| Commentary | Peer reviewed |
Cite as: Holst S, Yeske P, Pieters M. Elimination of Mycoplasma hyopneumoniae from breed-to-wean farms: A review of current protocols with emphasis on herd closure and medication. J Swine Health Prod. 2015;23(6):321–330.
Also available as a PDF.
SummaryMycoplasma hyopneumoniae is one of the most prevalent and economically significant respiratory pathogens in the swine industry. Economic losses related to M hyopneumoniae are associated with decreased feed efficiency, reduced average daily gain, and increased medication costs. In an effort to mitigate these economic losses, swine veterinarians and producers utilize several control measures, including optimizing management and housing, vaccination, and strategic antimicrobial medication. When control measures are insufficient, or eradication of M hyopneumoniae is preferred, swine veterinarians and producers may elect to eliminate M hyopneumoniae from affected sow farms. Herd closure and medication protocols have become widely used in North America to eliminate M hyopneumoniae from breed-to-wean farms. As vital principles for success, these protocols rely on no new animal introductions for at least 8 months, vaccination of the entire breeding herd, and medication of the breeding herd and piglets. Commonly, the breeding herd is medicated with oral antimicrobials delivered via the drinking water or feed, whereas the piglets are treated with injectable antimicrobials. In this commentary, we will review current M hyopneumoniae elimination protocols with an emphasis on the herd closure and medication protocols. | ResumenEl Mycoplasma hyopneumoniae es uno de los patógenos respiratorios más prevalentes y económicamente significativos de la industria porcina. Las pérdidas económicas relacionadas con el M hyopneumoniae están asociadas con la disminución de la eficiencia alimenticia, reducción en la ganancia diaria promedio, y el incremento en los costos de medicamento. En un esfuerzo por mitigar estas pérdidas económicas, los veterinarios y productores porcinos utilizan varias medidas de control, incluyendo la optimización del manejo y alojamiento, vacunación, y medicación antimicrobiana estratégica. Cuando las medidas de control son insuficientes, o se prefiere la erradicación del M hyopneumoniae, los productores y veterinarios porcinos pueden elegir eliminar el M hyopneumoniae de las granjas de hembras afectadas. El cierre de granja y los protocolos de medicación se han vuelto ampliamente utilizados en Norte América para eliminar el M hyopneumoniae de las granjas de cría a destete. Los principios importantes para el éxito de estos protocolos dependen de, no introducir nuevos animales por lo menos por 8 meses, vacunación del hato de cría completo, y medicación de los hatos de cría y lechones. Comúnmente, el hato de cría es medicado con antimicrobianos orales administrados vía agua de bebida o alimento, mientras que los lechones son tratados con antimicrobianos inyectables. En este comentario, revisaremos los protocolos actuales de eliminación del M hyopneumoniae actuales con énfasis en el cierre de granja y los protocoles de medicación. | ResuméMycoplasma hyopneumoniae est un des agents pathogènes les plus fréquents et économiquement importants dans l’industrie porcine. Les pertes économiques liées à M hyopneumoniae sont associées à une réduction de l’efficacité alimentaire, une diminution du gain moyen quotidien, et une augmentation des coûts de médication. Dans un effort de réduire ces pertes économiques, les vétérinaires porcins et les producteurs utilisent plusieurs mesures de contrôle, incluant l’optimisation de la gestion et de l’hébergement, la vaccination, et l’administration stratégique d’antimicrobiens. Lorsque les mesures de contrôle sont insuffisantes, ou que l’éradication de M hyopneumoniae est préférable, les vétérinaires et les producteurs peuvent décider d’éliminer M hyopneumoniae des troupeaux de truies affectées. La fermeture des troupeaux et des protocoles de médication sont couramment utilisés en Amérique du Nord pour éliminer M hyopneumoniae des fermes de type naisseur-sevrage. Comme principes essentiels à la réussite, ces protocoles se fient au fait qu’il n’y a aucune introduction de nouveaux animaux pour au moins 8 mois, que le troupeau entier des reproducteurs soit vacciné, et que les animaux reproducteurs et les porcelets soient médicamentés. De manière usuelle, le troupeau de reproducteurs est médicamenté par administration d’antimicrobiens oraux administrés via l’eau de boisson ou les aliments, alors que les porcelets sont traités par injections d’antimicrobiens. Dans le présent commentaire, nous ferons la revue des protocoles courants d’élimination de M hyopneumoniae avec une emphase sur la fermeture du troupeau et les protocoles de médication. |
Keywords: swine, Mycoplasma hyopneumoniae, elimination, herd closure, medication
Search the AASV web site
for pages with similar keywords.
Received: December 30, 2014
Accepted: June 16, 2015
Mycoplasma hyopneumoniae is one of the most prevalent and economically significant resp-iratory pathogens in the swine industry.1 Mycoplasma hyopneumoniae is the etiologic agent of enzootic pneumonia, a chronic respiratory disease in swine characterized by a chronic, non-productive cough.2,3 Pathogenicity of M hyopneumoniae stems from the organism’s ability to adhere to and damage the ciliary epithelium associated with the trachea, bronchi, and bronchioles of the respiratory tract.4 Economic losses related to M hyopneumoniae are associated with decreased feed efficiency, reduced average daily gain, and increased medication costs.1 In addition, M hyopneumoniae is considered to play a key role in porcine respiratory disease complex5 where it interacts with other respiratory pathogens.
Due to M hyopneumoniae’s ability to inflict economic losses independently, and its capability to interact with and increase the severity of other respiratory microorganisms, swine veterinarians and producers have attempted to mitigate losses through several control methods. These methods include, but are not limited to all-in, all-out (AIAO) production,1,6 sow and pig vaccination,1,7-17 gilt acclimatization,18 medicated and non-medicated early weaning,1,19-22 segregated parity production,23 and strategic antimicrobial medication.24-35 While these methods can decrease infection pressure and improve pig health, they do not assure the absence of M hyopneumoniae within a herd or flow of pigs.36
Mycoplasma hyopneumoniae elimination protocols
Mycoplasma hyopneumoniae elimination protocols can be implemented when control measures have been unsuccessful or if exclusion of the pathogen from a herd is desired. Various protocols for M hyopneumoniae elimination have been described, including depopulation and repopulation, partial depopulation, herd closure and medication, and whole-herd medication without herd closure. The herd closure and medication and whole-herd medication protocols will be emphasized in this commentary, as they are widely used in the United States.
Depopulation and repopulation is the most direct approach for M hyopneumoniae eradication, as it involves removal of the entire breeding herd and restocking with M hyopneumoniae-negative replacements.37 Advantages of depopulation and repopulation include the ability to eliminate more than one disease at once and the opportunity to improve genetics.37 However, there is a complete loss of production from the time the breeding herd is liquidated until replacement females begin farrowing. Furthermore, total depopulation of the breeding herd may be undesirable on farms with animals that have a high genetic potential (ie, genetic nucleus or multiplier farms).
The partial depopulation (Swiss) method gained recognition in the 1990s when Switzerland implemented a national program to eliminate M hyopneumoniae and Actinoba-cillus pleuropneumoniae.38 The following items are the framework for the Swiss method.38-41 First, remove all animals less than 10 months of age from the herd; second, cease farrowing for at least 2 weeks; and third, medicate remaining animals with an antimicrobial labeled for M hyopneumoniae during the non-farrowing period. Elimination projects in Norway and Denmark were also successful, with slight modifications to the Swiss partial depopulation protocol.42,43
Herd closure and medication protocols for M hyopneumoniae elimination are adaptations of the Swiss method. Modifications to the Swiss method allow for farrowing to continue during the medication period in order to minimize production losses. The herd closure and medication approach utilizes these key principles:37 first, exposure of all females, including replacement gilts, to M hyopneumoniae; second, closure of the herd for at least 8 months; third, entire herd vaccination with a M hyopneumoniae bacterin; and fourth, medication of the whole sow herd and piglets prior to introduction of M hyopneumoniae-negative replacement gilts. It is critical that all replacement gilts are exposed to M hyopneumoniae and colonized prior to beginning herd closure. Herd closure of at least 8 months is based on published research indicating that pigs can shed M hyopneumoniae up to 200 days post infection.44 Blanket vaccination of the whole sow herd with an M hyopneumoniae bacterin is usually performed to increase herd immunity. Finally, all sows and piglets on-site are medicated with an approved antimicrobial effective against M hyopneumoniae. Specific antimicrobial regimes commonly used in M hyopneumoniae elimination programs are discussed later in this commentary.
The whole-herd medication without herd closure protocol is the most recent M hyopneumoniae elimination protocol to be described.45,46 This protocol involves medicating the entire herd (gilts, sows, boars, and piglets) with a long-acting antimicrobial (typically administered via injection) with activity against M hyopneumoniae. The whole herd is treated via antimicrobial injection on day 1 of the elimination project, followed by another injection 2 weeks later. Additionally, piglets born 4 weeks after the initial whole-herd injection are treated at birth and at 14 days of age. Replacement gilt flow is maintained per normal farm protocol, and the farm remains open to new animal introductions, with the understanding that new animal introductions are from M hyopneumoniae-negative sources only. The advantage of whole-herd medication without herd closure, when successful, is that the herd has a faster return to M hyopneumoniae-negative status. However, this protocol has been less effective at eliminating M hyopneumoniae than the herd closure and medication protocol.46 A comparative summary of the key aspects of the four mentioned elimination protocols is presented in Table 1.
Table 1: Summary of the key aspects of Mycoplasma hyopneumoniae elimination protocols most commonly used in the United States
| Elimination protocol | Production time loss | Negative replacement gilts required before, during, or after elimination | Herd vaccination | Sow medication | Piglet medication | Animal introductions | Potential for other pathogens eliminated | |
|---|---|---|---|---|---|---|---|---|
| Feed or water | Injected | |||||||
| Depopulation/repopulation | Yes | Yes | No | No | No | No | NA | Yes |
| Partial depopulation* | Yes | No | No | Yes | No | No | NA | No |
| Herd closure and medication | Yes | Yes | Yes | Yes | Yes | Yes | Stop during elimination | Yes |
| Whole-herd medication† | No | Yes | No | No | Yes | Yes | Continue as usual | Yes |
* Swiss method.
† No herd closure.
NA = not applicable.
Specifics of herd closure and medication
Herd closure
Herd closure and rollover was first described as a disease elimination tactic by Torremorell et al47 for eliminating porcine reproductive and respiratory syndrome virus (PRRSV) from sow herds. Herd closure consists of ceasing introduction of replacement females into the breeding herd for an extended period of time (typically 6 to 9 months, depending on gilt supply and capacity). The rationale for stopping new introductions into the herd is to decrease the number of susceptible animals for the pathogen to replicate in,48,49 eventually reducing the number of susceptible animals to zero. The herd remains closed to new animal additions until sufficient time has passed for the pathogen to have infected all animals on the farm, and infected animals have had time to mount an immune response and clear the pathogen, and are no longer infectious. Following successful eradications of PRRSV using the herd-closure technique, veterinarians in the United States have adapted it for utilization in M hyopneumoniae elimination projects.37
In order to maintain a practical replacement rate and continue utilizing gilts in weekly or batch breeding groups, a 6- to 9-month supply (surplus) of gilts (depending on the desired length of the closure period) are stocked into an on-site isolation or gilt developer unit (GDU). The recommended length of herd closure for M hyopneumoniae elimination is at least 8 months; therefore, an 8-month supply of gilts would be required to avoid gaps in production. Additionally, it is recommended that the entire adult population be over 10 months of age when negative replacements are introduced37 to increase the likelihood that no animals will be infectious, assuming that animals have been exposed to M hyopneumoniae at an earlier age. Thus, to satisfy the 10-month age recommendation, gilts should be a minimum of 2 months of age when stocked into isolation or the GDU. Furthermore, gilts of various ages and weights should be included to avoid a surplus of gilts that are too old or too big at the end of the M hyopneumoniae elimination project.37
A potential obstacle is that not all sow farms have on-site isolation or GDU facilities at their disposal. If this is the case, an off-site breeding project could be considered as an alternative plan. An off-site breeding project allows gilts to be bred at a separate location and added back to the herd at the time of farrowing.50 The gilts should be bred in weekly groups, and breeding should be timed so that the first group of gilts is due to farrow shortly after the herd closure period is completed. This allows breeding and farrowing targets to be met and for pig flow to be maintained as consistently as possible once the herd closure is lifted.50
Vaccination and acclimation
Commercial M hyopneumoniae bacterins are widely used in swine production worldwide.9 Pigs can become colonized with M hyopneumoniae in the first weeks after birth;51-53 therefore, vaccination of piglets is the most common vaccination strategy utilized.1 Advantages of vaccinating growing pigs include increased average daily gain (ADG), improved feed efficiency, and potentially decreased mortality rate.1 While vaccination does have several advantages regarding increased production performance, it does not prevent M hyopneumoniae colonization.9,13,16,54 Other studies have shown that M hyopneumoniae vaccination is associated with a reduction in the number of organisms in the respiratory tract,13 as well as a decreased infection level within a herd.15
In addition to growing-pig M hyopneumoniae vaccination strategies, vaccination of sows has been utilized in an attempt to reduce vertical spread of the pathogen and to confer immunity to piglets via lactogenic transmission of maternal antibodies.7,14,55 Vaccination of sows during M hyopneumoniae elimination projects is aimed at bolstering herd immunity37 and has been implemented on a quarterly basis, prior to whole-herd antimicrobial medication, or on a pre-farrow schedule. Yeske37 described vaccinating the surplus gilts at 1 and 3 weeks post entry to the on-site isolation or GDU facility and vaccination of the entire breeding herd (including gilts) on a quarterly schedule after herd closure is initiated. Additionally, Yeske37 recommends exposing the surplus gilts to the most recently infected group of gilts as soon as possible to facilitate natural infection (if gilt surplus is negative to M hyopneumoniae prior to entry to the GDU).
Schneider56 documented vaccinating the breeding herd at 5 and 2 weeks prior to beginning the antimicrobial medication protocol. Moreover, Schneider described vaccinating sows 2 weeks prior to farrowing until testing for the presence of M hyopneumoniae post eradication was completed. Schneider recommends continuing this pre-farrow vaccine protocol indefinitely if the farm is at a medium to high risk of re-infection. Snider57 described whole-breeding-herd vaccination after the acute outbreak of M hyopneumoniae was diagnosed and again prior to beginning antimicrobial medication. Lorenzen58 documented vaccinating the entire breeding herd 1 week prior to antimicrobial medication, and an additional dose of vaccine 2 weeks later. A pre-farrow vaccination schedule was described by Alfonso et al,59 where sows were vaccinated at approximately 15 days prior to farrowing and the surplus gilts were vaccinated twice, at 55 and 220 days of age.
It is important to note that several different vaccination protocols have been described, with varying numbers and frequencies of vaccinations; however, to the knowledge of the authors, no published studies indicate an advantage of utilizing one protocol versus another.
In addition to sow and gilt vaccination during M hyopneumoniae elimination projects, it is important to continue routine farm-specific gilt acclimation protocols (other vaccinations, feed-back, estrus induction and synchronization, anthelmintic administration, disease surveillance, etc) to avoid disruptions in production or challenges with other diseases.
Medication
Virtually all Mycoplasma species are resistant to betalactam antimicrobials (penicillin, ampicillin, amoxicillin, and cephalosporins). Mycoplasmas lack a cell wall, which is the target of beta-lactam antimicrobials (Table 2).60 Classes of antimicrobials with potential activity against M hyopneumoniae include macrolides, lincosamides, tetracyclines, pleuromutilins, fluoroquinolones, amphenicols, and aminoglycosides (Tables 2 and 3). Many specific antimicrobials from within these classes have been utilized in M hyopneumoniae elimination projects.
Table 2: Common classes of antibiotics utilized in the US swine industry
| Antibiotic class | Mechanism of action | Bacterial target | Effect | Potential activity against M hyopneumoniae |
|---|---|---|---|---|
| Beta-lactams60 | Cell wall synthesis inhibition | Transpeptidase | Bactericidal | No |
| Macrolides61 | Protein synthesis inhibition | 50s ribosomal subunit | Bacteriostatic | Yes |
| Lincosamides61 | Protein synthesis inhibition | 50s ribosomal subunit | Bacteriostatic | Yes |
| Tetracyclines62 | Protein synthesis inhibition | 30s ribosomal subunit | Bacteriostatic | Yes |
| Pleuromutilins63 | Protein synthesis inhibition | 50s ribosomal subunit | Bacteriostatic | Yes |
| Fluoroquinolones64 | DNA synthesis inhibition | DNA gyrase | Bactericidal | Yes |
| Amphenicols64 | Protein synthesis inhibition | 50s ribosomal subunit | Bacteriostatic | Yes |
| Aminoglycosides65,66 | Protein synthesis inhibition | 30s ribosomal subunit | Bactericidal | Yes* |
| Sulfonamides67 | Folic acid synthesis inhibition | Dihydropteroate synthase | Bacteriostatic | No |
* Aminoglycosides have activity against Mycoplasma species but are poorly absorbed when administered orally. Withdrawal times are excessively long when delivered parenterally, rendering their use against M hyopneumoniae impractical.
Table 3: Antibiotics with potential activity against Mycoplasma hyopneumoniae
| Antibiotic class | Antibiotic | Route(s) | Dose (inclusion rate) | Label indication for M hyopneumoniae |
|---|---|---|---|---|
| Macrolides | Tylosin | Parenteral | 8.8 mg/kg BW | None |
| Feed | (40 or 100 mg/kg)* | None | ||
| Water | (66 mg/L) | None | ||
| Tilmicosin | Feed | (181.8 or 363.6 mg/kg)* | None | |
| Water | (200 mg/L) | None | ||
| Tulathromycin | Parenteral | 2.5 mg/kg BW | Treatment or control | |
| Tylvalosin | Water | (50 mg/L) | None | |
| Lincosamides | Lincomycin | Parenteral | 11 mg/kg BW | Treatment |
| Feed | (200 mg/kg) | Treatment | ||
| Water | 8.4 mg/kg BW (250 mg/L) | None | ||
| Tetracyclines | Oxytetracycline | Parenteral | 6.6 - 11 mg/kg BW | None |
| Feed | (22 mg/kg) | None | ||
| Water | (22 mg/L) | None | ||
| Chlortetracycline | Feed | (22 mg/kg) | None | |
| Water | (22 mg/L) | None | ||
| Pleuromutilins | Tiamulin | Feed | (200 mg/kg) | None |
| Water | (23.1 mg/L) | None | ||
| Fluoroquinolones | Enrofloxacin | Parenteral | 7.5 mg/kg BW | Treatment or control |
| Amphenicols | Florfenicol | Water | (100 mg/L) | None |
* Preventive or therapeutic dosage.
BW = body weight.
Documented medication programs
Kohne et al68 fed tilmicosin-medicated feed (15 mg per kg body weight [BW]) to all sows, boars, and gilts on-farm for a period of 4 weeks. Additionally, any breeding-stock animal that was sick or off feed was injected with one dose of tulathromycin (2.5 mg per kg BW). Piglets were injected with enrofloxacin (2.5 mg per kg BW) at birth and with tiamulin (2 mg per kg BW) every 2 days after 3 days of age (injectable tiamulin is not labeled for use in the United States). All piglets were weaned off-site by 21 days of age. To determine the success of the elimination project, Kohne et al68 collected tonsil swabs from animals that were born on-farm at 10, 14, 23, and 27 weeks following completion of the medication plan. All samples were negative on polymerase chain reaction (PCR) for M hyopneumoniae.
Snider57 described a medication plan using tulathromycin and lincomycin. The entire breeding herd was fed lincomycin (220 mg per kg medicated feed) for 3 weeks. After the lincomycin treatment, all breeding stock and piglets received a dose of tulathromycin (2.5 mg per kg intramuscularly [IM]) followed by an additional dose in 14 days. After the second dose of tulathromycin had been administered, lincomycin-medicated feed was again fed to the breeding herd for an additional 5 weeks. Piglets continued to receive tulathromycin (2.5 mg per kg BW, IM) at birth and at 12 to 14 days of age for a period of 5 months. Piglets were weaned at 18 to 21 days of age, with no piglets on-farm older than 24 days of age. Snider57 documented that piglets from the treated farm were comingled with M hyopneumoniae-naive piglets from another sow herd and that M hyopneumoniae was not detected clinically or by ancillary diagnostic modalities for 8 months.
Geiger et al69 also employed a tulathromycin and lincomycin treatment plan. Lincomycin (100 g per tonne) was fed to the whole breeding herd for 4 weeks. Piglets were injected with tulathromycin (2 mg per kg BW) at birth, beginning 2 weeks after initiation of the medicated-feed program, and during a 2-week period were weaned off-site by 10 days of age. Treatment of piglets with tulathromycin at birth was continued for 5 weeks. Monthly serological testing (Idexx and Dako ELISA reported69) of M hyopneumoniae-negative replacement gilts on the sow farm, commercial pigs in an on-site nursery, and commercial pigs in an off-site finisher was conducted to determine elimination success. Replacement gilts and on-site pigs were negative for 22 months and the off-site finisher was negative for 15 months (last time sampled). Additionally, quarterly slaughter checks and routine necropsies showed no signs of M hyopneumoniae infection.
Another tulathromycin and lincomycin treatment protocol was carried out by Geiger and Groth,70 where the breeding stock was treated with lincomycin delivered via the water system and piglets were injected with tulathromycin (2 mg per kg BW) at birth and again 11 days later; both treatment modalities were continued for 4 weeks. Piglet weaning age was left unchanged (average, 20.6 days). Monthly serological testing (Idexx and Dako ELISA reported70) of replacement gilts began 1 month after the first M hyopneumoniae-negative replacement gilts were introduced. Random serological testing, routine necropsies with diagnostic tissue submissions (M hyopneumoniae culture, PCR, and histopathology), and slaughter checks were conducted on the downstream flow of pigs. No evidence of M hyopneumoniae infection was found in the sow herd or downstream flow for 12 and 6 months, respectively (last times sampled).
Tylvalosin usage in M hyopneumoniae eliminations has been documented in Europe utilizing either the water or in-feed formulation to treat the breeding herd for 21 to 28 days.71,72 Additionally, some tylvalosin elimination projects have included tulathromycin injections to sows exhibiting reduced feed intake and to piglets (beginning at birth and continuing at 7- to 10-day intervals until weaning).
Schneider56 supplemented a chlortetracycline (CTC) and tiamulin feed-medication program with injectable oxytetracycline (OTC) and tylosin. The breeding herd diet was medicated with CTC (440 mg per kg) and tiamulin (100 mg per kg) for a period of 3 weeks. Sows that were off feed were injected daily with OTC (17.5 mg per kg BW) and tylosin (17.5 mg per kg BW) for 5 days, followed by 5 days of no injections, and then 5 additional days of antimicrobial injections. Piglets were weaned off-site by 12 days of age and did not receive antimicrobials while on farm. Schneider56 documented 14 sow herds where M hyopneumoniae eliminations were attempted between 1995 and 2001, utilizing a medication plan similar to the one described. The amount of time that those farms experienced freedom from M hyopneumoniae following elimination ranged from 14 months to 9 years.56
Geiger and Ragone73 utilized a medication protocol using two formulations of OTC. Lactation and gestation rations were medicated with OTC (500 mg per kg of feed) for 4 weeks. In addition, for a period of 3 weeks, piglets received injections of OTC (200 mg per piglet) at 3 and 7 days of age and were weaned by 10 days of age. Following the addition of M hyopneumoniae-negative replacement gilts (sentinels), 30 random sentinels were serologically tested (Dako ELISA reported73) on a monthly basis. Serologic tests were positive and mild clinical signs of M hyopneumoniae were detected approximately 4 months following the completion of the elimination protocol.
Alfonso et al59 utilized a medication protocol with tiamulin and tilmicosin. The gestation and lactation rations were medicated with tilmicosin (16 mg per kg of feed) for 2 weeks, then tiamulin (7 mg per kg BW) for an additional 2 weeks. Furthermore, while the breeding herd was fed the tiamulin-medicated feed, piglets were injected with tiamulin (6 to 8 mg per kg BW) at 1, 5, and 13 days of age (injectable tiamulin is not labeled for use in swine in the United States). Weaning age was not altered and remained 16 days of age. Ten M hyopneumoniae-negative sentinel gilts were added to the sow farm 1 week after the medication protocol had been completed. These 10 sentinels remained serologically negative (ELISA Tween 20 reported59) for the 5 months when they were tested.
Nielson et al74 utilized a medication protocol combining tilmicosin and enrofloxacin. The breeding herd diet was medicated with tilmicosin (16 mg per kg of feed) for a 2-week period. Additionally, piglets received an injection of enrofloxacin (5 mg per kg BW) at 1, 4, and 7 days of age and were weaned at 12 days of age. Mycoplasma hyopneumoniae-negative replacement gilts were not introduced to the farm for 3 months following completion of the medication plan. Blood samples were collected monthly from 20 replacement gilts at 5, 6, 7, 8, 9, and 10 months and showed no seroconversion to M hyopneumoniae.
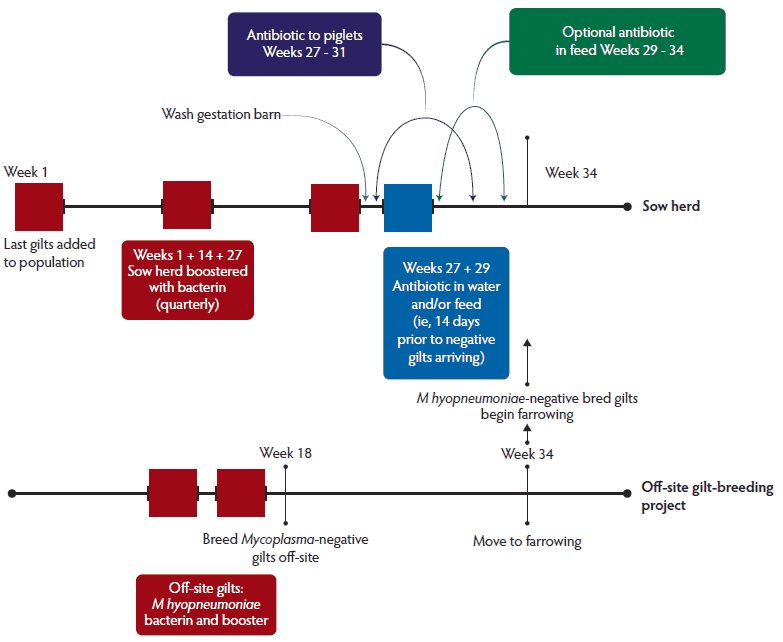
As a summary, an example timeline of action items associated with a herd closure and medication elimination protocol is shown in Box 1,37,56 and a diagram, including the parallel activities carried out in the sow herd and off-site breeding project, is presented in Figure 1. It is important to note that normal gilt acclimation procedures should be incorporated into the framework of the elimination protocol.
Box 1: Action items associated with a herd closure and medication protocol to eliminate Mycoplasma hyopneumoniae
Week 1 |
|---|
Acquire surplus supply of gilts and stock into on-site isolation or gilt development unit (GDU) |
Need enough gilts to close the herd for at least 240 days |
Gilts should be of various ages with the youngest at least 2 months of age |
Vaccinate gilts with an M hyopneumoniae bacterin |
If gilts are M hyopneumoniae-negative at the time of stocking, expose them to the most recently infected group of gilts to facilitate natural infection |
Week 3 |
Vaccinate gilts with second dose of M hyopneumoniae bacterin |
Week 4 |
Begin to introduce gilts into the sow herd (pending fulfillment of normal acclimation protocols) |
Flow gilts into sow herd as necessary to fulfill weekly breeding group needs |
Week 6 |
Vaccinate entire breeding herd with an M hyopneumoniae bacterin (quarterly vaccination schedule) |
Week 19 |
Vaccinate entire breeding herd with an M hyopneumoniae bacterin (quarterly vaccination schedule) |
Weeks 27-31 |
Stock M hyopneumoniae-naive or negative replacement gilts in isolation or GDU once all surplus gilts have entered the farm and isolation or GDU has been washed and disinfected (only if isolation or GDU is a separate air space from the breeding herd) |
Weeks 28, 29 |
Vaccinate entire breeding herd with an M hyopneumoniae bacterin (pre-medication vaccination schedule) |
Weeks 31, 32 |
Vaccinate entire breeding herd with an M hyopneumoniae bacterin (pre-medication vaccination schedule) |
Week 32 |
Vaccinate entire breeding herd with an M hyopneumoniae bacterin (quarterly vaccination schedule) |
Week 33 |
Wash and disinfect the breeding and gestation barns (shuffle sows row by row so that stalls are empty when washed and disinfected |
Weeks 33, 34 |
Begin medicating breeding herds via water or feed with antimicrobial approved for M hyopneumoniae |
Medicate breeding herd for 2 to 4 weeks depending on antimicrobial selected |
Begin treating on-farm piglets with an injectable antimicrobial approved for M hyopneumoniae at birth (or at first treatment) and again at a later time depending on the antimicrobial selected |
Week 35 |
M hyopneumoniae-naive or negative replacement gilts can begin to be introduced into the breeding herd |
Weeks 36, 37 |
Complete medication of breeding herd |
Weeks 37-41 |
Complete medication of piglets |
Weeks 38-42 |
Begin M hyopneumoniae testing to monitor success of elimination program (after completion of piglet medication) |
Figure 1: Diagram of a herd closure and medication timeline and activities, including an off-site breeding project, in a review of current M hyopneumoniae elimination protocols, with an emphasis on herd closure and medication protocols.
Discussion
The discussion of disease elimination from swine herds began back in the 1960s and 1970s with the implementation of the specific-pathogen-free (SPF) technique.75 Although the SPF program did not live up to expectations, it did lay the groundwork and encouraged veterinarians and producers that the elimination of certain diseases may be possible. A significant justification for all the time, effort, and resources dedicated to a disease elimination project may be best summarized in the benefits of disease-free populations of pigs, which will include improved animal welfare, increased production, decreased cost of production, reduction in preventative or therapeutic antimicrobial usage, and improved caretaker morale.75
Mycoplasma hyopneumoniae is a significant cause of economic loss to swine producers. In 2012, Haden et al76 quantified the economic impact of influenza A virus (IAV), PRRSV, and M hyopneumoniae on a large US production system over a 4-year period. The cost of uncomplicated M hyopneumoniae was determined to be $0.63 per head placed in grow-finish. Unfortunately, respiratory disease in growing pigs is usually not limited to one uncomplicated pathogen, but is rather a mixed infection of M hyopneumoniae, viruses, and bacteria.77,78 Haden and others76 also calculated the cost per head in situations in which M hyopneumoniae was complicated with PRRSV or IAV. The combination of PRRSV and M hyopneumoniae resulted in a loss of $9.69 per head, while co-infections of IAV and M hyopneumoniae inflicted a loss of $10.12 per head. The loss incurred due to the combination of IAV and M hyopneumoniae was only surpassed by IAV and PRRSV co-infections ($10.41 per head). While these loss calculations are specific to one particular system, similar losses are likely realized by other US swine producers as well. A 2005 and 2006 survey conducted by Holtkamp and colleagues79 attempted to estimate the impact of major swine health challenges in the United States. The participants in the survey comprised companies that produced more than 150,000 pigs per year, which accounted for approximately 50% of the total number of pigs produced annually in the United States. Results of the survey indicated that PRRSV, IAV, and M hyopneumoniae were the top three health challenges experienced in finishing herds.
Fortunately, swine producers and veterinarians have had success eliminating M hyopneumoniae using the protocols, principles, and techniques described in this commentary. A retrospective analysis of 46 herds that had undergone a M hyopneumoniae elimination project between 2003 and 2014 was completed in 2015.80 The analysis included farms located in upper midwestern US states, 33 of which utilized a herd closure and medication protocol and 13 that used a whole-herd medication protocol without herd closure. The overall success rates for elimination of M hyopneumoniae for the herd closure and medication and the whole-herd medication without closure protocols were 81% and 58%, respectively. The percentage of farms that experienced successful M hyopneumoniae elimination for a period greater than 1 year was 97% for the herd closure and medication protocol and 67% for the whole-herd medication without herd closure technique. Additionally, the average length of time that herds remained M hyopneumoniae-negative following elimination was 49 months for the herd closure and medication farms and 37 months for the whole-herd medication without closure farms.
This commentary would not be complete without discussing the costs associated with the implementation of a M hyopneumoniae elimination protocol. Yeske81 estimated $15.90 per sow as the cost of a herd closure and medication protocol utilizing quarterly sow vaccination, a 2-week course of lincomycin in the drinking water to treat the breeding herd, and, during a 4-week period, tulathromycin injections to piglets at birth and 14 days of age. Additionally, the estimated increase in wean-to-finish revenue, based on increased ADG, reduced mortality, and improved feed efficiency, was calculated to be $1.19 per pig. Furthermore, Yeske estimated that it would take a 2500-sow herd producing 25 pigs per sow per year approximately 4.5 months to recoup the financial investment in the elimination project. While $15.90 per sow is a significant amount to invest in a M hyopneumoniae elimination project, the potentially lower cost of wean-to-finish production and subsequently increased revenue allow that investment to be recovered in a reasonable amount of time. Additionally, if the hypothetical farm in Dr Yeske’s calculations81 were to remain M hyopneumoniae-free for the average of 31 months following elimination, it would realize an additional 26.5 months of reduced production costs after recovery of the initial investment.
One of the most debated aspects of the herd closure and medication protocol for M hyopneumoniae elimination is that of gilt vaccination and acclimation. Gilts have been indicted as the most likely source of M hyopneumoniae introduction into a herd and perpetuation of infection.18 Many M hyopneumoniae vaccination protocols have been described; however, to the knowledge of the authors, there is no published literature to support the use of one over another. Most gilt M hyopneumoniae vaccination and acclimation protocols are based on practitioner preference and experience. Additional research efforts are needed in this area.
It is also prudent to discuss the limitations associated with this commentary. First and foremost, many of the references utilized in this manuscript were non-peer-reviewed proceedings articles. Many of the findings described in these practitioner-authored proceedings articles are not the result of investigations subjected to the scientific rigor of studies published in peer-reviewed journals; however, they are accurate accounts of protocols, techniques, and strategies utilized in the field by practicing swine veterinarians and provide valuable information regardless of publication status. Also, there is no standardized testing protocol to determine the success of M hyopneumoniae elimination projects. Previous projects have been evaluated using one or more of the following: presence or absence of M hyopneumoniae determined by PCR on nasal or tonsil swabs, interpretation of serological screening results, and clinical signs (coughing). The lack of a standardized testing scheme makes it difficult to compare the outcomes of specific elimination protocols.
While no standard post-elimination testing protocol exists, multiple diagnostic modalities should be utilized to determine elimination success or failure. Previously described ante-mortem sampling methods for M hyopneumoniae (nasal swabs, tonsil swabs, and oral fluids)82 have been shown to lack sensitivity, and their utility in low-prevalence situations is less than satisfactory. However, a recently documented ante-mortem sampling method, laryngeal swabs, has demonstrated greater sensitivity82 and could be utilized as part of the post-elimination testing protocol. For example, serial collection and submission for M hyopneumoniae PCR testing of laryngeal swabs from a subset of M hyopneumoniae-negative replacement gilts (sentinels) that enter the farm following elimination is one testing option.
Serological screening of M hyopneumoniae-negative replacement gilts serving as sentinels would seem like a practical option for post-elimination testing; however, vaccination of replacement gilts with an M hyopneumoniae bacterin makes differentiating infection-induced antibody response from vaccine-induced antibody response difficult.4 Therefore, some farms that have undergone M hyopneumoniae elimination have elected to leave replacement gilts unvaccinated. This allows for easy interpretation of serological results; however, the risk for increased severity of M hyopneumoniae-related disease is greater if reinfection or novel infection were to occur.
Evaluation of clinical signs (coughing)4 in the downstream pig flow and replacement gilts that enter the farm following elimination should also be included in the post-elimination testing regime. Additionally, lung tissue collection and submission for M hyopneumoniae PCR testing from dead pigs in these populations rounds out a multipronged approach for post-elimination testing.
The authors’ goal for this commentary was not to recommend a specific M hyopneumoniae elimination protocol, but rather to review the basic principles, describe specific protocols utilized by practitioners, and discuss the merits of implementing an M hyopneumoniae elimination project. The elimination protocol best suited for a particular farm or system will hinge on the facilities, pig flow, gilt availability, location, production type, and other unique aspects specific to the farm or system. Due to the advantages of M hyopneumoniae-free production previously discussed, implementation of an M hyopneumoniae elimination project should be considered if it is feasible for a farm or system to adhere to the specific guidelines set forth by the elimination protocol. Interest in utilizing proven disease-elimination techniques has never been higher. Continued interest and focus on the development of innovative methods and strategies for disease elimination will be necessary to help combat health challenges faced by the swine industry, now and in the future.
Implications
• M hyopneumoniae is a significant cause of economic loss to swine producers, and successful elimination from a production system can result in improved animal welfare, increased production, decreased production costs, and reduced antimicrobial usage.
• The multitude of elimination protocols that have been described and successfully executed can be tailored to fit the unique aspects associated with a particular farm or system and their goals.
• Increased focus and effort on the development of novel disease elimination techniques and strategies will be vital to combat health challenges in the future.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Maes D, Segales J, Meyns T, Sibila M, Pieters M, Haesebrouck F. Control of Mycoplasma hyopneumoniae infections in pigs. Rev Vet Microbiol. 2008;126:297–309.
2. Mare CJ, Switzer WP. New species: Mycoplasma hyopneumoniae. A causative agent of virus pig pneumonia. Vet Med. 1965;60:841–846.
3. Goodwin RFW, Pomeroy AP, Whittlestone P. Production of enzootic pneumonia in pigs with a mycoplasma. Vet Rec. 1965;77:1247–1249.
4. Thacker E, Minion F. Mycoplasmal diseases. In: Straw B, Zimmerman J, D’Allaire S, Taylor DJ, eds. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Publishing; 2006:701–717.
5. Dee SA. The porcine respiratory disease complex: Are subpopulations important? [commentary]. J Swine Health Prod. 1996;4:147–149.
6. Clark LK, Armstrong CH, Freeman MJ, Scheidt AB, Sands-Freeman L, Knox K. Investigating the transmission of Mycoplasma hyopneumoniae in a swine herd with enzootic pneumonia. Vet Med. 1991;86:543–550.
7. Ruiz AR, Utrera V, Pijoan C. Effect of Mycoplasma hyopneumoniae sow vaccination on piglet colonization at weaning. J Swine Health Prod. 2003;11:131–134.
8. Baccaro MF, Hirose F, Umehara O, Goncalves LCB, Doto DS, Paixão R, Shinya LT, Moreno AM. Comparative efficacy of two single-dose bacterins in the control of Mycoplasma hyopneumoniae in swine raised under commercial conditions in Brazil. Vet J. 2006;172:526–531.
9. Haesebrouck F, Pasman F, Chiers K, Maes D, Ducatelle R, Decostere A. Efficacy of vaccines against bacterial diseases in swine: what can we expect? Vet Microbiol. 2004;100:255–268.
10. Hodgins DC, Shewen PE, Dewey CE. Influence of age and maternal antibodies on antibody responses of neonatal piglets vaccinated against Mycoplasma hyopneumoniae. J Swine Health Prod. 2004;12:10–16.
11. Maes D, Deluyker H, Verdonck M, Castryck F, Miry C, Lein A, Vrijens B, de Kruif A. Effect of vaccination against Mycoplasma hyopneumoniae in pig herds with a continuous production system. J Vet Med B. 1998;45:495–505.
12. Maes D, Deluyker H, Verdonck M, Castryck F, Miry C, Vrijens B, Verbeke W, Viaene J, de Kruif A. Effect of vaccination against Mycoplasma hyopneumoniae in pig herds with an all-in/all-out production system. Vaccine. 1999;17:1024–1034.
13. Meyns T, Dewulf, J, de Kruif A, Calus D, Haesebrouck F, Maes D. Comparison of transmission of Mycoplasma hyopneumoniae in vaccinated and non-vaccinated populations. Vaccine. 2006;24:7081–7086.
14. Sibila M, Bernal R, Torrents D, Riera P, Llopart D, Calsamiglia M, Segales J. Effect of sow vaccination against Mycoplasma hyopneumoniae on sow and piglet colonization and seroconversion, and pig lung lesions at slaughter. Vet Microbiol. 2008;127:165–170.
15. Sibila M, Nofrarias M, Lopez-Soria S, Segales J, Valero O, Espinal A, Calsamiglia M. Chronological study of Mycoplasma hyopneumoniae infection, seroconversion and associated lung lesions in vaccinated and non-vaccinated pigs. Vet. Microbiol. 2007;122:97–107.
16. Thacker EL, Thacker BJ, Boettcher TB, Jayappa H. Comparison of antibody production, lymphocyte stimulation, and protection induced by four commercial Mycoplasma hyopneumoniae bacterins. Swine Health Prod. 1998;6:107–112.
*17. Greiner L, Connor JF, Lowe JF. Comparison of Mycoplasma hyopneumoniae vaccination. Proc AASV. Phoenix, Arizona. 2011;245–248.
*18. Lowe JF. Mycoplasma hyopneumoniae: gilts, are they the problem? Proc AD Leman Conf. St Paul, Minnesota. 2012;83–86.
19. Dritz SS, Chengappa MM, Nelssen JL, Tokach MD, Goodband RD, Nietfeld JC, Staats JJ. Growth and microbial flora of nonmedicated, segregated, early weaned pigs from a commercial swine operation. J Vet Med. 1996;208:711–715.
20. Clark LK, Hill MA, Kniffen TS, VanAlstine W, Stevenson G, Meyer KB, Wu CC, Scheidt AB, Knox K, Albregts S. An evaluation of the components of medicated early weaning. Swine Health Prod. 1994;2(3):5–11.
*21. Clark LK. SEW-control or elimination of Mycoplasma hyopneumoniae and viruses. Swine Dis Conf Swine Pract. Ames, Iowa. 1995;69–73.
22. Dee SA. Apparent prevention of Mycoplasma hyopneumoniae infection in growing pigs with a low-cost modified-medicated-early-weaning program. Swine Health Prod. 1994;2(6):7–12.
*23. Moore C. Parity segregation, successes and pitfalls. Swine Dis Conf for Swine Pract. 2003;47–52.
24. Thacker EL, Thacker BJ, Wolff T. Efficacy of a chlortetracycline feed additive in reducing pneumonia and clinical signs induced by experimental Mycoplasma hyopneumoniae challenge. J Swine Health Prod. 2006;14:140–144.
25. Roberts E, Hammer JM, Lechtenberg K, Roycroft L, King S. Investigation of tiamulin hydrogen fumerate in-feed antibiotic for the control of porcine respiratory disease complex that includes Mycoplasma hyopneumoniae. J Swine Health Prod. 2011;19:218–225.
*26. VanBuren J, McDermid DK, Izeta J, Elfring G. LINCOMIX™ feed additive and Mycoplasma hyopneumoniae vaccination for treatment and control of naturally occurring Porcine Respiratory Disease Complex (PRDC). Proc AASV. Nashville, Tennessee. 2001;119–122.
27. Walter D, Holck JT, Sornsen S, Hagen C, Harris IT. The effect of metaphylactic pulse dosing in-feed antimicrobial strategy on finishing pig health and performance. Swine Health Prod. 2000;8:65–71.
*28. Thacker EL, Thacker BJ, Wolff T. Efficacy of Aureomycin chlortetracycline granular premix against experimental Mycoplasma hyopneumoniae challenge. Proc AASV. Nashville, Tennessee. 2001;83–85.
29. McKelvie J, Morgan JH, Nanjiani IA, Sherington J, Rowan TG, Sunderland SJ. Evaluation of tulathromycin for the treatment of pneumonia following experimental infection of swine with Mycoplasma hyopneumoniae. Vet Ther. 2005;6:197–202.
*30. Hammer JM, King S, Roberts E, Roycroft L. Efficacy of Denagard 10 (tiamulin hydrogen fumerate) in-feed antibiotic in a Mycoplasma hyopneumoniae infection model study. Proc IPVS. Vancouver, Canada. 2010;639.
31. Vicca J, Maes D, Jonker L, de Kruif A, Haesebrouck F. Efficacy of in-feed medication with tylosin for the treatment and control of Mycoplasma hyopneumoniae infections. Vet Rec. 2005;156:606–610.
*32. Harker JW, Watkins LE. Pulmotil efficacy against porcine respiratory disease complex in a commercial swine herd practicing AI/AO pigflow. Proc AASP. St Louis, Missouri. 1999;175–178.
*33. Jackson JA, Katz TL, Lockwood PW, Varma KJ. Comparison of florfenicol and tiamulin administered orally in the drinking water in the treatment of naturally occurring swine respiratory diseases in the USA. Proc IPVS. Melbourne, Australia. 2000;474.
34. Ciprián A, Palacios JM, Quintanar D, Batista L, Colmenares G, Cruz T, Romero A, Schnitzlein W, Mendoza S. Florfenicol feed supplemented decrease the clinical effects of Mycoplasma hyopneumoniae experimental infection in swine in Mexico. Res Vet Sci. 2012;92:191–196.
*35. Keil DJ, Charles S, Settje T. Studies to evaluate the effects of enrofloxacin on Mycoplasma hyopneumoniae and Bordetella bronchiseptica infections in swine. Proc AASV. San Diego, California. 2013;125–126.
*36. Painter T, Kuhn M, Wolff T, Pieters M, Senn M. Efficacy and duration of infection study for RespiSure® and Draxxin® against a Mycoplasma hyopneumoniae challenge in swine. Proc IPVS. Jeju, South Korea. 2012;207.
*37. Yeske P. Mycoplasma eradication strategies. Proc AASV. Orlando, Florida. 2007;367–370.
38. Stark KD, Miserez R, Siegmann S, Ochs H, Infanger P, Schmidt J. A successful national control programme for enzootic respiratory diseases in pigs in Switzerland. Rev Sci Tech. 2007;26:595–606.
*39. Bara MR. Eradication of Mycoplasma pneumonia: first reported Swiss depopulation in Australia. Proc IPVS. Ames, Iowa. 2002;107.
*40. Baekbo P. Procedures to eliminate M hyo and produce M hyo free pigs: an update. Proc AASP. St Louis, Missouri. 1999;479–481.
*41. Barcelo J, Oliva JE, Marinez J, Munoz A. Multiple eradication (PRRS, Mycoplasma and APP) without sow depopulation in a large farm. Proc ISSDE. 2001.
*42. Baekbo P, Madsen KS, Aagard M, Szancer J. Eradication of Mycoplasma hyopneumoniae from infected herds without restocking. Proc IPVS. Bangkok, Thailand. 1994;135.
*43. Lium B. An attempt to eradicate Mycoplasma hyopneumoniae from selected Norwegian farrowing to finishing herds. Proc IPVS. The Hague, the Netherlands. 1992;300.
44. Pieters M, Pijoan C, Fano E, Dee S. An assessment of the duration of Mycoplasma hyopneumoniae infection in an experimentally infected population of pigs. Vet Microbiol. 2009;134:261–266.
*45. Yeske P. Mycoplasma eradication from an acute outbreak using DRAXXIN® (tulathromycin). Proc IPVS. Durban, South Africa. 2008.
*46. Yeske P. Summary of Mycoplasma hyopneumoniae eradication experiences completed in upper Midwest USA. Proc IPVS. Vancouver, Canada. 2010;141.
*47. Torremorell M, Henry S, Moore C. Producing PRRSV negative herds and systems from PRRSV positive animals: the principles, the process and the achievement. Proc AASP. Indianapolis, Indiana. 2000;341–347.
48. Dee SA, Joo HS, Pijoan C. Controlling the spread of PRRS virus in the breeding herd through management of the gilt pool. Swine Health Prod. 1995;3:64–69.
49. Dee SA, Joo HS, Henry S, Tokach L, Park BK, Molitor T, Pijoan C. Detecting subpopulations after PRRS virus infection in large breeding herds using multiple serologic tests. Swine Health Prod. 1996;4:181–184.
*50. Yeske P. Economics of an off-site breeding project. Proc IPVS. Ames, Iowa. 2002;188.
51. Vicca J, Thermote L, Maes D, D’Hooghe I, Peeters J, Haesebrouck F, de Kruif A. Patterns of Mycoplasma hyopneumoniae infection in closed pig herds using serology and nested PCR on nasal samples. J Vet Med B. 2002;49:349–353.
52. Sibila M, Nofrarias M, Lopez-Soria S, Segales J, Riera P, Llopart D, Calsamiglia M. Exploratory field study on Mycoplasma hyopneumoniae infection in suckling pigs. Vet Microbiol. 2007;121:352–356.
*53. Holst S, Yeske P, Leuwerke B, Swalla R, Davies P, Pieters M. Effect of pre-farrow administration of tulathromycin injectable solution on Mycoplasma hyopneumoniae prevalence in suckling pigs at birth and weaning. Proc AASV. San Diego, California. 2013;89–90.
54. Pieters M, Fano E, Pijoan C, Dee S. An experimental model to evaluate Mycoplasma hyopneumoniae transmission from asymptomatic carriers to unvaccinated and vaccinated sentinel pigs. Can J Vet Res. 2010;74:157–160.
55. Bandrick M, Pieters M, Pijoan C, Molitor TW. Passive transfer of maternal Mycoplasma hyopneumoniae-specific cellular immunity to piglets. Clin Vac Immunol. 2008;15:540–543.
*56. Schneider P. Experiences with Mycoplasma hyopneumoniae and Transmissible Gastroenteritis eradication from sow herds. Proc AD Leman Conf. St Paul, Minnesota. 2006;82–86.
*57. Snider T. Mycoplasma eradication using Draxxin® (Tulathromycin), Lincomix 44 premix® (Lincomycin), and Respisure® in a 2750 sow isowean facility. Proc IPVS. Vancouver, Canada. 2010;642.
*58. Lorenzen JB. Eradication of Mycoplasma hyopneumoniae from an acutely infected Danish 2-site 390 sow herd without restocking. Proc IPVS. Melbourne, Australia. 2000;340.
*59. Alfonso A, Geiger JO, Freixes C, Fonz JC, Torremorell M. Mycoplasma hyopneumoniae and PRRSV elimination in a 1700 sow multi-site system. Proc IPVS. Hamburg, Germany. 2004;174.
60. Ter Laak EA, Pijpers A, Noordergraaf JH, Schoevers EC, Verheijden JHM. Comparison of methods for in vitro testing of susceptibility of porcine Mycoplasma species to antimicrobial agents. Antimicrob Agents Chem. 1991;35:228–233.
61. Vester B, Douthwaite S. Macrolide resistance conferred by base substitutions in 23S rRNA. Antimicrob Agents Chemother. 2001;45:1–12.
62. Prescott JF. Tetracyclines. In: Prescott JF, Baggot JD, Walker RD, eds. Antimicrobial Therapy in Veterinary Medicine. 3rd ed. Ames, Iowa: Iowa State University Press. 2000;275–289.
63. Prescott JF. Lincosamides, macrolides, and pleuromutilins. In: Prescott JF, Baggot JD, Walker RD, eds. Antimicrobial Therapy in Veterinary Medicine. 3rd ed. Ames, Iowa: Iowa State University Press. 2000;229–262.
64. Vicca J. Virulence and antimicrobial susceptibility of Mycoplasma hyopneumoniae isolates from pigs. PhD thesis. Faculty of Veterinary Medicine, Ghent University. 2005. ISBN 90-5864-086-8.
65. Kotra LP, Haddad J, Mobashery S. Aminoglycosides: perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrob Agents Chemother. 2000;44:3249–3256.
66. Prescott JF. Aminoglycosides and aminocyclotols. In: Prescott JF, Baggot JD, Walker RD, eds. Antimicrobial Therapy in Veterinary Medicine. 3rd ed. Ames, Iowa: Iowa State University Press. 2000;191–228.
67. Henry RJ. The mode of action of sulfonamides. Bacteriol Rev. 1943;7:175–262.
*68. Kohne K, Kriegler W, Zabke J. Elimination of Mycoplasma hyopneumoniae and PRRS in a 1800 sow herd with tilmicosin. Proc IPVS. Copenhagen, Denmark. 2006;399.
*69. Geiger JO, Donovan T, Janssen J. Elimination of Mycoplasma hyopneumoniae from 560 sow herd using parity structure, lincocin, and tulathromycin. Proc IPVS. Durban, South Africa. 2008.
*70. Geiger JO, Groth D. Mycoplasma hyopneumoniae elimination in a 3800 sow, multi-site system. Proc IPVS. Vancouver, Canada. 2010;640.
*71. Roozen M, Kaptur R, Rosener D, Marco E. Recent experiences with tylvalosin (Aivlosin®) intervention for the eradication of Mycoplasma hyopneumoniae in commercial swine operations. Proc AASV. Dallas, Texas. 2014;479–480.
*72. Marco E, Quiroga M, Menjon R, Bollo JM, Calvo E, Donadeu M, Cia C, Duran O. Eradication of Mycoplasma hyopneumoniae in a herd using Aivlosin. Proc IPVS. Durban, South Africa. 2008.
*73. Geiger JO, Ragone PA. Elimination of PRRS and M hyopneumoniae from a 950 sow system. Proc IPVS. Copenhagen, Denmark. 2006;393.
*74. Nielson E, Kongsted KK, van Aken N. Attempted elimination of Actinobacillus pleuropneumoniae (serovar 2&6), Mycoplasma hyopneumoniae, and PRRS-EU by depopulation and tilmicosin treatment. Proc IPVS. Ames, Iowa. 2002;276.
*75. Pijoan C. Disease eradication: should we go there? Proc AD Leman Conf. St Paul, Minnesota. 2001;7–9.
*76. Haden CD, Painter T, Fangman T, Holtkamp D. Assessing production parameters and economic impact of swine influenza, PRRS, and Mycoplasma hyopneumoniae on finishing pigs in a large production system. Proc AASV. Denver, Colorado. 2012;75–76.
77. Choi YK, Goyal SM, Joo HS. Retrospective analysis of etiologic agents associated with respiratory diseases in pigs. Can Vet J. 2003;44:735–737.
*78. Thacker EL. Immunology of the porcine respiratory disease complex. Vet Clin North Am Food Anim Pract. 2001;173:551–565.
*79. Holtkamp D, Rotto R, Garcia R. The economic cost of major health challenges in large US swine production systems. Proc AASV. Orlando, Florida. 2007;85–90.
*80. Yeske P. Mycoplasma hyopneumoniae elimination: Swine Vet Center experience. Proc AASV. Orlando, Florida. 2015;505–508.
*81. Yeske P. Cost of eradicating diseases according to method. Proc AASV. Omaha, Nebraska. 2010;15–18.
*82. Pieters M, Rovira A. Comparison of various sample types for detection of Mycoplasma hyopneumoniae in recently infected pigs. Proc AD Leman Conf. St Paul, Minnesota. 2013;75–76.
* Non-refereed references.
