentally infected with PRRSV could cause sufficient contamination of a model transport trailer to infect naive sentinel pigs with PRRSV.1 High-pressure washing of an experimentally PRRSV-contaminated model transport trailer was not effective at preventing sentinel pigs from being exposed to viable PRRSV.2,12 To the knowledge of the authors, research focusing on the risk of transportation vehicles in TGEV spread has not been published.
Sanitation procedures to decrease the pathogen load in the standard equipment utilized in swine transportation have been described,1,2,12 but trailers, sorting panels, and chutes are not always thoroughly cleaned between transport events and thus may contain organic debris as well as bacterial and viral agents. Reasons for failure to properly clean all soiled areas include lack of perceived risk, lack of proper cleaning tools, cost, and time (P Schneider, unpublished data.). These concerns are particularly warranted when discussing sanitation of trucks that haul market hogs because of the frequency of transportation events required.
Currently, a number of disinfectants are available for use in livestock facilities and onboard livestock transport vehicles; however, little research has been done to understand the effectiveness of commonly used disinfectants against swine pathogens in the presence of feces and with short disinfectant contact times. This scenario is similar to the conditions commonly found in field settings for trucks that haul multiple loads of pigs in a single day. Studies evaluating disinfection of fomites, such as boot-washing stations, showed the presence of organic material greatly reduced the bactericidal effect of many disinfectant agents.13,14 Generally, bactericidal action improved when fecal material was mechanically removed and when contact time with disinfectant products was increased.
Multiple disinfectants, including quaternary ammoniums, phenolic agents, aldehydes, and peroxygen compounds, have been studied for effectiveness against viral agents. One study compared the use of a combined glutaraldehyde and quaternary ammonium product (Synergize; Preserve International, Reno, Nevada) and a mixed chemical and heavy-metal disinfectant (Stalosan F powder; Vitfoss, Graasten, Denmark) when used against PRRSV on soiled boots at multiple time points post contamination.15 Eighty percent of samples collected from boots contaminated with PRRSV and treated with Stalosan F powder in the presence of organic material tested negative for PRRSV by polymerase chain reaction (PCR). When contaminated boots treated with Synergize were similarly tested at the same time points, 42% of samples were PCR-negative. Dee et al16 demonstrated that sodium hypochlorite (bleach) in boot baths was effective for decontamination of PRRSV on disposable plastic boots. For that study, investigators stepped first into feces, then into an aqueous pool formed by melting snow that had been spiked with PRRSV prior to melting. Investigators then entered a bath of 6% sodium hypochlorite and swabbed the soles of the boots immediately after exiting the bath. PRRS virus was not mixed into the contaminating fecal material, nor was time given to allow fecal material to dry.
Properties of an ideal disinfectant for the swine industry would include being quick acting and maintaining activity in the presence of large amounts of feces, wood shavings, and other organic material commonly present when limited or inferior cleaning practices are implemented. Accel (Virox Technologies Inc, Oakville, Ontario, Canada) is an accelerated hydrogen peroxide product with Environmental Protection Agency label claims as a disinfectant, cleaner, and deodorant with a broad spectrum of action that may have these ideal properties. The product contains low concentrations of certain food-grade anionic and non-ionic surfactants that interact with hydrogen peroxide to enhance microbiocidal activity. The label recommendation for usage against viral agents as a one-step disinfectant is at a concentration of 236 mL in 3.8 L of water with 5 minutes of contact time. Synergize is the most commonly utilized disinfection product in the swine industry and has been shown in previous testing to be effective against PRRSV.1 Synergize is labeled as a cleaner and broad-spectrum disinfectant with a required contact time of 10 minutes and label concentration of 14.8 mL per 3.8 L. The purpose of this research was to evaluate the efficacy of an accelerated hydrogen peroxide disinfectant (AHP) and a combined glutaraldehyde and quaternary ammonium disinfectant (GQA) against PRRSV and TGEV under field conditions using model trailers and after a high-pressure wash. The first specific objective was to evaluate the efficacy of the disinfection procedures by testing environmental samples collected from model trailers intentionally contaminated with PRRSV and TGEV at specific time points pre- and post disinfection. The second objective was to determine if the virus remaining in the model trailers at 15 and 60 minutes post disinfection was infective by using the samples collected from the model trailers in a swine bioassay.
Materials and methods
Investigation of both study objectives was conducted at the Iowa State University Veterinary Medical Research Institute. The study protocol was reviewed and approved for use by the Iowa State University Institutional Animal Care and Use Committee.
Objective 1
Experimental design. The experimental design for Objective 1 was an incomplete block design with three replicates per block (Table 1). The experimental unit in each block was the model trailer. Four treatment groups were evaluated. Model trailers in the first treatment group were contaminated with feces spiked with PRRSV and TGEV, then washed using a high-pressure wash with cold water and disinfected with an AHP (AHP group). The same process was repeated for the second treatment group with the exception that a GQA disinfectant was used after the high-pressure wash (GQA group). Model trailers for the third group were contaminated with virus-spiked feces then washed using a high-pressure wash with cold water and sham disinfected with water (Pos Control group). The final group utilized feces not spiked with virus to contaminate trailers, which then were washed using a high-pressure wash with cold water and no disinfection (Neg Control group). Detergent was not utilized in the washing process for any treatment group. Tap water obtained from the City of Ames Water Plant (City of Ames, Ames, Iowa) was used for the high-pressure washing procedure and for sham disinfection for the Pos Control group. The RAND function in Microsoft Excel (1999; Microsoft Corporation, Redmond, Washington) was used to randomly assign treatments to trailers within each block. The Pos Control group was randomly assigned to one model trailer in each block, and two of the remaining three treatment groups (AHP, GQA, Neg Control) were randomly assigned to the other two model trailers. In total, 10 blocks were conducted for the study. Eight replicates were performed for the AHP and GQA groups and four replicates for the Neg Control group. Ten replicates of the Pos Control group were planned, but an error resulted in an extra replicate for the GQA group being performed. The extra GQA replicate was removed from the study and only nine Pos Control replicates were performed.
Table 1: Incomplete block design to evaluate the efficacy of disinfection procedures for swine transport trailers by collecting environmental samples from model trailers pre- and post disinfection and testing for PRRSV and TGEV by PCR (Objective 1 of the study)*
| Trailer 1 | Trailer 2 | Trailer 3 | |
|---|---|---|---|
| Block 1 | GQA | AHP | Pos Control |
| Block 2 | GQA | Pos Control | AHP |
| Block 3 | AHP | Neg Control | Pos Control |
| Block 4 | Neg Control | Pos Control | GQA |
| Block 5 | ND | AHP | Neg Control |
| Block 6 | GQA | Neg Control | Pos Control |
| Block 7 | Pos Control | GQA | AHP |
| Block 8 | GQA | AHP | Pos Control |
| Block 9 | Pos Control | AHP | GQA |
| Block 10 | GQA | AHP | Pos Control |
* Three 1:61 scale model aluminum livestock trailers were enrolled in the study. Four sites in each trailer were contaminated with a total of 50 mL of a feces slurry containing PRRSV and TGEV. Additional fecal material free of PRRSV and TGEV was spread on the trailer floor and wall surfaces to mimic conditions in a trailer that had hauled pigs. After 1 hour in a 4°C cooler, trailers were again contaminated with fecal material, washed with a high-pressure washer, then treated with disinfectant. Samples were collected from each of the four sites contaminated with viruses at five time points for each replicate: immediately after the second contamination process; after the washing process; and 15, 30, and 60 minutes post treatment. Three replicates per block were conducted, with a total of 10 blocks and four treatment groups: accelerated hydrogen peroxide disinfectant (AHP; n = 8); combined glutaraldehyde and quaternary ammonium disinfectant (GQA; n = 8); positive control with a virus-contaminated trailer and sham disinfection with water (Pos Control; n = 9); and negative control with no virus contamination and no disinfection (Neg Control; n = 4).
PRRSV = porcine reproductive and respiratory syndrome virus; TGEV = transmissible gastroenteritis virus; PCR = polymerase chain reaction; ND = not done (a Pos Control was planned for this block but in error a GQA treatment was performed and removed from the study).
Description of trailer model. Three 1:61 scale model trailers (Figure 1) were utilized for Objective 1. The models were designed by a commercial livestock-transport trailer manufacturer (EBY Inc, Story City, Iowa) and had been used in a prior study17 to evaluate disinfection protocols for porcine circovirus type 2. The model trailers measured 0.62 m wide × 0.82 m tall × 1.11 m long. Total floor area in the trailers was 0.69 m2. The models were designed to represent a standard livestock trailer used to transport commercial swine. Materials used in the design and construction of the model trailers were identical to those used in full-sized livestock transport trailers. The models were constructed using aluminum alloy diamond-plate flooring, welded and riveted to rectangular aluminum tubing cross-members. The side walls and roof were made of flat aluminum sheeting, with the side walls containing punched holes for ventilation. An inner aluminum dividing gate and an aluminum roll up door in the rear of the model trailer were attached with hinges and a latch.
Figure 1: The 1:61 scale model aluminum trailers utilized to evaluate the efficacy of disinfection procedures for swine transport trailers by collecting environmental samples from model trailers pre- and post disinfection and testing for porcine reproductive and respiratory syndrome virus and transmissible gastroenteritis virus by polymerase chain reaction. The trailers measured 0.62 m wide × 0.82 m tall × 1.11 m long and contained characteristics commonly found in commercial livestock trailers. Trailers were constructed of aluminum materials and included a dividing gate and rear roll-up door. Study details described in Table 1.

Fecal collection. Approximately 56.8 L of feces from 6-month-old pigs were collected from a commercial swine wean-to-finish barn that had no previous clinical signs of PRRS or TGE and had tested negative for PRRSV on multiple oral-fluid and serum tests since the pigs had been placed. To further confirm that no previous exposure to PRRSV had occurred, oral-fluid samples were collected from the swine herd at the time of fecal collection and tested for PRRSV by enzyme-linked immunosorbent assay (ELISA) and PCR at the Iowa State University Veterinary Diagnostic Laboratory (ISU-VDL; Ames, Iowa). Both tests were negative. Samples of collected fecal material were tested and confirmed negative for PRRSV and TGEV by PCR at the ISU-VDL.
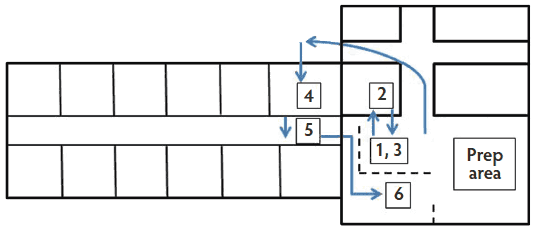
Procedure. To conduct the study, a coordinated sequence of events was staged in designated areas within the research facility (Figure 2). Trailers were first contaminated by applying feces to the designated areas in Location 1 and then moved into a 4°C cooler, identified as Location 2, for 60 minutes. A second contamination procedure and pre-wash sampling were performed at Location 3. Model trailers were moved outside the research facility to enter the designated wash area (Location 4). Post-wash sampling and disinfection with an AHP, a combined GQC, or sham disinfection was executed in a hallway adjacent to the wash area (Location 5). Three post-disinfection samplings at 15, 30, and 60 minutes post disinfection for the AHP and GQA treatment groups and at 15, 30, and 60 minutes post wash for the Pos Control and Neg Control groups were carried out in Location 6.
Figure 2: Diagram representing the movement of the trailer through the Iowa State University Veterinary Medical Research Institute building during Objective 1 of the study described in Table 1. Locations 1 and 3, trailer contamination area; Location 2, 4°C cooler; Location 4, washing room; Location 5, disinfection area; Location 6, post-decontamination sampling area. Arrows show the movement of the trailer. Dotted lines represent barricades preventing movement between the two areas.
Contamination of trailer models with PRRSV and TGEV. Contamination of the model trailers was achieved using a diluted feces mixture with PRRSV SDSU73 strain and TGEV Purdue strain (ATCC VR-763). The PRRSV SDSU73 was initially isolated from a sow herd with a high prevalence of abortions and higher than usual sow mortality in 1996, and has been used previously in experimental challenge studies.18,19 Two mL of PRRSV SDSU73, with a final concentration of 3 × 105 per mL on a median tissue culture infective dose (TCID50) assay, and 2 mL of TGEV Purdue strain (ATCC VR-763), with a final concentration of 105.25 TCID50 per mL, were mixed with 46 mL of 1:1 feces:deionized water, resulting in a mixture containing a concentration of 104.08 TCID50 per mL of PRRSV and 103.85 TCID50 per mL of TGEV. PRRS virus and TGEV at the doses used in this study have been shown to be infectious in previous studies.20-22 The feces and virus mixture was manually applied with a gloved hand to four designated areas inside the trailer (Figure 3). The designated areas included an approximately 12-cm × 65-cm area on the left side wall immediately inside the rear door; an approximately 10-cm × 10-cm area on the floor in the front half of the model trailer along the right side wall; the rear surface of the dividing gate latch; and an approximately 16-cm × 16-cm area on the inside surface of the roll-up rear gate.
Figure 3: Model trailers were each contaminated with 50 mL of a feces and virus mixture at four internal sites for Objective 1 for the AHP, GQA, and Pos Control treatment groups (groups and study described in Table 1). The four sites of contamination inside the model livestock trailer included the rear roll door (Panel A), on the floor in the front half of the right side wall (Panel B), dividing gate latch (panels B and C), and the rear left side wall (Panel C).



The remaining floor surface and various areas on the model trailer walls and rear door were covered by hand with 1 L of undiluted feces that did not contain PRRSV or TGEV, to simulate the amount of organic matter that would commonly be found in a livestock trailer after hauling pigs. Trailers were placed in a walk-in cooler for a period of 60 minutes at 4°C to simulate colder environmental conditions more commonly associated with PRRS and TGE disease breaks. The contamination procedure was repeated once the trailer was removed from the cooler to create a mixture of partially dried and fresh feces as would typically occur under field conditions. To prevent contamination of the study area, investigators changed disposable gloves immediately after the contamination procedure was completed. If the investigator’s clothing came in contact with feces within the model trailer, the investigator also changed clothing.
Cleaning procedure. Trailers were moved from the contamination area into a room at 20°C immediately after the initial sampling period. Each trailer underwent a limited wash with a standard high-pressure washer (Hotsy Corporation, Englewood, Colorado) at a pressure of 1500 pounds per square inch (psi) (105 kg per cm2) using cold water for 90 seconds. Under these circumstances, significant amounts of grossly visible fecal matter consistently remained after the pressure wash, closely representing conditions often found in transport vehicles that haul commercial growing and market pigs after washing (Figure 4). One investigator was designated the washer for the entirety of the study. The designated investigator was blinded to the placement of the virus-contaminated feces and to treatment assignments.
Figure 4: After contamination, model trailers in Objective 1 (study described in Table 1) were washed with a high-pressure washer (1500 pounds per square inch [103 bar; 10.3 MPa]) using cold water. Shown are a contaminated model trailer prior to washing (Panel A) and model trailers after high-pressure washing (panels B and C).



Disinfection procedure. All disinfectants were applied with a Model 25 Compact Airless Foamer (Ogena Solutions, LLC, Stoney Creek, Ontario, Canada). Due to the foamer design, both disinfectants were applied at slightly higher concentrations than those on the label. The AHP disinfectant was utilized at a rate of 266 mL per 3.8 L; the combined GQA disinfectant was applied at a rate of 20.7 mL per 3.8 L. The researcher applying the disinfectant was not blinded to the locations of the virus and feces slurry.
Once the wash was finished, trailers were removed from the wash room to a separate corridor where all disinfectants were applied. A single investigator who was not blinded to the treatment group for each trailer was designated to apply the disinfectant to all trailers throughout the study. The designated investigator changed gloves between trailers to prevent contamination. Trailers in the Pos Control group were sham disinfected with tap water using the same foamer used to apply the disinfectants. No disinfectant was applied to the Neg Control replicates. Separate disinfectant vessels were used for the AHP, GQA, and Pos Control treatment groups. The foamer was rinsed with cold tap water between applications. To mimic field conditions, disinfectants were not rinsed from the model trailers after application.
Model trailer and working-area decontamination. At the conclusion of each block, model trailers were thoroughly cleaned and disinfected to prevent contamination of future replicates. Each model trailer was individually washed with a high-pressure washer (1500 psi) using 48.9°C water. The interior and exterior of the model trailers were scrubbed with dish soap (Dawn Ultra Antibacterial Dishwashing Liquid; Procter and Gamble, Cincinnati, Ohio), rinsed with cold water, and disinfected with Quatricide PV (Pharmacal Research Laboratories Co, Waterbury, Connecticut) at a concentration of 52.5 mL per 3.8 L of water using a liquid concentrate sprayer (ACE Hardware, Oak Brook, Illinois). Trailers were manually dried using a separate new bath towel for each trailer.
Site 4 in Figure 2, the washing room for all model trailers, was washed using a high-pressure washer (1500 psi) and 48.9°C water. All visible organic debris was removed and the room was disinfected with Quatricide PV at 52.5 mL per 3.8 L of water concentration.
Contamination and sampling areas were also cleaned between blocks. A low-pressure nozzle (49.9 psi; 3.5 kg per cm2) attached to a garden hose was used to remove visible organic debris from the floors and walls. Virkon S (E. I. du Pont de Nemours and Company, Wilmington, Delaware) was applied at a concentration of 5 g per L of water using a liquid concentrate sprayer.
Detection of PRRSV and TGEV. To identify the presence of TGEV or PRRSV, one sample was collected from each of the four designated areas in the model trailers at five different time points using nylon flocked dry swabs (FLOQSwabs; Copan Diagnostics, Inc, Murrieta, California). Time points were immediately after the second feces-virus mixture was applied, immediately after the completion of the washing procedure, and at 15, 30, and 60 minutes after completion of the treatment protocol. After sample collection, each swab was stored in a 5-mL snap-cap tube (Becton, Dickinson and Company, Franklin Lakes, New Jersey) containing 2 mL of minimum essential medium plus 1× antibiotic, containing 0.05 mg per mL gentamicin, 10 units per mL penicillin, 10 mg per mL streptomycin, and 0.25 mg per mL amphotericin, plus 2% fetal bovine serum. After collection, samples were immediately chilled on ice. All samples were placed in a -80°C freezer within 1 hour of collection and stored until testing. For each replicate, samples from each of the four designated areas in a trailer were pooled for each time point and tested for PRRSV and TGEV by respective PCRs at the ISU-VDL. Briefly, viral nucleic acid was extracted from the samples using a MagMAX bead-based method (Life Technologies, Carlsbad, California) following the manufacturer’s procedures. A commercial PRRSV real-time reverse-transcriptase quantitative PCR (RT-qPCR) assay (Tetracore Inc, Rockville, Maryland) was used for testing PRRSV per manufacturer’s instructions. A series of plasmid-derived RNA standards with known concentrations were used to generate a standard curve in each PRRSV PCR plate. A transmissible gastroenteritis virus S-gene-based real-time RT-PCR was set up using a Path-ID Multiplex One-Step RT-PCR Kit (Life Technologies) and primers (forward primer 5ʹ-AACCATAAGTTCCCTATAT GTCCTT-3ʹ, reverse primer 5ʹ-CCAGACCATTGATTTTCAAAAC TAATAC-3ʹ) and probe (5ʹ-6FAM-CACCATGTAAATAAGCAACAA-3ʹMGB). The RT-PCR was run on an ABI 7500 Fast instrument (Life Technologies) with the following conditions: one cycle of 48°C for 10 minutes, one cycle of 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds and 60°C for 45 seconds.
Objective 2
Swine bioassay. To determine if the environmental samples collected for Objective 1 contained infectious PRRSV and TGEV, a swine bioassay was conducted. The environmental samples collected for Objective 1 were used to prepare the inoculum for the bioassay. For the AHP group in Objective 1, the environmental samples collected from all of the replicates at 15 minutes post disinfection were pooled to form the AHP15 group for the bioassay, and all of the replicates at 60 minutes post disinfection were pooled to form the AHP60 group. For the GQA group in Objective 1, the environmental samples collected from all of the replicates at 15 minutes post disinfection were pooled to form the GQA15 group for the bioassay, and all of the replicates at 60 minutes post disinfection were pooled to form the GQA60 group. For the Pos Control and Neg Control groups in Objective 1, the environmental samples collected from all of the replicates at 15 minutes post wash were pooled to form the Pos15 and Neg15 groups. The resulting pools were allowed to settle, and the overlying supernatant was collected and used to inoculate all pigs by both oral gavage and intramuscular (IM) injection for the bioassay.
Study pigs and housing. Twenty-four 10-week-old pigs were obtained from a known PRRSV-negative and TGEV-negative herd. Pigs were numbered and tagged and then assigned to treatment groups using a random number generator. All pigs in a treatment group were housed together in a single room. Treatment groups were purposively assigned to rooms to minimize the risk of moving virus from one room to the next.
Pigs were received 3 days prior to inoculation. Blood and fecal samples were collected from all pigs 2 days after arrival (day -1) and submitted to the ISU-VDL to confirm the pigs were negative for PRRSV by qPCR and TGEV by PCR, and for PRRSV and TGEV antibodies by ELISA. A commercial PRRS X3 ELISA Kit (Idexx Laboratories Inc, Westbrooke, Maine) was used to test for anti-PRRSV antibody and a commercial TGEV/PRCV-Ab ELISA kit (Boehringer Ingelheim Svanova, Uppsala, Sweden) was used to test for anti-TGEV antibody. For the duration of the study, four pigs were housed in pens that were 1.68 m wide and 3.05 m long, with solid concrete floors. Each pen had a single water nipple. Pigs were fed a non-medicated, complete-feed ration adequate for their nutritional needs and were monitored daily for clinical signs of PRRS or TGE. All clinical observations were recorded.
Inoculation. Study pigs were manually restrained by an investigator for inoculation. For intramuscular injection, 4 mL of supernatant was administered per pig. Oral gavage was performed using 7 mL of supernatant per pig for all groups except for the GQA15 group; to utilize all available supernatant, 8 mL per pig was used for oral gavage in the GQA15 group. Fewer negative control replicates for Objective 1 resulted in less supernatant available for inoculation of Neg15 study pigs: 2 mL of supernatant was utilized for IM injection and 4 mL for oral gavage in the Neg15 group. Oral gavage was performed using a speculum and 16-cm, 18 Fr rubber urethral catheter (Tyco Healthcare Group, Mansfield, Massachusetts). To prevent potential cross contamination between study groups, the investigators changed coveralls, disposable plastic boots, and nitrile gloves between study rooms. Investigators always visited the Neg15 group first and the Pos15 group last, with the remaining four treatment groups placed in rooms between the aforementioned groups.
Blood and fecal sampling. Blood samples and fecal swabs were collected from all pigs on days 7 and 14 post inoculation. Blood samples were collected by venipuncture of the jugular using a separate Vacutainer (Becton Dickinson ) for each pig. Fecal samples were collected using a Copan Liquid Amies Elution Swab Collection and Transport System (Copan Italia, Brescia, Italy). The blood was centrifuged, and serum and feces were stored at -80°C until tested. All samples were submitted to the ISU-VDL to be tested for PRRSV by qPCR and for TGEV by PCR, and antibodies to PRRSV and TGEV by ELISA immediately after collection. Determination of whether a minimum infectious dose of live infectious virus was present in the inoculum was based on the PCR and ELISA results on day 7 and day 14 post inoculation as an indicator of whether the pigs were infected with either PRRSV or TGEV.
Euthanasia and necropsy. Study pigs were necropsied on day 14 post inoculation after collection of a fecal sample and blood. Euthanasia was performed by administering Fatal-Plus (Pentobarbital sodium; Vortech Pharmaceuticals, Ltd, Dearborn, Michigan) at a dose of 1 mL per 4.54 kg of body weight via the jugular vein. Each pig was necropsied and multiple sections of small and large intestine were collected for fixation in 10% neutral-buffered formalin. Necropsies were performed in exactly the same order as investigators visited bioassay groups during the course of the study. Sections were submitted to ISU-VDL for immunohistochemistry following previously described procedures.23-25
Statistical analysis
Statistical analysis of the data was performed using SAS statistical software (SAS version 9.2, SAS Institute, Inc, Cary, North Carolina). For the PRRSV qPCR and TGEV PCR results in Objective 1, pairwise comparisons of the number of positive replicates between treatment groups at each time point were performed using Fisher’s exact test. Values for the number of genomic copies of PRRSV were transformed by log10 (x + 1) so that 0 values for samples that were negative by PCR were transformed to 0. A mixed model using PROC GLIMMIX in SAS was utilized for analysis of the transformed results, with trailer and block set as random effects and treatment and time as fixed effects.
A power calculation was performed prior to the beginning of the study to understand the number of pigs needed for each group in the bioassay. It was determined that a sample size of eight was more than sufficient to detect a difference of 80% in the proportion of pigs positive for the bioassay between the positive control (Pos15) group and each of the treatment groups with a 5% level of significance and 80% power.
Results
Objective 1 environmental PCR
No significant difference was identified in the numbers of PRRSV qPCR-positive replicates found for the AHP, GQA, or Pos Control treatment groups at any sampling time (Table 2). The Neg Control group did not have any positive replicates by PRRSV qPCR at any time point. The least squares mean of the number of PRRSV genomic copies per mL found in replicates of AHP was significantly lower than for the GQA and the Pos Control treatments (P < .001) at time point 3, and for AHP versus the Pos Control treatment (P < .001) at time point 4.
Table 2: PRRS virus polymerase chain reaction (PCR) results and number of genomic copies of PRRS virus (least squares means [LSM] and standard error [SE]) for environmental samples collected from each model livestock trailer at five designated time points for Objective 1*
| Treatment group | PCR results† | No. of genomic copies/mL (LSM)‡ | SE |
|---|---|---|---|
| Time 1 | |||
| AHP | 8/8a | 5.0a | 0.25 |
| GQA | 8/8a | 5.1a | 0.25 |
| Pos Control | 9/9a | 5.0a | 0.24 |
| Neg Control | 0/4b | 0b | NA |
| Time 2 | |||
| AHP | 8/8a | 3.2a | 0.25 |
| GQA | 8/8a | 3.3a | 0.25 |
| Pos Control | 9/9a | 3.1a | 0.24 |
| Neg Control | 0/4b | 0b | NA |
| Time 3 | |||
| AHP | 6/8ab | 1.5a | 0.25 |
| GQA | 8/8b | 3.0b | 0.25 |
| Pos Control | 9/9b | 3.3b | 0.24 |
| Neg Control | 0/4a | 0c | NA |
| Time 4 | |||
| AHP | 7/8a | 1.8a | 0.25 |
| GQA | 8/8a | 2.1ab | 0.25 |
| Pos Control | 9/9a | 2.7b | 0.24 |
| Neg Control | 0/4b | 0c | NA |
| Time 5 | |||
| AHP | 7/8a | 2.0a | 0.25 |
| GQA | 6/8ab | 2.1a | 0.25 |
| Pos Control | 9/9a | 2.1a | 0.24 |
| Neg Control | 0/4b | 0.0b | NA |
* Study described in Table 1. Treatment groups evaluated for Objective 1 included disinfection of virus-contaminated trailers with an accelerated hydrogen peroxide disinfectant (AHP), or a combined glutaraldehyde and quaternary ammonium disinfectant (GQA), a positive control group (Pos Control) with a virus-contaminated trailer and sham disinfection with water, and a negative control group (Neg Control) with no virus contamination and no disinfection. Time 1, immediately after the second feces-virus mixture was applied; Time 2, immediately after completion of the washing procedure; and times 3, 4, and 5 at 15, 30, and 60 minutes after disinfection for treatment groups AHP and GQA and after washing for treatment groups Neg Control and Pos Control.
† No. of replicates positive for PRRS virus by PCR/no. of replicates tested. No. of positive replicates were compared among groups using Fisher’s exact test.
‡ Differences in least squares means of number of genomic copies were compared among treatment groups using a general linear mixed model, with trailer and block set as random effects and treatment and time as fixed effects. Values for the number of genomic copies of PRRS virus were transformed by log10 (x+1). Least squares means of the number of genomic copies are reported on a log10 scale.
a,b,c Within a column and a time point, values with different superscripts are statistically different (P < .05; Fisher’s exact test, general linear mixed model).
PRRS = porcine reproductive and respiratory syndrome; NA = not applicable.
Significantly fewer TGEV PCR-positive replicates were found for AHP than for the Pos Control at time points 2 and 4 (P < .05) (Table 3). A significant difference in the number of TGEV-positive replicates was not found between AHP, GQA, or Pos Control at any other time point. No replicates were positive on TGE PCR at any time point tested for the Neg Control group.
Table 3: Results of PCR testing for TGEV in virus-contaminated model livestock trailers representing field conditions and disinfected using either an accelerated hydrogen peroxide disinfectant (AHP) or a combined glutaraldehyde and quaternary ammonium disinfectant (GQA) or contaminated with virus, washed, and sham disinfected (Pos Control), or washed but neither contaminated with virus nor disinfected (Neg Control)
| Treatment group* | PCR results† |
|---|---|
| Time 1 | |
| AHP | 8/8a |
| GQA | 8/8a |
| Pos Control | 9/9a |
| Neg Control | 0/4b |
| Time 2 | |
| AHP | 4/8ab |
| GQA | 8/8bc |
| Pos Control | 9/9c |
| Neg Control | 0/4a |
| Time 3 | |
| AHP | 1/8a |
| GQA | 4/8a |
| Pos Control | 5/9a |
| Neg Control | 0/4a |
| Time 4 | |
| AHP | 0/8a |
| GQA | 2/8ab |
| Pos Control | 5/9b |
| Neg Control | 0/4ab |
| Time 5 | |
| AHP | 1/8a |
| GQA | 3/8a |
| Pos Control | 2/9a |
| Neg Control | 0/4a |
* Model livestock trailers were contaminated twice with PRRSV- and TGEV-spiked feces. Time point 1: immediately after the second feces-virus mixture was applied; time point 2: immediately after completion of the washing procedure; and time points 3, 4, and 5 at 15, 30, and 60 minutes after disinfection, respectively, for treatment groups AHP and GQA, and after washing for Neg Control and Pos Control treatment groups.
† No. of replicates positive/no. of replicates tested.
a,b,c Significant differences between groups within each time point in the proportion of replicates that were PCR-positive for TGEV (P < .05; Fisher’s exact test).
PCR = polymerase chain reaction; TGEV = transmissible gastroenteritis virus; PRRSV = porcine reproductive and respiratory syndrome virus.
The 90-second wash time utilized for the model trailers consistently resulted in some visible fecal matter remaining in the trailers, as can be seen in panels B and C of Figure 4. Visual differences were also noted in the appearance of the AHP and GQA treatments post application. Figure 5 shows the AHP disinfectant and the combined GQA disinfectant 60 minutes after the foaming step was completed. A noticeably larger amount of foam was present for the AHP treatment than for the GQA treatment.
Figure 5: Model trailers after disinfectants were applied with a disinfectant foamer after the washing procedure had been completed. Panel A: accelerated hydrogen peroxide disinfectant 60 minutes after disinfection application; Panel B: combined glutaraldehyde and quaternary ammonium disinfectant 60 minutes after application.


Objective 2 swine bioassay
All four pigs in the Pos15 treatment group were positive for PRRSV by PCR at 7 and 14 days post inoculation. ELISA testing confirmed that four of four pigs were positive for PRRSV antibodies in the Pos15 group at day 14 post inoculation. All pigs in the Neg15, AHP15, AHP60, GQA15, and GQA60 groups remained negative for PRRSV by qPCR at 7 and 14 days post inoculation and negative for antibodies to PRRSV by ELISA on day 14 post inoculation. Diarrhea was noted in all testing groups except Neg15. Most pigs began showing loose stools within the first 7 days post inoculation. Signs resolved in all groups by 10 days post inoculation. Pigs in GQA15 had noticeable diarrhea on day -1, but signs resolved by the day of inoculation. No pigs in the study tested positive for TGEV by PCR at either 7 or 14 days post inoculation. Serum ELISA testing showed no seroconversion for TGEV in any bioassay group. Immunohistochemistry staining for the presence of TGEV was negative on all intestinal tissue samples collected on day 14 post inoculation. No other etiologies that may have caused the diarrhea were explored, and the cause of the diarrhea remained unidentified.
Discussion
The conditions under which this study was conducted closely resembled field conditions to better understand how the risk of disease transmission can be mitigated in field settings. The pressure wash resulted in incompletely cleaned model transport trailers that closely represented conditions often found in transport vehicles that haul commercial growing and market pigs after a wash is completed. Washing times from previous transport vehicle research1,2,12 were based on the amount of time required to clean transportation vehicles used to haul breeding stock and genetically valuable swine. For this study, the wash time was selected to replicate field conditions under which growing and market pigs are transported. The design characteristics of the model transport vehicle created areas within the model that were harder to clean and prone to contamination with washed debris. Areas such as gate latches, hinges, and cross beams responsible for supporting an upper deck were represented in the model, creating challenges due to the small size and accessibility to good washing angles.
High-pressure washing with tap water did result in a reduction in the number of PRRSV genomic copies, but did not prevent infection of pigs with PRRSV when paired with a sham disinfection with tap water. Both the AHP disinfectant and a combined GQA disinfectant sufficiently eliminated viable PRRSV and prevented infection after 15 minutes of contact time. The combined GQA disinfectant results are similar to those found in previous research.2
No bioassay study pigs developed TGEV antibodies, were found to be shedding TGEV by PCR testing, or were positive by IHC, including those in the Pos15 group. Therefore, it was not possible to determine the efficacy of the AHP or GQA treatments against TGEV. The number of positive control replicates that were positive for TGEV by PCR in Objective 1 declined over time, with only two of nine replicates remaining positive at 60 minutes after washing. Multiple factors could have contributed to these results. The mixture of virus with feces and subsequent cleaning with high-pressure washing and drying of the model trailer may have resulted in removal and desiccation of a large portion of the TGEV. Transmissible gastroenteritis virus Purdue strain VR-763 has been used to induce clinical lesions of TGE in previous research.26 Additional passages of the virus may have caused adaptation of the virus to the cell line, reduced viral stability outside of cell culture, and reduced infectivity. It is also possible, though less likely, that sampling at 7 days post inoculation may have been too late to detect TGEV by PCR. Previous research showed that by 7 days post inoculation, fewer than 50% of inoculated 4-week-old piglets were shedding TGEV as measured by cell culture.27
The foaming characteristics of the AHP disinfectant evaluated may be beneficial in trailers where longer disinfectant contact times are desired. The relative lack of foam does not infer that the combined GQA disinfectant is no longer active, but continued contact of foam on walls, ceilings, and other surfaces may increase the likelihood of continued disinfectant activity.
Some limitations exist in extrapolating results to field conditions commonly experienced within the swine industry. Due to the characteristics of the disinfectant foamer utilized in the study, both disinfectants were applied at a rate slightly greater than their labeled concentrations. Labelled rates of application for the two products are a 1:16 concentration for the AHP and 1:256 for the GQA. The AHP product was applied at a rate equivalent to one part of AHP to 14.3 parts water and the GQA product was applied at a rate of one part GQA to 183.6 parts water. It may be possible that the increase in applied concentrations above label rate may be equivalent to results achieved with either product when used at the label rate. Future studies may benefit from utilizing a disinfectant foamer with the ability to achieve the labelled concentration for each product.
Investigators used 10-week-old pigs in the bioassay phase of the study for inoculation with environmental supernatant collected from PRRSV- and TGEV-contaminated model trailers. It is possible that younger pigs would have been more sensitive to lower concentrations of infectious TGEV and may have served as better bioassay candidates in this study.11 Subsequent studies may opt to utilize younger pigs for bioassay testing.
Testing the negative control samples was meant to serve as a check on the effectiveness of the decontamination process used between blocks to decontaminate the model trailers. Investigators felt that poor decontamination processes for model trailers would have resulted in positive samples being found in at least one negative control replicate. Positive results were not found in any negative control replicates in Objective 1. The investigators acknowledge that, in future testing, it may be beneficial to complete testing between blocks for all model trailers rather than relying on the Neg Control treatment group to evaluate the decontamination process. The bioassay remained the primary outcome of interest for this study, and the investigators did not feel that testing between blocks significantly changed the outcome of those results.
To standardize the research, the investigators chose to use a 4°C cooler to simulate cold, winter-like conditions for contaminated model trailers. The investigators also understand that these conditions vary between regions of North America, depending on latitude and regional geographical characteristics, and that different weather conditions may positively or negatively affect viability of infectious PRRSV or TGEV onboard livestock trailers. A cooler or freezer that was able to maintain a colder temperature setting was not available to the investigators. Future studies may identify the impact of subfreezing temperatures on the effectiveness of the sanitation and disinfectant processes utilized in this study.
Further research may be beneficial to identify whether an AHP disinfectant is effective at eliminating TGEV and other swine pathogens, including porcine epidemic diarrhea virus and Brachyspira species, from transport vehicle settings. Research is also needed to investigate alternative methods for transport-vehicle sanitation that could be performed more rapidly, with greater ease, and for less cost. Additionally, the availability of truck-wash facilities is limited in some parts of the country. Devising additional sanitation methods that resolve these challenges is important to increase compliance among truck drivers and to improve transportation biosecurity.
Implications
• Under the conditions of this study, environmental samples from model trailers cleaned with a cold-water, high-pressure wash and disinfected with either an accelerated hydrogen peroxide disinfectant or a combined glutaraldehyde and quaternary ammonium disinfectant do not consistently test negative by qPCR for PRRSV.
• In conditions equivalent to those experienced in this research, a cold-water, high-pressure wash to remove most, but not all, organic matter, paired with application of an accelerated hydrogen peroxide disinfectant or a combined glutaraldehyde and quaternary ammonium disinfectant, with at least 15 minutes of contact time, is able to inactivate PRRSV onboard experimentally contaminated model transport trailers.
• Under the conditions of this study, a cold-water, high-pressure wash alone is not effective at eliminating virulent PRRSV from a model transportation trailer.
Acknowledgments
The researchers would like to thank Boehringer-Ingelheim Vetmedica Inc (St Joseph, Missouri), through a PRRS Research Award and Virox Technologies Inc (Oakville, Ontario, Canada), for providing funding for this study.
Conflict of interest
Funding, in part, for this study was provided by Virox Technologies Inc, the manufacturer of Accel.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Dee SA, Deen J, Otake S, Pijoan C. An experimental model to evaluate the role of transport vehicles as a source of porcine reproductive and respiratory syndrome virus to susceptible pigs. Can J Vet Res. 2004;68:128–133.
2. Dee S, Deen J, Burns D, Douthit G, Pijoan C. An assessment of sanitation protocols for commercial transport vehicles contaminated with porcine reproductive and respiratory syndrome virus. Can J Vet Res. 2004;68:208–214.
3. Loula T. Mystery pig disease. Agri-practice. 1991;12:23–34.
4. Christianson WT, Joo HS. Porcine reproductive and respiratory syndrome: A review. Swine Health Prod.1994;2:10–28.
5. Holtkamp DJ, Kliebenstein JB, Neumann EJ, Zimmerman JJ, Rotto HF, Yoder TK, Wang C, Yeske PE, Mowrer CL, Haley CA. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J Swine Health Prod. 2013;21:72–84.
6. Li Y, Wang X, Bo K, Wang X, Tang B, Yang B, Jiang W, Jiang P. Emergence of a highly pathogenic porcine reproductive and respiratory syndrome virus in the mid-eastern region of China. Vet J. 2007;174:577–584.
7. Bierk MD, Dee SA, Rossow KD, Otake S, Collins JE, Molitor TW. Transmission of porcine reproductive and respiratory syndrome virus from persistently infected sows to contact controls. Can J Vet Res. 2001;65:261–266.
*8. Halbur PG, Bush E. Update on abortion storms and sow mortality [Report]. Swine Health Prod. 1997;5:73.
9. Mengeling WL, Lager KM, Vorwald AC, Koehler KJ. Strain specificity of the immune response of pigs following vaccination with various strains of porcine reproductive and respiratory syndrome virus. Vet Micro. 2003;93:13–24.
10. Haelterman EO, Hutchings LM. Epidemic diarrheal disease of viral origin in newborn swine. Ann New York Acad Sci. 1956;66:189–190.
11. Saif LJ, Pensaert MB, Sestak K, Yeo SG, Jung K. Coronaviruses. In: Zimmerman J, Karriker L, Ramirez A, Schwartz K, Stevenson G. Diseases of Swine. 10th ed. Ames, Iowa; John Wiley and Sons, Inc; 2012:501–524.
12. Dee SA, Deen J. Evaluation of an industry-based sanitation protocol for transport vehicles contaminated with porcine reproductive and respiratory syndrome virus. J Swine Health Prod. 2006;14:126–132.
13. Amass SF, Vyverberg BD, Ragland D, Dowell CA, Anderson CD, Stover JH, Beaudry DJ. Evaluating the efficacy of boot baths in biosecurity protocols. Swine Health Prod. 2000;8:169–173.
14. Amass SF, Ragland D, Spicer P. Evaluation of the efficacy of a peroxygen compound, Virkon® S, as a boot bath disinfectant. J Swine Health Prod. 2001;9:121–123.
*15. Rabbe C, Murray D, Sponheim A. An evaluation of Stalosan® F powder for deactivation of PRRSv. Proc AASV. Denver, Colorado. 2012;97–100.
16. Dee S, Deen J, Pijoan C. Evaluation of 4 intervention strategies to prevent the mechanical transmission of porcine reproductive and respiratory syndrome virus. Can J Vet Res. 2004;68:19–26.
17. Patterson AR, Baker RB, Madson DM, Pintar AL, Opriessnig T. Disinfection protocols reduce the amount of porcine circovirus type 2 in contaminated 1:61 scale model livestock transport vehicles. J Swine Health Prod. 2011;19:156–164.
18. Mengling WL, Lager KM, Vorwald AC. Clinical consequences of exposing pregnant gilts to strains of porcine reproductive and respiratory syndrome (PRRS) virus isolated from field cases of “atypical” PRRS. Am J Vet Res. 1998; 59:1540–1544.
19. Opriessnig T, McKeown NE, Harmon KL, Meng XJ, Halbur PG. Porcine circovirus type 2 infection decreases the efficacy of a modified live porcine reproductive and respiratory syndrome virus vaccine. Clin Vaccine Immunol. 2006;13: 923–929.
20. Bernard S, Laude H. Site-specific alteration of transmissible gastroenteritis virus spike protein results in markedly reduced pathogenicity. J Gen Virol. 1995;76:2235–2241.
21. Zhou J, Huang F, Hua X, Cui L, Zhang W, Shen Y, Yan Y, Chen P, Ding D, Mou J, Chen Q, Lan D, Yang Z. Inhibition of porcine transmissible gastroenteritis virus (TGEV) replication in mini-pigs by shRNA. Virus Res. 2010;149:51–55.
22. Yoon KJ, Zimmerman JJ, Chang CC, Cancel-Tirado S, Harmon KM, McGinley MJ. Effect of challenge dose and route on porcine reproductive and respiratory syndrome virus (PRRSV) infection in young swine. Vet Res. 1999;30:629–638.
23. Halbur PG, Andrews JJ, Huffman EL, Paul PS, Meng XJ, Niyo Y. Development of a streptavidin-biotin immunoperoxidase procedure for the detection of porcine reproductive and respiratory syndrome virus antigen in porcine lung. J Vet Diagn Invest. 1994;6:254–257.
24. Halbur PG, Miller LD, Paul PS, Meng XJ, Huffman EL, Andrews JJ. Immunohistochemical identification of porcine reproductive and respiratory syndrome virus (PRRSV) antigen in the heart and lymphoid system of three-week-old colostrum-deprived pigs. Vet Path. 1995;32:200–204.
25. Shoup DI, Swayne DE, Jackwood DJ, Saif LJ. Immunohistochemistry of transmissible gastroenteritis virus antigens in fixed paraffin-embedded tissues. J Vet Diagn Invest. 1996;8:161–167.
26. Kim B, Chae C. Experimental infection of piglets with transmissible gastroenteritis virus: A comparison of three strains (Korean, Purdue, and Miller). J Comp Path. 2002;126:30–37.
27. Wesley RD, Mengling WL, Lager KM. Prior infection of nursery-age pigs with porcine reproductive and respiratory syndrome virus does not affect the outcome of transmissible gastroenteritis virus challenge. J Vet Diagn Invest. 1998;10:221–228.
*Non-refereed references.
