| Original research | Peer reviewed |
Cite as: Varagka N, Lisgara M, Skampardonis V, et al. Pathological evaluation of claw lesions in culled sows from a Greek herd. J Swine Health Prod. 2016;24(2):72–80.
Also available as a PDF.
SummaryObjectives: To characterize macroscopic claw lesions of culled sows, describe the histologic characteristics observed in the laminar corium and investigate their associations with lesion severity, and compare the morphometric characteristics of horn tubules among claws according to lesion severity. Materials and methods: One front and the opposite rear foot from 74 culled sows of one herd were examined for lesions. From each claw, a tissue sample consisting of dermis and epidermis was examined histologically for changes suggesting laminitis. Slices from the lateral claws of the rear feet of 48 sows were examined morphometrically to evaluate the density and size of horn tubules. Results: The most frequent lesions were those located on the heel, wall, and white line, with 146 (49.3%), 94 (31.8%), and 81 (27.4%) affected claws, respectively, among the 296 examined. Lamellar hyperplasia was the most frequently recorded characteristic in the epidermis of 87 of 296 claws (29.4%) in 51 of the 74 examined sows (68.9%). The total lesion score of the claw was higher (P < .001) when lamellar hyperplasia was recorded than when no histologic change was recorded. The density of horn tubules was lowest (P = .018) and the size was largest (P < .001) among animals with severe wall lesions, compared to those without wall lesions. Implications: The histologic changes observed in the dermis and epidermis of the sows’ claws have been described in cases of equine and bovine laminitis. Sow laminitis may frequently occur, causing production of low quality hoof horn. | ResumenObjetivos: Caracterizar las lesiones macroscópicas de las pezuñas de hembras desechadas, describir las características histológicas observadas en el corium laminar e investigar las asociaciones con la severidad de la lesión, y comparar las características morfométricas de los túbulos de cuerno entre las pezuñas de acuerdo a la severidad de la lesión. Materiales y métodos: Se examinaron las lesiones de una pata delantera y la pata trasera opuesta de 74 hembras desechadas de un hato. De cada pezuña, se examinó histológicamente, una muestra de tejido de dermis y epidermis en busca de cambios sugerentes de laminitis. Se examinaron morfometricamente cortes laterales de pezuña de la pata trasera de 48 hembras para evaluar la densidad y tamaño de los túbulos de cuerno. Resultados: Las lesiones más frecuentes fueron aquellas localizadas en el talón, pared, y la banda blanca, con 146 (49.3%), 94 (31.8%), y 81 (27.4%) pezuñas afectadas, respectivamente, entre las 296 examinadas. La hiperplasia laminar fue la característica más frecuentemente registrada en la epidermis de 87 de 296 pezuñas (29.4%) en 51 de las 74 hembras examinadas (68.9%). El puntaje total de lesión de la pezuña fue mayor (P < .001) cuando se registró hiperplasia laminar que cuando no se registró cambio histológico. La densidad de túbulos de cuerno fue menor (P = .018) y el tamaño fue mayor (P < .001) entre los animales con lesiones severas de pared, comparado con aquellos sin lesiones en la pared. Implicaciones: Los cambios histológicos observados en la dermis y la epidermis de las pezuñas de las hembras se han descrito en casos de laminitis bovina y equina. La laminitis de hembra puede ocurrir frecuentemente, produciendo pezuña de baja calidad. | ResuméObjectifs: Caractériser les lésions macroscopiques des onglons de truies réformées, décrire les caractéristiques histologiques observées dans le chorion laminaire et étudier les associations avec la sévérité des lésions, et comparer les caractéristique morphométriques des tubules cornés parmi les onglons selon la sévérité des lésions. Matériels et méthodes: Une patte avant et la patte arrière opposée provenant de 74 truies réformées d’un troupeau ont été examinées pour la présence de lésions. Pour chaque onglon, un échantillon de tissu composé du derme et de l’épiderme a été soumis à un examen histologique pour vérifier la présence de lésions suggestives de laminite. Des tranches des onglons latéraux des pattes arrière de 48 truies ont été examinées par morphométrie pour évaluer la densité et la dimension des tubules cornés. Résultats: Les lésions les plus fréquentes étaient celles localisées au talon, sur la muraille, et la ligne blanche, avec 146 (49,3%), 94 (31,8%), et 81 (27,4%) onglons affectés, respectivement, parmi les 296 examinés. L’hyperplasie lamellaire était la caractéristique la plus fréquemment enregistrée dans l’épiderme de 87 des 296 onglons (29,4%) chez 51 des 74 (68,9%) des truies examinées. Le pointage total des lésions des onglons était supérieur (P < 0,001) lorsque l’hyperplasie lamellaire était notée comparativement à l’absence de changement histologique. La densité des tubules cornés était plus faible (P = 0,18) et la dimension plus grande (P < 0,001) parmi les animaux avec des lésions sévères de la muraille, comparativement à ceux sans lésion à la muraille. Implications: Les changements histologiques observés dans le derme et l’épiderme des onglons des truies ont été décrits dans des cas de laminite chez les chevaux et les bovins. La laminite chez les truies peut survenir fréquemment, causant une production de corne des onglons de piètre qualité. |
Keywords: swine, claw lesions, laminitis
Search the AASV web site
for pages with similar keywords.
Received: May 18, 2015
Accepted: September 18, 2015
Claw lesions, which are an important underlying cause of locomotor disorders in pigs,1 have been associated with lameness and can result in culling from the herd or euthanasia.2,3 In studies conducted in modern herds in Belgium, Greece, and the United States, almost every sow had at least one claw lesion.4-6
From an economic viewpoint, lameness reduces the productivity of a farm by reducing sow longevity and the number of pigs produced per sow per year due to increased involuntary culling rate of sows.7 Lameness can be costly for the producer because of sow replacement costs and increased treatment costs. Moreover, lifetime reproductive and financial performance is better in herds having a higher proportion of high-parity females.8,9
The hoof horn is produced through a complex process of epidermal cell differentiation, which ends with their transformation into dead horn cells.10 The latter become connected by the intercellular cementing substance. Functional hoof horn integrity essentially depends on proper keratinization of hoof epidermal cells, which depends on nutrient and oxygen flow to the epidermal cells. The epidermis itself is an avascular tissue; thus, keratinocytes are dependent on receiving oxygen and nutrients from the fine microvasculature of the corium by diffusion across the basement membrane.11 Inflammation in the corium or localized trauma may interfere with the supply of nutrients,12 resulting in production of low-quality horn that is more susceptible to environmental effects. Mechanical strength and hoof horn quality depend on the density and diameter of horn tubules.10,11 Each horn tubule consists of an outer cortex, originating from the living epidermis located around the dermal papilla, and an inner medulla, originating from the epidermis over the tip of the papilla. The diameter and density of tubules, as well as the ratio between cortex and medulla, determine the quality of hoof horn.10 Hoof horn of poor structural integrity and mechanical strength is likely to be susceptible to separation and bacterial invasion, with consequential pain and suffering for the affected animal.10
In dairy cows, the most common ailment within the horny tissues of the hoof is laminitis, an inflammation of the laminar corium of the hoof.13,14 Laminitis is the generic term for conditions in which the sensitive dermal structures between the pedal bone and the hoof horn are damaged.15,16 Laminitis, which causes production of poor quality horn, is associated with impaired synthesis or disturbed chemical binding of keratins, the structural proteins of the hoof, with resultant deterioration of the macromolecular organization that gives the horn mechanical strength.16 Thus, laminitis is associated with hoof lesions, such as sole ulcer or white line separation, which may not become visible for 2 to 3 months.17 In sows, laminitis has been investigated radiographically.18 However, radiography detects distal phalanx rotation, which is found only in the chronic phase of laminitis.18,19 Initial pathological changes of acute laminitis, ie, hyperemia, hemorrhage, and edema,20 can be detected only by histopathologic evaluation.19
Therefore, in the present study, we attempted to associate visible claw lesions with histologic and morphologic characteristics suggestive of damage to the dermal corium of sow claws.
Materials and methods
Sample collection
The feet examined in the present study were collected from a Greek abattoir which operates in accordance with the European legislation (93/119/EC) for slaughtering animals without unnecessary suffering.
Sampled sows, which were culled at weaning, originated from a Greek indoor farrow-to-finish herd with 800 sows of Hermitage genotype (The Pig Breeding Company Hermitage Genetics, Kilkenny, Ireland; http://www.hermitagegenetics.ie). Their parities ranged from one to 10 (median, sixth parity) and they were individually housed during all previous gestations. For participation in the study, the only criterion was the owner’s written consent. Neither the health status of the sows’ feet nor the frequency of locomotor disorders was considered for herd selection.
One front and the opposite rear foot from 74 sows were collected from May to October 2013, alternating selection between left and right front foot of successively sampled sows. The technician collecting the feet was blinded to the purpose of the study and had not been trained to recognize claw lesions, reducing bias towards selection of claws with more lesions. In addition, the technician recorded sow identification number and parity from the herd management software. After collection of samples, the feet and the ear tag of each sow were placed in the same plastic bag. All bags were placed in polystyrene cooling boxes and transferred, within 1 day, to the Aristotle University of Thessaloniki, School of Veterinary Medicine, Department of Pathology. On the day of arrival, claws were macroscopically examined, sectioned, and fixed in 10% neutral buffered formaldehyde.
Macroscopic examination
The medial and lateral claws of the 148 front and rear feet that were collected were macroscopically examined for lesions and scored by one of the authors (VP). The scoring system applied has been described in detail.6 Briefly, for each claw, five anatomical sites were examined: the heel (soft keratinized epidermis on the ventral surface of the claw towards the posterior end); the sole (hard keratinized epidermis anterior to the heel on the ventral surface of the claw including the junction between heel and sole); the white line (junction between sole and wall), the wall (hard keratinized epidermis on the dorsal surface of the claw); and the coronary band. These five anatomical sites of the claw were examined for the presence of cracks, erosions, ulcers, bruises, separation along the white line, and hyperkeratinization. The evaluation of the anatomical sites of the claw involved a severity scale ranging from 0 to 2, where score 0 was assigned to claw sites with no lesions or very small superficial ones. For the sole and heel, score 1 was assigned to claws with erosions and score 2 to claws with ulcers. For the white line, score 1 was assigned to claws with superficial separation and score 2 to claws with deep separation. For the wall, score 1 was assigned when bruises were observed and score 2 when cracks were noted. For the coronary band, score 0 was assigned to claws with no lesions, and score 1 to claws with lesions of any kind.
Histologic examination
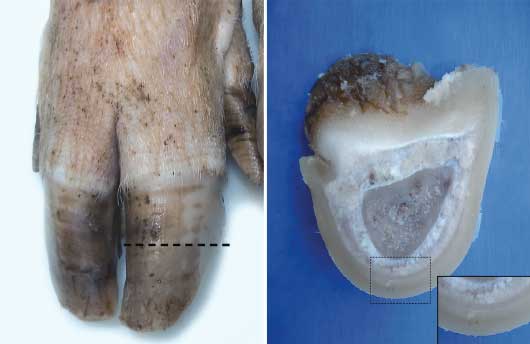
A slice (width 0.5 cm) was cut with a band saw perpendicular to the dorsal wall of each of the 296 claws (74 sows × 2 feet × 2 claws) that had been previously collected. The central point of the slice was at the midpoint between the coronary band and the weight-bearing area of the wall, at the junction of the wall and sole. From the extracted slice of tissue, a sample (0.5 cm × 0.5 cm × 0.5 cm) was cut from the wall segment of the claw. The isolated tissue sample, which consisted of dermis and epidermis, was separated from the underlying bone by a scalpel incision through the dermis as close to the pedal bone as possible (Figure 1).
Figure 1: 74 sows of Hermitage genotype originating from a Greek farrow-to-finish sow herd were culled between May and October, 2013, and feet were collected at slaughter for histologic examination. For collection of a 0.5-cm slice from the dorsal wall of each claw, two transverse parallel cuts were made with a band saw perpendicular to the wall (dashed line). The isolated tissue sample (insert), which consisted of dermis and epidermis, was separated from the underlying bone by a scalpel incision through the dermis as close to the third phalanx as possible.
Samples were fixed for 1 week, then dehydrated through graded concentrations of ethanol and xylene using an automatic tissue-processing machine (Shandon 2LE tissue processor; Shandon Southern Products Ltd, Astmoor, Runcorn, Cheshire, England), and embedded in paraffin wax. A sledge microtome was used to cut 5-μm horizontal sections from each sample. The sections were stained with hematoxylin and eosin and examined under a light microscope at ×10, ×20, and ×40 magnification. After reviewing the literature for equine and bovine laminitis, we formed a list of characteristics which were considered to represent possible pathologic changes of tissue affected by laminitis.19,21-25 In horses, chronic laminitis is characterized by hyperplasia of the laminar epidermis.26,27 Therefore, we recorded the number of suprabasal cell layers along the cornified part of the epidermal lamellae. One or two layers were classified as normal, whereas three or more were classified as increased (lamellar hyperplasia). In addition, the presence of white blood cells, hyperemia, hemorrhage, edema, and necrosis of the dermis were recorded. In normal claws, the capillaries of the dermis appear small and their lumina are usually empty. Reactive hyperemia is the first physiologic event of acute laminitis.21 In this study, hyperemia was recorded when the vessels were filled with red blood cells up to the tips of the laminae. Hemorrhage was noted if blood components (plasma and hemosiderin) were found inside tubules. Edema was noted if normal tissue components were spread apart, giving the tissue a less dense appearance. Necrosis was noted when pyknosis or karyolysis of several cells were observed.
Morphometric examination
Due to laboratory limitations, a convenience sample of slices from the lateral claw of the rear foot of 48 sows was used to evaluate the morphological features of the horn tubules. The slices morphometrically evaluated were from 19 claws without wall lesions (score 0), 20 claws with bruises or superficial cracks on the wall (score 1), and nine claws with deep wall cracks (score 2). Slices were selected from the lateral claws of the rear feet because they were most commonly and severely affected. Three zones of morphologically different tubules were identified: an outer zone with flattened tubules (zone A), an intermediate zone with round to oval tubules (zone B), and an inner zone with tiny horn tubules (zone C). Two representative fields magnified ×10 and two magnified ×20 in each zone of each sample were captured using a Nikon eclipse 50i microscope and a Nikon DS-5 M-L1 digital camera (Nikon Instruments Inc, Melville, New York). At the lower magnification, the tubules in each image were counted using the cell count plug-in of the ImageJ image processing and analysis program (NIH, Bethesda, Maryland), and at the higher magnification, the largest and smallest diameter of three representative tubules were measured.
The histopathologic and morphometric evaluations were performed by one of the authors (NV), who was blinded to the results of the macroscopic examination.
Statistical analysis
All statistical analyses were performed using Stata 13.1 (Stata Statistical Software, College Station, Texas).
Macroscopic examination. For each claw site, the frequency of lesions and their severity was calculated by claw and foot. For each claw or foot, the total lesion score, which could range from 0 to 9 or from 0 to 18, respectively, was calculated as the sum of the scores of the five sites for either claw or both claws, respectively. Paired t tests were used to compare the mean total lesion scores between medial and lateral claws on the same foot (front or rear) and between front and rear foot for the same claw (medial or lateral). The mean total lesion score was also compared between front and rear feet.
Histologic examination. For each foot and claw, the frequency of pathological changes recorded in tissue samples of dermis and epidermis of claw sections from the midpoint of the dorsal wall was calculated. The total lesion score of the claw was associated with lamellar hyperplasia, which was the most frequently recorded pathological change, in a multi-level linear regression model in GLAMM.28,29 In this model, the total lesion score was the dependent variable, whereas lamellar hyperplasia, the foot (front or rear), the claw (medial or lateral), and sow parity were the independent variables. Furthermore, a random-effect term for sow and a random-effect term for foot nested within sow were included in order to account for the multiple measurements on the same animal and foot. Similar analytical models were not used for the other pathological changes because they were either infrequently recorded (necrosis, hemorrhage, hyperemia, presence of white blood cells) or frequently recorded but usually co-existing with lamellar hyperplasia (edema).
Morphometric examination. The density and the horizontal and vertical diameters of the horn tubules were summarized by wall macroscopic score and zone. Then the three measurements were associated with wall score in three multi-level linear regression models in GLAMM.28,29 In these models, score, zone, and sow parity were included as fixed-effect terms, field as a random-effect term nested within zone, and sow as a random-effect term.
Results
Macroscopic examination
The frequency of lesions recorded and their severity scale by site and claw (medial or lateral), as well as the mean of the total lesion score by foot (front or rear), are shown in Table 1. The most frequently observed lesions were those located on the heel, the wall, and the white line, with 146 (49.3%), 94 (31.8%), and 81 (27.4%) affected claws, respectively, of the 296 examined. Specifically, for lesions located on the heel, 53 of 148 (35.8%) examined claws of the front foot and 93 of 148 (62.8%) examined claws of the rear foot were affected. For lesions located on the wall, 40 of 148 (27.0%) examined claws of the front foot and 54 of 148 (36.5%) examined claws of the rear foot were affected. For lesions on the white line, 35 of 148 (23.6%) and 46 of 148 (31.1%) examined claws of the front and the rear foot, respectively, were affected. The mean total lesion score was higher (P = .04) on rear than on front feet, and also higher on lateral compared to medial claws on either front (P = .045) or rear feet (P < .001).
Table 1: Frequency (%) of lesions on 296 claws from 74 culled sows by anatomical site and lesion severity score and mean of the total lesion score, presented by foot (front or rear) and claw (medial or lateral)*
| Score | Sole | Heel | White line | Wall | Coronary band | Mean total score | |||
|---|---|---|---|---|---|---|---|---|---|
| Front foot | Lateral claw | 0 | 78.38 | 50.00 | 71.62 | 67.57 | 98.65 | 5.00Aa | 2.70Ba,Da |
| 1 | 20.27 | 24.32 | 21.62 | 29.73 | 1.35 | ||||
| 2 | 1.35 | 25.68 | 6.76 | 2.70 | NA | ||||
| Medial claw | 0 | 82.43 | 63.51 | 74.33 | 71.63 | 100 | 2.30Bb,Ea | ||
| 1 | 17.57 | 21.62 | 22.97 | 24.32 | 0.00 | ||||
| 2 | 0.00 | 14.87 | 2.70 | 4.05 | NA | ||||
| Rear foot | Lateral claw | 0 | 67.57 | 24.33 | 66.22 | 59.46 | 98.65 | 5.70Ab | 3.50Ca,Db |
| 1 | 22.97 | 31.08 | 25.67 | 33.78 | 1.35 | ||||
| 2 | 9.46 | 44.59 | 8.11 | 6.76 | NA | ||||
| Medial claw | 0 | 86.49 | 64.86 | 78.38 | 74.33 | 98.65 | 2.20Cb,Ea | ||
| 1 | 12.16 | 25.68 | 16.22 | 21.62 | 1.35 | ||||
| 2 | 1.35 | 9.46 | 5.40 | 4.05 | NA | ||||
* 74 sows of Hermitage genotype from a Greek farrow-to-finish herd were culled at weaning between May and October 2013, and feet were collected at slaughter. Study described in Figure 1.
A,B,C,D,E Uppercase superscripts define the compared pairs of mean total scores. A = front and rear feet; B = lateral and medial claws of front foot; C = lateral and medial claws of rear foot; D = lateral claws of front and rear feet; and E = medial claws of front and rear feet.
ab Pairs of mean total scores with different lowercase superscript letters are significant different (P < .05; paired t test).
NA = not applicable (lesion score ranged from 0 to 1).
Histologic examination
The frequency of pathologic changes recorded by foot and claw are shown in Table 2. In many samples there was marked disruption of the normal architecture of the epidermal lamellae (figures 2 and 3). Lamellar hyperplasia, leading to lamellar widening, was the most frequently recorded characteristic in the epidermis of 87 of 296 claws (29.4%) in 51 of 74 sows (68.9%). Among claws without lesions, one or more pathologic changes were recorded in 34 of 91 claws (37.4%), while hyperplasia was noted in 18 of 91 claws (19.8%). Moreover, in 36 of 87 samples (41.4%) with lamellar hyperplasia, a proliferative “cap horn” (partially keratinized epidermal cells and small tubules over the tips of the dermal lamellae) was also noted. In addition, isolated round islands of dermal tissue, some of them vascular, were noted inside the cap horn in 45 of 87 samples (51.7%) with lamellar hyperplasia. Widening and disruption of the dermal lamellae due to edema was noted in the dermis of 63 of 296 claws (21.3%) in 44 of 74 sows (59.5%). White blood cells were found in the dermis of 35 of 296 claws (11.8%) in 30 of 74 sows (40.5%). Evidence of hemorrhage (densely stained material and hemosiderin) were found inside tubules of 34 of 296 claws (11.5%) in 26 of 74 sows (35.1%). Extensive necrosis in the dermis and epidermis was noted in 14 of 296 claws (4.7%) in 12 of 74 sows (16.2%). In these cases, hyperplasia was not identified. Hyperemia in the dermis was observed in only four of 296 claws (1.4%) in three of 74 sows (4.1%). The total lesion score of the claw was higher (P < .001) by almost one unit when lamellar hyperplasia was recorded in the epidermis than when no lesion was recorded in the dermis or epidermis.
Table 2: Number and frequency (%) of pathological changes recorded in tissue samples of dermis and epidermis of claw sections from the midpoint of the dorsal wall of 296 claws of 74 culled sows, presented by foot (front or rear) and claw (medial or lateral)*
| Pathological change | Lamellar hyperplasia (%) | Edema (%) | Necrosis (%) | Hemorrhage (%) | Hyperemia (%) | Presence of WBCs (%) | |
|---|---|---|---|---|---|---|---|
| Front foot | Medial claw n = 74 | 17 (22.97) | 14 (18.92) | 2 (2.70) | 6 (8.11) | 2 (2.70) | 8 (10.81) |
| Lateral claw n = 74 | 21 (28.37) | 19 (25.67) | 5 (6.76) | 9 (12.16) | 0 (0.00) | 5 (6.76) | |
| Rear foot | Medial claw n = 74 | 20 (27.03) | 11 (14.86) | 3 (4.05) | 7 (9.46) | 1 (1.35) | 7 (9.46) |
| Lateral claw n = 74 | 29 (39.19) | 19 (25.67) | 4 (5.41) | 12 (16.22) | 1 (1.35) | 15 (20.27) | |
* Study described in Figure 1.
WBCs = white blood cells.
Figure 2: Normal architecture of the lamellar tissue in a sow’s foot in the study described in Figure 1. Stained with hematoxylin and eosin; ×4 magnification.
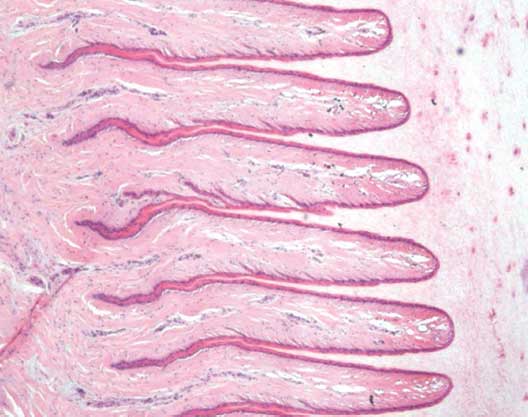
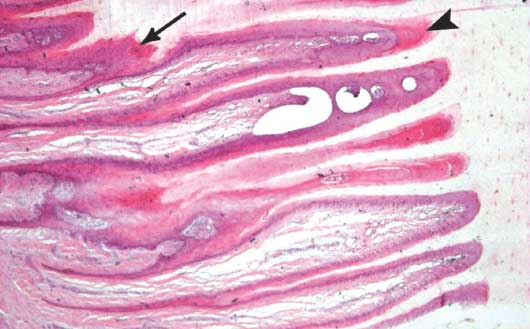
Figure 3: Marked disruption of the architecture of the lamellar tissue of a sow’s foot in the study described in Figure 1. Several layers of suprabasal cells surround the dermal lamellae, which are irregular in length (arrow). A proliferative “cap horn” fills the arcades between adjacent epidermal lamellae (arrowhead). Stained with hematoxylin and eosin; ×4 magnification.
Morphometric examination
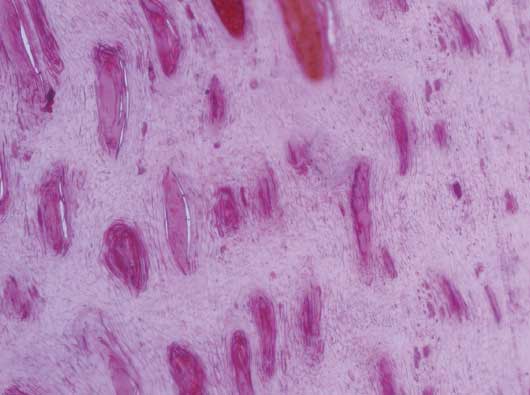
The density and the horizontal and vertical diameters of the tubules are summarized by wall score and zone in Table 3. The density of the tubules was lowest (P = .02) among animals with score 2 versus those with score 0 (figures 4 and 5). It did not differ (P = .08 and P = .40, respectively) among animals with score 2 versus 1 and 1 versus 0. The horizontal diameter of the tubules was largest (P < .001) among animals with score 2 versus those with score 0. Also, the diameter was larger (P = .01 and P < .001, respectively) among animals with score 2 versus 1 and 1 versus 0. Lastly, the vertical diameter of the tubules was largest (P = .01) among animals with score 2 compared with those with score 0; larger (P = .02) among those with score 2 versus score 1; and did not differ (P = .70) between those with score 1 or 0.
Table 3: Mean tubular density (number of horn tubules per field at ×10 magnification) and the mean horizontal and vertical diameter of horn tubules (µm), and their SDs by wall score and zone, as observed and measured in histologic slices from the wall of the lateral claw of the rear foot of 48 of 74 culled sows*
| Wall score | 0 | 1 | 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Zone | A | B | C | A | B | C | A | B | C |
| Tubular density (± SD) | 65.75 (± 10.44) | 62.14 (± 7.89) | 64.11 (± 13.26) | 63.82 (± 13.53) | 61.12 (± 12.98) | 59.45 (± 17.70) | 59.11 (± 12.48) | 55.27 (± 13.27) | 51.00 (± 15.48) |
| Horizontal diameter (± SD) | 52.52 (± 17.19) | 41.01 (± 16.21) | 40.21 (± 12.56) | 49.53 (± 17.32) | 50.66 (± 20.47) | 42.19 (± 13.10) | 69.47 (± 38.82) | 64.05 (± 32.99) | 41.37 (± 13.93) |
| Vertical diameter (± SD) | 17.31 (± 5.26) | 20.17 (± 5.46) | 20.64 (± 5.02) | 15.98 (± 4.38) | 21.88 (± 6.36) | 21.82 (± 7.90) | 21.83 (± 11.86) | 22.73 (± 10.13) | 19.29 (± 8.30) |
* Study described in Figure 1. Area of wall sectioned shown in Figure 1. Slices for morphometric evaluation were selected from the lateral claws of the rear feet because they were most commonly and severely affected. Three zones of morphologically different tubules were identified: Zone A, an outer zone with flattened tubules; Zone B, an intermediate zone with round to oval tubules; and Zone C, an inner zone with tiny horn tubules. Wall score 0 = no lesions; score 1 = bruising or superficial cracks; and score 2 = deep cracks.
SD = standard deviation.
Figure 4: Normal structure of tubules in a sow’s foot without macroscopic lesions on the hoof wall in the study described in Figure 1. Stained with hematoxylin and eosin; ×10 magnification.
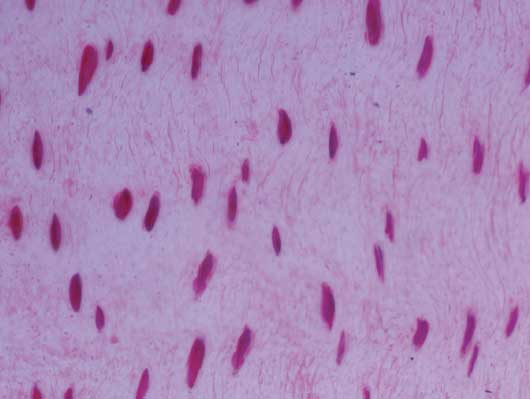
Figure 5: Reduced density and increased vertical and horizontal diameter of tubules from a sow’s foot showing severe macroscopic lesions on the hoof wall in the study described in Figure 1. Stained with hematoxylin and eosin; ×10 magnification.
Discussion
The high prevalence of claw lesions in modern sows may be linked with the intensive farming of sows on concrete floors, with minimal or no bedding, and the selection towards highly productive sows in today’s swine industry.4,30 As treatment of claw disorders in sows is frequently unrewarding, there is merit in working towards prevention and management.31 Since prevention should include measures to discourage the development of claw lesions, there is need for better understanding of the pathogenesis and determining causes and significance of claw lesions in breeding pigs. In cattle with inflammatory disease of the corium, hooves may have wall grooves, cracks, and white-line separations.17 Although these gross changes may also be observed in swine, an association with a primary inflammatory condition is less clearly determined in pigs due to the few descriptive histologic studies reported in the peer-reviewed literature for swine.18,19
In this study, we macroscopically examined and scored lesions of the claws of one front and one rear foot of 74 sows culled at weaning. Lesion scores were recorded for five anatomic sites of the claws, namely the wall, the sole, the white line, the heel and the coronary band. Similarly to findings elsewhere reported, the heel, the wall, and the white line were the most frequently affected claw sites.4,5,32,33 The severity of lesions was greater on rear than front feet and on lateral than medial claws, which has also been noted in previous studies.4,32,33 Inequality of the size of the claws and varying tissue strength between medial and lateral claws contribute to the difference in susceptibility.31,32,34-36 Lateral claws tend to be larger than medial claws, with the discrepancy in size being more pronounced on rear feet than on front feet and increasing as pigs age.37-39 As the difference in size between lateral and medial claws becomes larger, the frequency of claw lesions increases.40 In addition to different claw size, the greater severity of lesions on lateral compared to medial claws may also be due to sow weight distribution.36,40,41
The histopathologic changes observed in the examined claws of the culled sows have been described in cases of equine and bovine laminitis.20,21,42,43 Lamellar hyperplasia was observed in the claws of almost 70% of the sampled sows. Furthermore, tubules in the cap horn, such as noted in this study, have been described as indicators of laminitis in both pigs44 and horses.45 The sporadic areas of cap horn observed may represent the first stage of wedge formation, which is often described as a hallmark of chronic laminitis.26,46 Edema was noted in the claws of almost 60% of the sampled sows. Similar observations have been made in both cattle47 and pigs.44 Lamellar tissues are normally devoid of white blood cells, but laminitis promotes an early influx of white blood cells into both the dermal and epidermal compartments.25,46,48,49 White blood cells, mainly lymphocytes, were found in almost 40% of the sows. Lastly, the presence of blood or blood products in the horn is evidence of damage to both the blood vessels in the corium and the basement membrane of the coronary band. During laminitis, different degrees of injury can occur, ranging from a slight increase in permeability of capillary walls, permitting leakage of plasma, through a breach of capillaries allowing the passage of cells, to extensive damage to larger vessels resulting in the loss of greater amounts of blood.47 We recorded evidence of hemorrhage in 35% of the sows examined.
An association between lamellar hyperplasia and higher total lesion score of the claw was found. Moreover, almost 20% of the claws without clinically evident lesions had lamellar hyperplasia. Other less frequent characteristics were also recorded in claws without lesions. Therefore, the histologic changes may be regarded as the causes and not the consequences of claw lesions.50 They may indicate a prodromal phase of laminitis in sows similar to that of horses and cattle, in which the disease develops before the symptomatic phase.51,52
In the present study, three zones of morphologically different tubules were identified. These findings were in agreement with those in cattle53 and horses,54,55 suggesting that the tubular architecture of the pig’s claw may resemble that of the equine and bovine hooves.
Negative correlations have been found between measures of hoof hardness and lameness and lesion severity scores in cattle.43,56,57 Gunther et al58 and Geyer and Tagwerker,59 for cattle hoof and pig claw, respectively, suggested that “hardness” was related to tubule density. We found that claws with severe wall lesions had less tubular density than those with no lesions and that the size of the tubules, measured by their horizontal and vertical diameters, was increasing with increasing severity of wall lesions. Increased diameter of the tubules has been implicated in the genesis of qualitatively inferior horn.60-63 Hinterhofer et al64 found that, in cattle with chronic laminitis, the low horn quality was attributable to the malformed tubular and lamellar structure of the diseased dermis. Moreover, Reilly et al54 suggested that increased tubular density across the hoof wall offers smooth energy transfer as well as crack-stopping properties.
Implications
• The histologic changes previously described in cases of equine and bovine laminitis can also be observed in the dermis and epidermis of the claws of sows.
• Under the conditions of this study in a Greek herd, sow laminitis may frequently occur and lead to production of low-quality horn.
• Histologic changes in claws without macroscopic lesions may indicate a subclinical phase of laminitis in sows prior to a symptomatic phase.
Acknowledgments
This work was financially supported by the European Regional Development fund and the Greek Ministry of Education and Religious Affairs (action SYNERGASIA 2011).
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Dewey CE, Friendship RM, Wilson MR. Clinical and postmortem examination of sows culled for lameness. Can Vet J. 1993;34:555–556.
2. Sanz M, Roberts JD, Perfumo CJ, Alvarez RM, Donovan T, Almond GW. Assessment of sow mortality in a large herd. J Swine Health Prod. 2007;15:30–36.
3. Engblom L, Lundeheim N, Strandberg E, del P Schneider M, Dalin AM, Andersson K. Factors affecting length of productive life in Swedish commercial sows. J Anim Sci. 2008;86:432–441.
4. Anil SS, Anil L, Deen J, Baidoo SK, Walker RD. Factors associated with claw lesions in gestating sows. J Swine Health Prod. 2007;15:78–83.
5. Pluym L, Van Nuffel A, Dewulf J, Cools A, Vangroenweghe F, Van Hoorebeke S, Maes D. Prevalence and risk factors of claw lesions and lameness in pregnant sows in two types of group housing. Veterinarni Medicina. 2011;56:101–109.
6. Lisgara M, Skampardonis V, Kouroupides S, Leontides L. Hoof lesions and lameness in sows in three Greek swine herds. J Swine Health Prod. 2015;23:244–251.
7. Anil SS, Anil L, Deen J. Evaluation of patterns of removal and associations among culling because of lameness and sow productivity traits in swine breeding herds. JAVMA. 2005;226:956−961.
8. Lucia T, Dial GD, Marsh WE. Lifetime reproductive and financial performance of female swine. JAVMA. 2000;216:1802−1809.
9. Stalder KJ, Knauer M, Baas TJ, Rothschild MF, Mabry JW. Sow longevity. CAB Reviews: Pig News and Information. 2004;25:53−74.
10. Tomlinson DJ, Mülling CH, Fakler TM. Invited review: formation of keratins in the bovine claw: Roles of hormones, minerals, and vitamins in functional claw integrity. J Dairy Sci. 2004;87:797–809.
11. Mulling CKW, Bragulla HH, Reese S, Burdas KD, Steinberg W. How structures in bovine claw epidermis are influenced by nutritional factors. Anatomia, histologia, embryologia. 1999;28:103−108.
*12. Hoblet KH, Weiss W. Metabolic hoof horn disease. Claw horn disruption. Vet Clin N Am. 2001;17:111−127.
*13. Greenough PR. Breeding for disease resistance in farm animals. In: Owens JB, Axford RFE, eds. Conference Proceedings Book. Wallingford, UK: CAB International; 1991:371–393.
14. Kempson SA, Logue DN. Ultrastructural observations of hoof horn from dairy cows: The structure of the white line. Vet Rec. 1993;132:499–502.
*15. Mülling C, Lischer CJ. New aspects of etiology and pathogenesis of laminitis in cattle. Recent advances in bovine medicine. World Buiatrics Conf. Hannover, Germany. 2002;236–247.
16. Hendry KAK, MacCallum AJ, Knight CH, Wilde CL. Laminitis in the dairy cow: a cell biological approach. J Dairy Res. 1997;64:475−486.
17. Bergsten C. Causes, risk factors, and prevention of laminitis and related claw lesions. Acta Vet Scand. 2003;44:157−166.
18. Guimaraes AMS, Althaus LK, Tullio DM, Deconto I, Silva AW, Ferrari MV, Biondo AW, Alberton GC. Laminitis in culled sows from commercial swine farms of Southern Brazil. Arch Vet Sci. 2008;13:140–144.
19. Newman SJ, Rohrbach BW, Wilson ME, Torrison J, van Amstel S. Characterization of histopathologic lesions among pigs with overgrown claws. J Swine Health Prod. 2015;23:91–96.
20. MacLean CW. The histopathology of laminitis in dairy cows. J Comp Pathol. 1971;81:563–570.
21. Obel N. Studies on the histopathology of acute laminitis [PhD dissertation]. Uppsala, Sweden: Swedish University of Agricultural Sciences; 1948.
22. Nilsson SA. Clinical, morphological and experimental studies of laminitis in cattle. Acta Vet. Scand. 1963;4(suppl 1):188–222.
23. Andersson L, Bergman A. Pathology of bovine laminitis especially as regards vascular lesions. Acta Vet Scand. 1980;21:559–566.
24. Boosman R. Bovine laminitis: Histopathologic and arteriographic aspects, and its relation to endotoxaemia [PhD dissertation]. Utrecht, The Netherlands: University of Utrecht; 1991.
25. Pollitt CC. Basement membrane pathology: a feature of acute equine laminitis. Equine Vet J. 1996;28:38−46.
26. Roberts ED, Ochoa R, Haynes PF. Correlation of dermal-epidermal laminar lesions of equine hoof with various disease conditions. Vet Pathol. 1980;17:656−666.
27. Stashak TS. Laminitis. In: Stashak TS. Adams’ Lameness in Horses. 4th ed. Philadelphia: Lea and Febiger Publications; 1987:468–499.
28. Rabe-Hesketh S, Skrondal A, Pickles A. Maximum likelihood estimation of limited and discrete dependent variable models with nested random effects. J Econometrics. 2005;128:301−323.
29. Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling using Stata. 2nd ed. College Station Texas: Stata Press; 2008:295−309.
30. Cameron R. Integumentary system: skin, hoof and claw. In: Zimmerman J, Karriker L, Ramirez A, Schwartz K, Stevenson G, eds. Diseases of Swine. West Sussex: Wiley-Blackwell; 2012:264–269.
31. Pluym L, Van Nuffel A, Maes D. Treatment and prevention of lameness with special emphasis on claw disorders in group-housed sows. Livest Sci. 2013;156:36–43.
32. Gjein H, Larssen RB. Housing of pregnant sows in loose and confined systems – a field study. 2. Claw lesions: morphology, prevalence, location and relation to age. Acta Vet Scand. 1995;36:433–442.
33. Kirk RK, Svensmark B, Ellegaard LP, Jensen HE. Locomotive disorders associated with sow mortality in Danish pig herds. J Vet Med A. 2005;52:423–428.
34. Webb NG, Penny RCH, Johnston AM. Effect of a dietary supplement of biotin on pig hoof horn strength and hardness. Vet Rec. 1984;114:185–189.
35. Tubbs RC. Lameness in sows: Solving a preventable problem. Vet Med. 1988;83:610–616.
36. Kroneman A, Vellenga L, van der Wilt FJ, Vermmer HM. Review of health problems in group housed sows, with special emphasis on lameness. Vet Q.1993;15:26–29.
37. Penny RHC, Osborne AD, Wright AI. The causes and incidence of lameness in store and adult pigs. Vet Rec. 1963;75:1225–1235.
38. Bradley CL, Frank JW, Maxwell CV, Johnson ZB, Powell JG, van Amstel SR, Ward TL. Characterization of claw lesions associated with lameness in the University of Arkansas sow herd. Arkansas Animal Science Department Report. 2007:106–110.
39. van Amstel S. Claw horn growth and wear rates, toe length, and claw size in commercial pigs: A pilot study. J Swine Health Prod. 2010;18:239–243.
40. Kornegay ET, Bryant KL, Notter DR. Toe lesion development in gilts and sows housed in confinement as influenced by toe size and toe location. Appl Agric Res. 1990;5:327–334.
41. Enokida M, Sasaki Y, Hoshino Y, Saito H, Koketsu Y. Claw lesions in lactating sows on commercial farms were associated with postural behavior but not with suboptimal reproductive performance or culling risk. Livest Sci. 2011;136:256–261.
42. Wattle O. Studies on the cytokeratins of the equine hoof wall, chestnut and skin with special reference to laminitis [PhD dissertation]. Uppsala, Sweden: Swedish University of Agricultural Sciences; 2000.
43. Karikoski NP, McGowan CM, Singer ER, Asplin KE, Tulamo RM, Patterson-Kane JC. Pathology of natural cases of equine endocrinopathic laminitis associated with hyperinsulinemia. Vet Pathol. 2014. doi:10.1177/0300985814549212.
*44. Kempson SA, Johnston AM. Changes in the hoof horn of pigs with laminitis: a scanning and transmission electron microscopic study. Proc First World Congr Vet Dermatol. Dijon, France. 27-30 September 1989. In: Tscharner C, Halliwell KEW, eds. Advances in Veterinary Dermatology. Vol 1. London: Bailliere Tindall; 1990.
45. Marks G. Makroskopische, licht- und elektronen mikroskopische Untersuchungen zur Morphologie des Hyponichiums bei der Hufrehe des Pferdes [PhD dissertation]. [Macroscopic, light and electron microscopic Examination of the Morphology of the Hyponychium with Laminitis in Horses]. Berlin: The Free University of Berlin; 1984.
*46. Morgan SJ, Grosenbaugh DA, Hood DM. The pathophysiology of chronic laminitis. Pain and anatomic pathology. Vet Clin N Am: Equine Pract. 1999;15:395−417.
47. Leach KA. Lesions and microscopic structure of claw horn in dairy cattle [PhD dissertation]. Scotland, United Kingdom: The University of Edinburgh; 1996.
48. French KR, Pollitt CC. Equine laminitis: loss of hemi desmosomes in hoof secondary epidermal lamellae correlates to dose in an oligo fructose induction model: an ultrastructural study. Equine Vet J. 2004;36:230−235.
49. Black SJ, Lunn DP, Yin C, Hwang M, Lenz SD, Belknap JK. Leukocyte emigration in the early stages of laminitis. Vet Immunol Immunopathol. 2006;109:161−166.
50. Tarlton JF, Holah DE, Evans KM, Jones S, Pearson GR, Webster JF. Biomechanics and histopathological changes in the support structures of bovine hooves around the time of first calving. Vet J. 2002;163:196−204.
*51. Pollitt CC. Equine laminitis: a revised pathophysiology. Proc Amer Assoc Equine Path. 1999;188−192.
52. Mendes HMF, Casagrande FP, Lima IR, Souza CH, Gontijo LD, Alves GES, Vasconcelos AC, Faleiros RR. Histopathology of dairy cows’ hooves with signs of naturally acquired laminitis. Pesquisa Veterinária Brasileira. 2013;33:613−619.
53. Franck A, Cocquyt G, Simoens P, De Belie N. Biomechanical properties of bovine claw horn. Biosystems engineering. 2006;93:459–467.
54. Reilly JD, Cottrell DF, Martin RJ, Cuddeford D. Tubule density in equine hoof horn. Biomimetics. 1996;4:23−35.
55. Lancaster LS, Bowker RM, Mauer WA. Equine hoof wall tubule density and morphology. J Vet Med Sci. 2013;75:773–778.
56. Borderas TF, Pawluczuk B, Passille AM, Rushen J. Claw hardness of dairy cows: relationship to water content and claw lesions. J Dairy Sci. 2004;87:2085−2093.
57. Tranter WP, Morris RS, Dohoo R, Williamson NB. A case control study of lameness in dairy cows. Prev Vet Med. 1993;15:191−203.
58. Gunther M, Anton W, Kastner R. Klauenkrankheiten. [Diseases of the bovine foot]. Jena: Gustav Fisher, Verlag; 1983.
59. Geyer H, Tagwerker F. The Pig’s Hoof: Its Structure and Alterations. Bade, Switzerland: Hoffmann-La Roche; 1986:27.
60. Kempson SA. Scanning electron microscope observations of hoof horn from horses with brittle feet. Vet Rec. 1987;120:568−570.
61. Leu U. Vergleichende Untersuchungen Uber den Einfluß von oral verobreichtem Biotin auf das Hufhorn beim Pferd [dissertation med vet]. [Comparative Studies on the Influence of oral Biotin on the Hoof of the Horse]. Zurich, Switzerland; 1987.
62. Rosskopf M, Geyer H. Mikroskopische anatomie der klauen epidermis des schafes. [Microscopic anatomy of the epidermis of the hoof in sheep]. Berliner und Munchener Tierarztliche Wochenschrift (Berlin).1987;100:373−377.
63. Geyer H, Schulze J. The long term influence of biotin supplementation on hoof horn quality in horses. Schweizer Archiv für Tierheilkunde.1994;136:137−149.
64. Hinterhofer C, Apprich V, Ferguson JC, Stanek C. Modulus of elasticity and dry-matter content of bovine claw horn affected by the changes of chronic laminitis. Vet J. 2007;174:605−609.
* Non-refereed references.
