| Original research | Peer reviewed |
Cite as: Jeong J, Kang HS, Park C, et al. Comparative efficacy of concurrent administration of a porcine circovirus type 2 (PCV2) vaccine plus a porcine reproductive and respiratory syndrome virus (PRRSV) vaccine from two commercial sources in pigs challenged with both viruses. J Swine Health Prod. 2016;24(3):130–141.
Also available as a PDF.
SummaryObjective: To compare clinical, virologic, immunologic, and pathologic parameters in pigs each concurrently administered a porcine circovirus type 2 (PCV2) and a porcine reproductive and respiratory syndrome virus (PRRSV) vaccine from one of two commercial sources and challenged with field strains of both viruses. Materials and methods: One group of pigs administered concurrently Fostera PCV and Fostera PRRS (Zoetis, Florham Park, New Jersey) and another group administered concurrently Ingelvac CircoFLEX and Ingelvac PRRS MLV (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri) at study day -28 (21 days of age) were challenged with both viruses at study day 0 (49 days of age). Serum samples were tested for viremia by real-time polymerase chain reaction (PCR), and for antibodies by a commercial enzyme-linked immunosorbent assay and a virus neutralization test. Peripheral blood mononuclear cells were tested for interferon-γ secreting cells (IFN-γ-SC) by enzyme-linked immunospot assay. Lung and lymphoid tissues were tested for lesions and viral antigen by histopathology and immunohistochemistry. Results: Significant differences were observed between vaccinated, challenged and unvaccinated, challenged groups in clinical (average weight gain and clinical signs), virologic (PCR testing), immunologic (antibodies, IFN-γ-SC, and interleukin-10), pathologic (lesions and viral antigen) outcomes. No significant differences were observed between the two vaccinated, challenged groups in clinical, virologic (except PCV2 viremia at day 14), immunologic, and pathologic outcomes. Implications: Under the conditions of this study, it makes no difference to protection whether PCV2 and PRRSV vaccines are administered concurrently. Concurrent vaccination is efficacious for controlling co-infection with PCV2 and PRRSV. | ResumenObjetivo: Comparar los parámetros clínicos, virológicos, inmunológicos, y patológicos en cerdos a los que se les administró simultáneamente una vacuna una de circovirus porcino tipo 2 (PCV2 por sus siglas en inglés) y del virus del síndrome reproductivo y respiratorio porcino (PRRSV por sus siglas en inglés) de una de las dos casas comerciales y fueron retados con cepas de campo de ambos virus. Materiales y métodos: Los dos grupos de cerdos, uno al que se le administró sim-ultáneamente Fostera PCV y Fostera PRRS (Zoetis, Florham Park, New Jersey) y otro, al que se le administró Ingelvac CircoFLEX e Ingelvac PRRS MLV (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri) en el día -28 del estudio (21 días de edad) fueron retados con ambos virus en el día 0 del estudio (49 días de edad). Las muestras de suero se analizaron para medir viremia por medio de la reacción en cadena de polimerasa en tiempo real (PCR), y en busca de anticuerpos por medio de ensayo por inmunoabsorción ligado a enzimas comercial y una prueba de neutralización de virus. Se probaron las células mononucleares de sangre periférica en busca de células que secretan interferón γ (IFN-γ-SC, por sus siglas en inglés) por medio del ensayo inmunospot ligado a enzimas. Se probaron los tejidos linfoides y de pulmón en busca de antígenos virales, y lesiones por medio de histopatología e inmunohistoquímica. Resultados: Se observaron diferencias significativas entre los grupos retados sin vacunar y los retados vacunados en evaluación clínica (ganancia de peso promedio y signos clínicos), virológicos (pruebas de PCR), inmunológicos (anticuerpos, IFN-γ-SC, e interleukina-10), patológicos (lesiones y antígeno viral). No se observaron diferencias significativas entre los dos grupos retados y vacunados en la evaluación de resultados clínicos, virológicos (excepto viremia contra PCV2 en el día 14), inmunológicos, y patológicos. Implicaciones: Bajo las condiciones de este estudio, no hay diferencia en la protección si las vacunas de PCV2 y PRRSV se administran simultáneamente. La vacunación simultánea es eficaz para controlar la coinfección con PCV2 y PRRSV. | ResuméObjectif: Comparer les paramètres cliniques, virologiques, immunologiques, et pathologiques chez des porcs ayant été simultanément vacciné avec un vaccin contre le circovirus porcin de type 2 (CVP2) et le virus du syndrome reproducteur et respiratoire porcin (VSRRP) d’une de deux sources commerciales et soumis à une infection défi avec des souches de champs des deux virus. Matériels et méthodes: Un groupe de porcs a reçu simultanément Fostera PCV et Fostera PRRS (Zoetis, Florham Park, New Jersey) et un autre a reçu simultanément Ingelvac CircoFLEX et Ingelvac PRRS MLV (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri) au jour -28 de l’étude (21 jours d’âge) et les animaux infectés avec les deux virus au jour 0 de l’étude (49 jours d’âge). Des échantillons de sérum ont été testés par réaction d’amplification en chaine par la polymérase (ACP) pour détecter une virémie, et par une épreuve immuno-enzymatique commerciale ainsi qu’un test de neutralisation virale pour détecter des anticorps. Les cellules mononucléaires du sang périphérique ont été testées pour la présence de cellules productrices d’interféron-γ (IFN-γ-SC) au moyen d’une épreuve immuno-enzymatique par tache. Les tissus lymphoïde et pulmonaire ont été examinés pour la présence de lésions et d’antigène viral par histopathologie et immunohistochimie. Résultats: Des différences significatives ont été observées entre les groupes d’animaux vaccinés et infectés et les animaux non-vaccinés et infectés du point de vue clinique (gain moyen quotidien et signes cliniques), virologique (épreuve ACP), immunologique (anticorps, IFN-γ-SC, et interleukine-10), et pathologique (lésions et antigène viral). Aucune différence significative ne fut notée entre les deux groupes d’animaux vaccinés et infectés pour ce qui est des aspects clinique, virologique (sauf la virémie CVP2 au jour 14), immunologique et pathologique. Implications: Dans les conditions expérimentales de la présente étude, aucune différence dans la protection ne fut causée par l’administration simultanée des vaccins CVP2 et VSRRP. La vaccination simultanée est efficace pour limiter la co-infection par CVP2 et VSRRP. |
Keywords: swine, porcine circovirus-associated diseases, porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, vaccine, PCVAD, PCV, PRRSV
Search the AASV web site
for pages with similar keywords.
Received: August 26, 2015
Accepted: December 29, 2015
Porcine respiratory disease complex (PRDC) is a serious health problem in growing and finishing pigs, typically approximately 16 to 22 weeks of age, and is characterized by slow growth, poor feed efficiency, lethargy, anorexia, fever, cough, and dyspnea.1 Pathogens involved in PRDC can be viral, bacterial, or both. Among them, a co-infection with porcine circovirus type 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) is the most common etiology of PRDC.2 Therefore, controlling both PCV2 and PRRSV infection is a high priority for the swine industry globally. Since vaccination is one of the major tools to control PCV2 and PRRSV infection, vaccination of pigs with both PCV2 and PRRSV is necessary to control PRDC efficiently.
Recently, a new commercial modified-live PRRSV vaccine (Fostera PRRS; Zoetis, Florham Park, New Jersey) was introduced into the international market to control respiratory disease in growing pigs. In the field, swine producers usually administer both single-dose PCV2 and PRRSV vaccines concurrently to control PRDC. Hence, comparing use of single-dose PCV2 and PRRSV vaccines administered concurrently mirrors field conditions. However, to the knowledge of the authors, no one has reported comparing clinical, virologic, immunologic, and pathologic parameters when commercial single-dose PCV2 and PRRSV vaccines are administered concurrently. The objective of this study was to compare growth performance and virologic, immunologic, and pathologic parameters in wean-to-finish pigs concurrently vaccinated with a PCV2 vaccine plus a PRRSV vaccine, respectively, from two commercial sources.
Materials and methods
All animal protocols were approved by the Seoul National University Institutional Animal Care and Use Committee.
Experimental study
Sixty colostrum-fed, crossbred, conventional piglets were purchased at 5 days of age from a commercial Korean farm. Upon arrival at a research facility, all piglets in this study tested negative for PCV2 and PRRSV by serological testing (PCV2 Ab Mono Blocking ELISA; Synbiotics, Lyon, France, and PRRS X3 Ab test; Idexx Laboratories Inc, Westbrook, Maine). All piglets also tested negative for PCV2 and PRRSV viremia by real-time polymerase chain reaction (PCR).3,4
A total of 60 pigs were randomly divided into four groups using the random number generation function in Excel (Microsoft Corporation, Redmond, Washington) (Table 1). Sample size was calculated assuming a 90% power (1 - β = .90) of detecting a difference at the 5% level of significance (α = .05), which was based on expected results of ELISA antibody titers (PCV2 and PRRSV), virus load (PCV2 and PRRSV) determined by real-time PCR, and lung and lymphoid lesions represented by scores.5 The treatment timeline is shown in Table 1. Pigs in Group 1 were administered one 2.0 mL dose of Fostera PCV (Zoetis) and one 2.0 mL dose of Fostera PRRS (Zoetis) intramuscularly in the right and left sides of the neck, respectively, at study day -28 (21 days of age) according to the manufacturer’s label instructions. Pigs in Group 2 were administered one 1.0-mL dose of Ingelvac CircoFLEX (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri) and one 2.0-mL dose of Ingelvac PRRS MLV (Boehringer Ingelheim Vetmedica Inc) intramuscularly in the right and left sides of the neck, respectively, at study day -28 according to the manufacturer’s label instructions. At study day 0 (49 days of age), each pig in groups 1, 2, and 3 was inoculated intranasally with 2 mL of PCV2b (strain SNUVR000463; 5th passage; 1.2 × 105 median tissue culture infective doses (TCID50) per mL). In the afternoon of the same day, the same pigs were inoculated intranasally with 2 mL of PRRSV (strain SNUVR090851; 5th passage; 1.2 × 105 TCID50 per mL). Co-infection with these PCV2b and PRRSV strains induced severe interstitial pneumonia and lymphoid depletion of lymph nodes in infected pigs.6 Group 3 pigs served as the positive-control group (challenged but not vaccinated), and Group 4 pigs served as the negative-control group (unchallenged and unvaccinated). Groups were housed in separate rooms (five pigs per room) within the same facility. Blood samples were collected at study days -42, -28, 0 (49 days of age), 14, 28, 63, 91, and 126 (175 days of age). Each pig was sedated with an intravenous injection of azaperon (Stresnil; Janssen Pharmaceutica, Beerse, Belgium) and then euthanized for necropsy at study day 126. Lung and lymph nodes were collected for histopathologic and immunohistochemistry examination.
Table 1: Means (with standard deviations) of lymphoid and pulmonary lesion scores and numbers of cells positive for lymphoid porcine circovirus type 2 (PCV2) antigen and pulmonary porcine reproductive and respiratory syndrome virus (PRRSV) antigen in pigs vaccinated concurrently with PCV2 and PRRSV vaccines and challenged with PCV2 and PRRSV*
| Group | Vaccination (21 days of age) | Challenge (49 days of age) | Lymph node | Lung | ||||
|---|---|---|---|---|---|---|---|---|
| Lesion score† | No. of PCV2+ cells‡ | Lesion score† | No. of PRRSV+ cells‡ | No. of PCV2+ cells‡ | ||||
| 1 | Fostera PCV and Fostera PRRSV | PCV2 and PRRSV | 0.43 (0.53)a | 3.15(3.52)a | 1.15 (0.23)a | 1.76 (3.87)a | 1.65 (2.19)a | |
| 2 | Ingelvac CircoFLEX and Ingelvac PRRS MLV | PCV2 and PRRSV | 0.71 (0.59)a | 7.05(5.45)a | 1.23 (0.38)a | 1.54 (3.43)a | 2.09 (2.60)a | |
| 3 | None | PCV2 and PRRSV | 2.11 (0.73)b | 20.70(8.17)b | 2.23 (0.44)b | 2.95 (3.31)b | 6.78 (5.21)b | |
| 4 | None | None | 0.28 (0.41)a | 0 | 0.11 (0.54)c | 0 | 0 | |
* Group 1 pigs were concurrently administered Fostera PCV and Fostera PRRS vaccines (Zoetis, Florham Park, New Jersey) and Group 2 pigs were concurrently administered Ingelvac CircoFlex and Ingelvac PRRS MLV vaccines (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri), and both groups were challenged with both viruses. The body weight of each pig was measured at study days -28 (21days of age), 0, 21 (70 days of age), 63, and 126 (175 days of age). Blood samples with EDTA were collected from pigs for interferon-γ secreting cells and without anticoagulant for serologic testing at study days -42, -28, 0, 14, 28, 63, 91, and 126. Nasal swabs were also collected on these study days.
† Pigs in all groups were euthanized at 175 days of age. Superficial inguinal lymph node and lung were collected for histopathologic examination and immunohistochemical testing. Lymphoid lesion scores: 0 = no lymphoid depletion or granulomatous replacement; 1 = mild lymphoid depletion; 2 = moderate lymphoid depletion; and 3 = severe lymphoid depletion and histiocytic replacement. Lung lesion scores: 0 = no microscopic lesions; 1 = mild interstitial pneumonia; 2 = moderate multifocal interstitial pneumonia; 3 = moderate diffuse interstitial pneumonia; and 4 = severe interstitial pneumonia. Scores were compared among groups using Fisher’s exact test.
‡ Numbers of lymphoid and pulmonary cells positive for PCV2 antigen, and of pulmonary cells positive for PRRSV antigen, per unit area (0.25 mm2) of lung were counted using an NIH Image J 1.45s program (http://imagej.nih.gov/ij/download.html). Numbers of positive cells were compared among groups using Tukey’s test.
abc Within a column, values with different superscript letters are significantly different (P < .05).
Clinical evaluation
Beginning on the day when groups 1, 2, and 3 were inoculated (day 0), all pigs were monitored daily for physical condition and scored weekly for clinical respiratory disease severity using scores ranging from 0 (normal) to 6 (severe dyspnea, abdominal breathing, and death).7 Observers were blinded to vaccination status. Rectal body temperature was recorded daily from day 0 through 21.
Assessment of growth performance
Body weight of each pig in groups 1, 2, 3, and 4 was measured at study days -28, 0, 21, 63, and 126. Average daily gain (ADG; grams per pig) was analyzed over four time periods: between day -28 and 0; 0 and 21; 21 and 63; and 63 and 126, respectively. The ADG during these various production stages was calculated as the difference between the starting and final weights divided by the duration of the stage. Data from dead pigs were included in the calculation.
PCV2 serological testing
Serum samples were tested using a commercial PCV2 ELISA (Synbiotics) and serum virus neutralization using the heterologous challenging PCV2b (strain SNUVR000463).8 Serum samples were considered positive for anti-PCV2 antibody if the reciprocal ELISA titer was > 350, according to the manufacturer’s instructions. Neutralizing antibody (NAb) data were converted to base 2 logarithms for analysis.
PRRSV serological testing
Serum samples were tested using a commercial PRRSV ELISA (Idexx Laboratories Inc) and serum virus neutralization using the heterologous challenging PRRSV (strain SNUVR090851).9 Serum samples were considered positive for anti-PRRSV antibody if the sample-to-positive (S:P) ratio was > 0.4, according to the manufacturer’s instructions. The NAb data were converted to base 2 logarithms for analysis.
Quantification of PCV2 DNA
QIAamp DNA Mini Kit (Qiagen Inc, Valencia, California) was used to extract DNA from serum samples. The DNA extracts were used to quantify numbers of PCV2 genomic DNA copies by real-time PCR as previously described.3 The numbers of genomic copies of PCV2 DNA per mL of serum were converted to base 10 logarithms for analysis.
Quantification of PRRSV RNA
A QIAamp RNA Mini Kit (Qiagen Inc) was used to extract RNA from serum samples. The RNA extracts were used to quantify numbers of PRRSV genomic RNA copies by real-time PCR as previously described.4 The numbers of genomic copies of PRRSV RNA per mL of serum were converted to base 10 logarithms for analysis.
Enzyme-linked immunospot assay
The numbers of PCV2- and PRRSV-specific interferon-γ secreting cells (IFN-γ-SC) were determined in peripheral blood mononuclear cells (PBMC) by the enzyme-linked immunospot (ELISPOT) method as previously described.6,10 Whole PCV2b and PRRSV (the strains used for challenge), each at a multiplicity of infection of 0.01, were used to stimulate PBMC. Phytohemagglutinin (10 µg per mL; Roche Diagnostics GmbH, Mannheim, Germany) and phosphate buffered saline were used as positive and negative controls, respectively. The results were expressed as the numbers of IFN-γ-SC per million PBMC.
Interleukin-10
The protein concentrations of interleukin-10 (IL-10) were quantified in the supernatants of PBMC cultures (2 × 106 cells per well; 250 μL) in vitro for 20 hours with the challenging PRRSV (multiplicity of infection of 0.01) or phytohemagglutinin (10 μg per mL) using commercial ELISA kits (Pig Interleukin-10 ELISA kit; Cusabio Biotech, Wuhan, China) according to the manufacturer’s instructions. The detection limit for IL-10 was 1.5 pg per mL.
Histopathologic examination
For morphometric analysis of histopathologic lesion scores in lymph nodes, the superficial inguinal lymph node was collected from each pig, and three sections of that lymph node were examined blindly as previously described.11,12 Lymphoid lesions were scored on a scale from 0 to 3: 0, no lymphoid depletion or granulomatous replacement; 1 = mild lymphoid depletion; 2 = moderate lymphoid depletion; and 3 = severe lymphoid depletion and histiocytic replacement.11
For morphometric analysis of histopathologic lesion scores in lung, eight samples of lung tissue (two from the right cranial lobe, two from the right middle lobe, one from the ventromedial part of the right caudal lobe, one from the dorsomedial part of the right caudal lobe, one from the mid-lateral part of the right caudal lobe, and one from the accessory lobe) were collected from each pig and three sections of that lung tissue were examined histologically by one of the authors (JJ), blinded to the animal IDs, as previously described.7 Lung lesions were scored on a scale from 0 to 4: 0 = no microscopic lesions; 1 = mild interstitial pneumonia; 2 = moderate multifocal interstitial pneumonia; 3 = moderate diffuse interstitial pneumonia; and 4 = severe interstitial pneumonia.7
Immunohistochemical examination for PCV2 antigen was performed using PCV2 polyclonal antibody (Iowa State University, Ames, Iowa).13 Immunohistochemical examination for PRRSV antigen was performed using SR30 monoclonal antibody (Rural Technologies Inc, Brookings, South Dakota).14 Numbers of lymphoid cells positive for PCV2 antigen in lymph node12 and of pulmonary cells positive for PRRSV and PCV2 antigen in lung per unit area (0.25 mm2)15 were counted using an NIH Image J 1.45s program (http://imagej.nih.gov/ij/ download.html).
Statistical analysis
Continuous data (rectal body temperature; body weight; PCV2 DNA (log10 PCV2 genomic copies per mL) determined by real-time PCR; PPRSV RNA (log10 PRRSV genomic copies per mL) determined by real-time PCR; PCV2 and PRRSV serum titer; number of IFN-γ-SC per 106 PBMC determined by ELISPOT assay; numbers of lung sections positive for PRRSV antigen and PCV2 antigen; and lymph-node sections positive for PCV2 antigen per unit area (0.25 mm2; determined by immunohistochemistry) were analyzed using repeated measures ANOVA for each time point. If the ANOVA showed a significant effect, Tukey’s test for multiple comparisons was performed at each time point. Fisher’s exact test was used for discrete data (clinical respiratory score and lung and lymphoid lesion scores). A chi-square test was used for mortality rate. A value of P < .05 was considered significant.
Results
Clinical evaluation
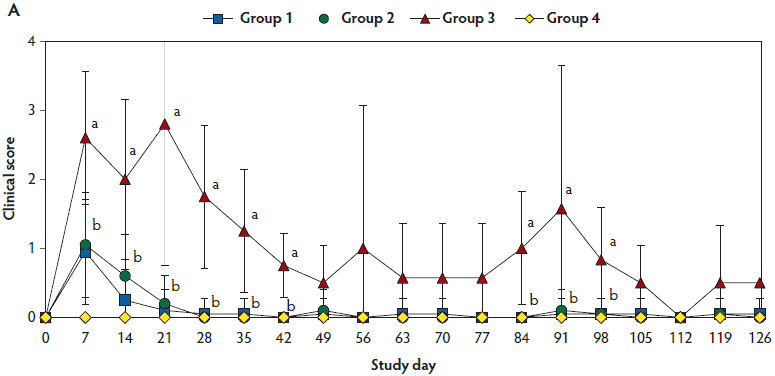
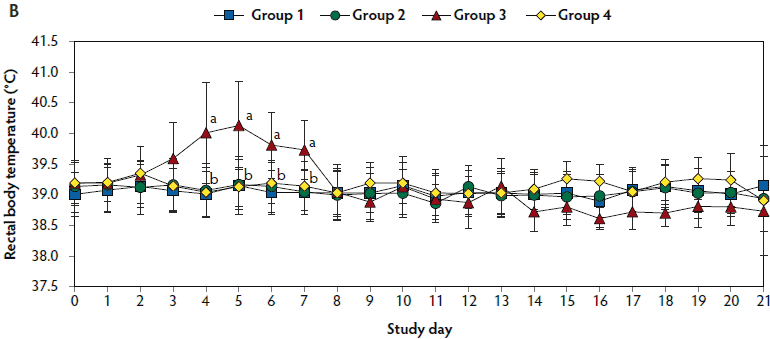
Mean respiratory scores were significantly higher (P < .05) in unvaccinated, challenged pigs (Group 3) than in vaccinated, challenged pigs (Group 1 and Group 2) from day 7 to 42 and from day 84 to 98 (Figure 1A). Mean rectal temperature (ranging from 39.7°C to 40.2°C) was significantly higher (P < .05) in unvaccinated, challenged pigs (Group 3) than in vaccinated, challenged pigs (Group 1 and Group 2) from day 4 to 7 (Figure 1B). Overall mortality rates were 5% (one of 20 pigs) in Group 1, 10% (two of 20 pigs) in Group 2, 30% (three of 10 pigs) in Group 3, and 0% (0 of 10 pigs) in Group 4. There was no significant difference in mortality rate between vaccinated, challenged pigs (Group 1 and Group 2) and unvaccinated, challenged pigs (Group 3). Diagnostic results indicated that pig deaths were primarily related to severe pneumonia.
Figure 1: Means (with standard deviations) of the scores for clinical signs (Panel A) and rectal body temperature (Panel B) in pigs in the study described in Table 1. Different letters (a,b) indicate significant differences among groups (Panel A, P < .05; Fisher’s exact test and Panel B, P < .05; one-way ANOVA).
Growth performance
Mean ADGs were significantly higher (P < .05) in vaccinated, challenged pigs (Group 1 and Group 2) and unvaccinated, unchallenged pigs (Group 4) than in unvaccinated challenged pigs (Group 3) throughout the experiment. However, mean ADG did not differ between the two groups of vaccinated, challenged pigs (Group 1 and Group 2) (Table 2).
Table 2: Means (with standard deviation) of average daily gain (ADG) in pigs in the study described in Table 1
| Period between study days* | Age (days) | ADG (g/day) | |||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | ||
| -28 to 0 | 21-49 | 329 (31) | 330 (28) | 326 (25) | 340 (27) |
| 0 to 21 | 49-70 | 629 (33)a | 612 (35)a | 519 (24)b | 626 (43)a |
| 21 to 63 | 70-112 | 792 (44)a | 785 (47)a | 672 (45)b | 804 (39)a |
| 63 to 126 | 112-175 | 734 (43)a | 718 (39)a | 650 (33)b | 728 (42)a |
| -28 to 126 | 21-175 | 662 (33)a | 651 (34)a | 579 (39)b | 664 (43)a |
* The body weight of each pig in each group was measured at study days -28 (21days of age), 0, 21 (70 days of age), 63, and 126 (175 days of age) and ADGs were compared among groups using Tukey’s test.
ab Within a row, values with different superscript letters are significantly different (P < .05).
Quantification of PCV2 DNA in serum samples
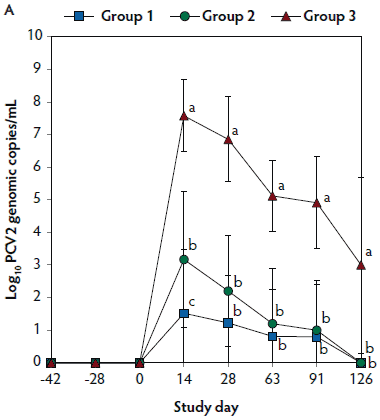
No PCV2 DNA was detected in the serum samples of pigs tested at days -42, -28, and 0. On days 14 through 126, the numbers of genomic copies of PCV2 in serum were significantly lower (P < .05) in Group 1 and Group 2 (vaccinated, challenged pigs) than in Group 3 (unvaccinated, challenged pigs). Numbers of genomic copies of PCV2 in serum differed between the two groups of vaccinated, challenged pigs (Group 1 and Group 2) at day 14 (Figure 2A). No PCV2 DNA was detected in serum samples of Group 4 pigs (unvaccinated, unchallenged pigs) throughout the experiment.
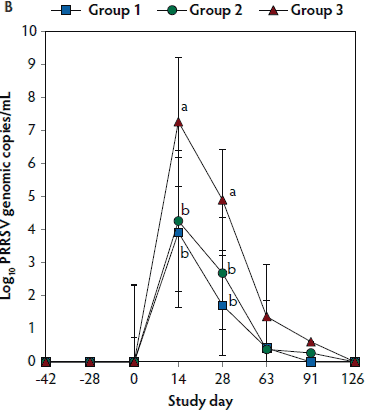
Figure 2: Means (with standard deviations) of the number of genomic copies of PCV2 DNA (transformed to base 10 logarithms; Panel A) and PRRSV RNA (transformed to base 10 logarithms; Panel B) in serum samples from pigs in the study described in Table 1. Different letters (a,b) indicate significant differences among groups (P < .05; repeated measures ANOVA).
Quantification of PRRSV RNA in sera
No PRRSV RNA was detected in the serum samples of pigs tested at days -42, -28, and 0. On days 14 and 28, the numbers of genomic copies of PRRSV in serum were significantly lower (P < .05) in Group 1 and Group 2 (vaccinated, challenged pigs) than in Group 3 (unvaccinated, challenged pigs). Numbers of genomic copies of PRRSV in serum did not differ between the two groups of vaccinated, challenged pigs (Group 1 and Group 2) (Figure 2B). No PRRSV RNA was detected in serum of Group 4 pigs (unvaccinated, unchallenged pigs) throughout the experiment.
Immunological responses to PCV2
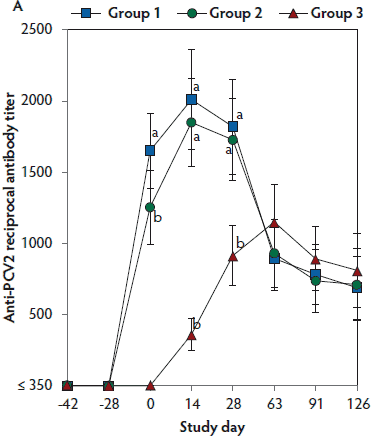
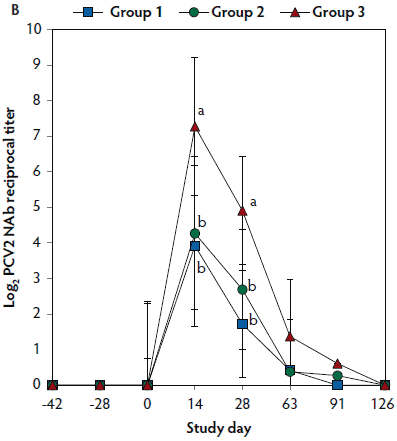
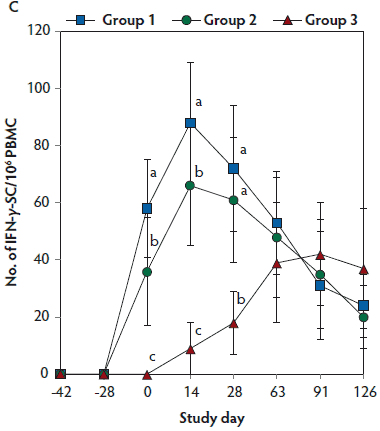
On days 0 through 28, anti-PCV2 antibody titers were significantly higher (P < .05) in Group 1 and Group 2 (vaccinated, challenged pigs) than in Group 3 (unvaccinated, challenged pigs). Anti-PCV2 antibody titers differed between the two groups of vaccinated, challenged pigs (Group 1 and Group 2) at day 0 (Figure 3A). On days 0 through 91, mean NAb titers were significantly higher (P < .05) in Group 1 and Group 2 (vaccinated, challenged pigs) than in Group 3 (unvaccinated, challenged pigs). Mean NAb titers differed between the two groups of vaccinated, challenged pigs (Group 1 and Group 2) at day 14 (Figure 3B). On days 0 through 28, numbers of PCV2-specific IFN-γ-SC were significantly higher (P < .05) in Group 1 and Group 2 (vaccinated, challenged pigs) than in Group 3 (unvaccinated, challenged pigs). Numbers of PCV2-specific IFN-γ-SC differed between the two groups of vaccinated, challenged pigs (Group 1 and Group 2) at days 0 and 14 (Figure 3C). No anti-PCV2 antibodies or PCV2-specific NAb or IFN-γ-SC were detected in Group 4 (unvaccinated, unchallenged pigs).
Figure 3: Mean (with standard deviation) for anti-PCV2 reciprocal ELISA antibody titers (Panel A); group means transformed to base 2 logarithms (with standard deviation) for neutralizing antibody (NAb) reciprocal titers (Panel B); and mean (with standard deviation) of PCV2-specific interferon-γ secreting cells (IFN-γ-SC) in peripheral blood mononuclear cells (PBMC) (Panel C) in the study described in Table 1. Different letters (a,b) indicate significant differences among groups (P < .05; repeated measures ANOVA).
Immunologic responses to PRRSV
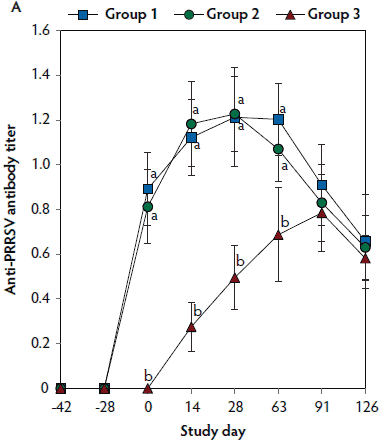
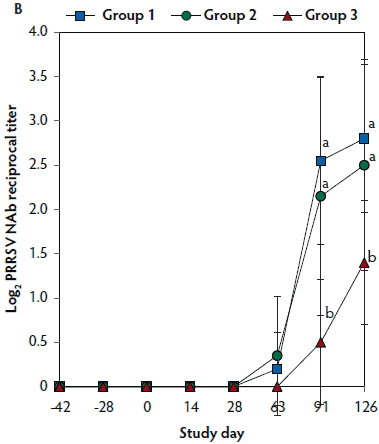
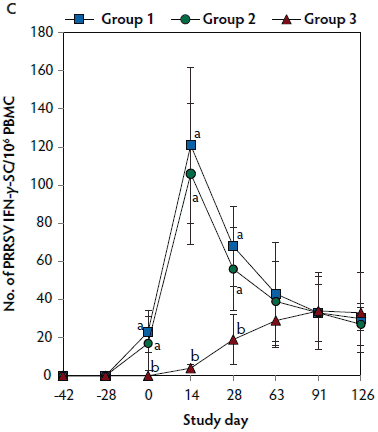
On days 0 thorugh 63, anti-PRRSV antibody titers were significantly higher (P < .05) in Group 1 and Group 2 (vaccinated, challenged pigs) than in Group 3 (unvaccinated, challenged pigs) (Figure 4A). On days 91 and 126, mean NAb titers were significantly higher (P < .05) in Group 1 and Group 2 (vaccinated, challenged pigs) than in Group 3 (unvaccinated, challenged pigs) (Figure 4B). On days 0 through 28, numbers of PRRSV-specific IFN-γ-SC were significantly higher (P < .05) in Group 1 and Group 2 (vaccinated, challenged pigs) than in Group 3 (unvaccinated, challenged pigs) (Figure 4C). No anti-PRRSV antibodies or PRRSV-specific NAb or IFN-γ-SC were detected in Group 4 (unvaccinated, unchallenged pigs).
Figure 4: Means (with standard deviation) of commercial PRRSV ELISA sample-to-positive (S:P) ratio (Panel A); group means transformed to base 2 logarithms (with standard deviation) for neutralizing antibody (NAb) reciprocal titers (Panel B); and mean (with standard deviation) of PRRSV-specific interferon-γ secreting cells (IFN-γ-SC) in peripheral blood mononuclear cells (PBMC) (Panel C) in the study described in Table 1. Different letters (a,b) indicate significant differences among groups (P < .05; repeated measures ANOVA).
PRRSV-specific IL-10
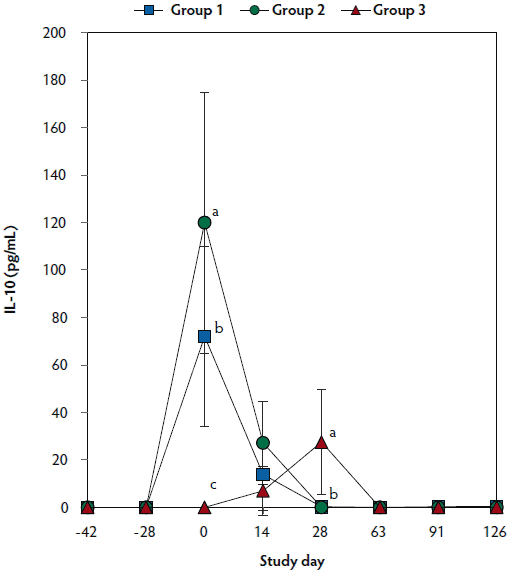
On day 0, IL-10 levels were significantly higher (P < .05) in Group 1 and Group 2 (vaccinated, challenged pigs) than in Group 3 (unvaccinated, challenged pigs). Concentrations of IL-10 differed between the two groups of vaccinated, challenged pigs (Group 1 and Group 2) at day 0. On day 28, IL-10 concentrations were significantly higher (P < .05) in Group 3 (unvaccinated, challenged pigs) than in Group 1 and Group 2 (vaccinated, challenged pigs) (Figure 5). No IL-10 was detected in Group 4 (unvaccinated, unchallenged pigs).
Figure 5: Mean (with standard deviation) for PRRSV-specific IL-10 concentrations in serum samples from pigs in the study described in Table 1. Different letters (a,b) indicate significant differences among groups (P < .05; repeated measures ANOVA).
Pathologic testing
Lymphoid and pulmonary lesion scores were significantly lower (P < .05) in Group 1 and Group 2 (vaccinated, challenged pigs) than in Group 3 (unvaccinated, challenged pigs). The numbers of lymphoid cells positive for PCV2 antigen (Figure 6), and pulmonary cells positive for PRRSV antigen (Figure 7) and PCV2 antigen (Figure 8) were significantly lower (P < .05) in Group 1 and Group 2 (vaccinated, challenged pigs) than in Group 3 (unvaccinated, challenged pigs) (Table 1).
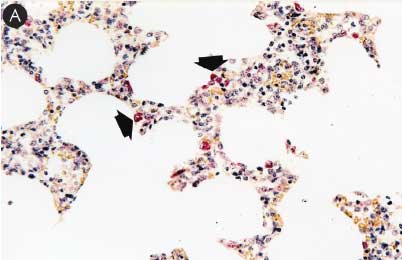
Figure 6: Immunohistochemical testing to detect porcine circovirus type 2 (PCV2) antigen in lymph nodes of pigs in the study described in Table 1 was performed using PCV2 polyclonal antibody (Iowa State University, Ames, Iowa). Few PCV2 antigen-positive cells (arrowheads) were detected in macrophages in Group 1 pigs (Panel A). Numerous PCV2 antigen-positive cells were detected in macrophages in Group 3 pigs (Panel B) (magnification × 400).
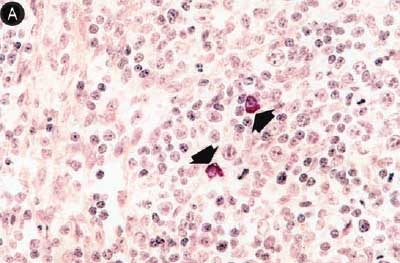
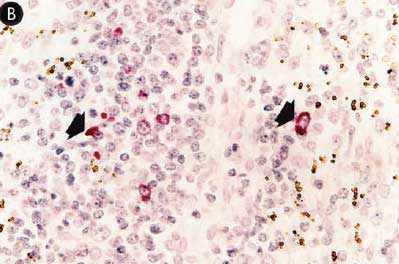
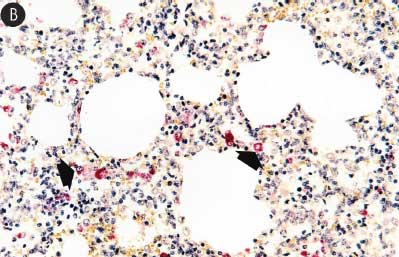
Figure 7: Immunohistochemical testing to detect porcine reproductive and respiratory syndrome virus (PRRSV) antigen in lungs of pigs in the study described in Table 1 was performed using SR30 monoclonal antibody (Rural Technologies Inc, Brookings, South Dakota). Few PRRSV antigen-positive cells (arrowheads) were detected in macrophages in pigs from Group 1 (Panel A). Numerous PRRSV antigen-positive cells were detected in macrophages in pigs from Group 3 (Panel B) (magnification × 200).
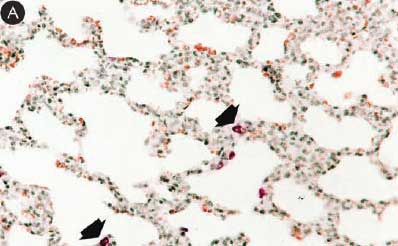
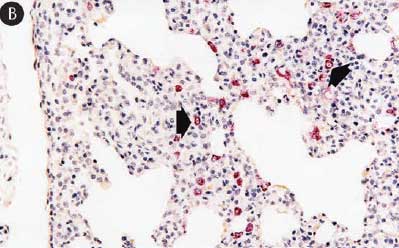
Figure 8: Immunohistochemical testing to detect porcine circovirus type 2 (PCV2) antigen in lungs of pigs in the study described in Table 1 was performed using PCV2 polyclonal antibody (Iowa State University, Ames, Iowa). Few PCV2 antigen-positive cells (arrowheads) were detected in macrophages in pigs from Group 1 (Panel A). Numerous PCV2 antigen-positive cells were detected in macrophages in pigs from Group 3 (Panel B) (magnification × 200).
Discussion
This study demonstrated that the single-dose vaccination regimen for PCV2 and PRRSV vaccine is efficacious for controlling co- infection with PCV2 and PRRSV. Regardless of types of vaccines, ADG was higher and mortality rate was lower in the vaccinated, challenged animals than in the unvaccinated, challenged animals.
Porcine circovirus type 2 viremia is correlated with the severity of PCV2-induced lymphoid lesions.16,17 Therefore, PCV2 viremia is an appropriate parameter to evaluate a PCV2 vaccine. A lower number of genomic copies of PCV2 DNA correlates with induction of PCV2-specific NAb and IFN-γ-SC.16-20 In the current study, only vaccinated animals exhibited PCV2-specific NAb and IFN-γ-SC. Pigs immunized with the Fostera PCV and Fostera PRRSV vaccine (Group 1) had higher titers of PCV2-specific NAb and higher numbers of IFN-γ-SC than did pigs immunized with the Ingelvac CircoFLEX and Ingelvac PRRS MLV vaccines (Group 2). These differences likely influenced the lower numbers of genomic copies of PCV2 DNA in Group 2. These results agree with previous findings that the Fostera PCV vaccine results in significantly lower numbers of genomic copies of PCV2 DNA and greater protective immunity (higher titers of PCV2-specific NAb and higher numbers of IFN-γ-SC) when compared to the Ingelvac CircoFLEX vaccine.21
The number of genomic PRRSV RNA copies in serum samples is a critical parameter to evaluate the efficacy of vaccines in control of PRRSV infection.22 In the present study, PRRSV viremia had resolved before neutralizing antibodies were developed. Therefore, neutralizing antibodies are not essential for the lower number of genomic PRRSV RNA copies as reported in previous studies.23,24 In addition, there is no evidence that PRRSV antibodies detected by ELISA play a role in protection against infection with PRRSV.25 In contrast, a lower number of PRRSV genomic RNA copies coincided with the appearance of PRRSV-specific IFN-γ-SC in vaccinated, challenged animals. Therefore, PRRSV-specific IFN-γ-SC are responsible for PRRSV clearance, although the role of IFN-γ-SC in a lower number of PRRSV RNA copies is still conflicting.23,26 In the present study, no significant differences were observed in the ability of the two tested PRRSV vaccines to induce PRRSV-specific IFN-γ-SC and reduce PRRSV viremia, as a previous study showed.10
Pathologic evaluation is another critical parameter to determine the efficacy of the PCV2 and PRRSV vaccines under experimental conditions. The characteristic microscopic lesions caused by co-infection with PCV2 and PRRSV were severe interstitial pneumonia and lymphoid depletion in the unvaccinated, challenged animals in the present and previous studies.2,27 Single-dose vaccination with PCV2 and PRRSV at 21 days of age was effective in lowering scores for lung and lymphoid lesions in the vaccinated, challenged animals, compared to the unvaccinated, challenged animals, without significant differences between Fostera PCV-PRRS and Ingelvac CircoFLEX-PRRS MLV.
There is interest in the possible interference with the efficacy of one vaccine by another, because animals received both PCV2 and PRRSV vaccines at the same time in this study. Especially, induction of IL-10 by PRRSV vaccine raised concerns that vaccination with PRRSV may interfere with the efficacy of a PCV2 vaccine.28 Interleukin-10 is a well-known cytokine synthesis inhibiting factor and inhibits cell-mediated immune responses.29 Both PRRSV vaccines used in this study induced maximal levels of IL-10 at study day 0 (28 days post vaccination) that thereafter decreased rapidly. Nevertheless, PCV2- and PRRSV-specific IFN-γ-SC increased gradually, beginning at study day 0 and reaching a peak at study day 14, even in the presence of IL-10. These results suggest that induction of IL-10 by PRRSV vaccines may not interfere with cell-mediated immunity induced by PCV2 vaccines. This information is clinically meaningful, as swine producers prefer to administer both vaccines at the same time, saving labor and resulting in less stress to the animals.
Vaccination is still considered the most effective tool for controlling PRDC caused by co-infection with PCV2 and PRRSV, although co-infection can still be controlled by other practices, such as improved management, pig flow, biosecurity measures, and housing conditions. The results of this study may provide swine practitioners and producers with another option in controlling PRDC, through concurrent administration of single-dose PRRSV and PCV2 vaccines.
Implications
- Under the conditions of this study, it makes no difference to protection whether single-dose PCV2 and PRRSV vaccines are administered concurrently.
- Under the conditions of this study, concurrent vaccination of pigs with PCV2 and PRRSV is efficacious for controlling co-infection with PCV2 and PRRSV.
Acknowledgements
This research was supported by Zoetis Ltd; contract research funds from the Research Institute for Veterinary Science (RIVS) of the College of Veterinary Medicine, Seoul National University; and Brain Korea 21 Plus Program for Creative Veterinary Science Research in the Republic of Korea. The first two authors contributed equally to this work.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Chae C. A review of porcine circovirus 2-associated syndromes and diseases. Vet J. 2005;169:326–336.
2. Chae C. Porcine circovirus type 2 and its associated diseases in Korea. Virus Res. 2012;164:107–113.
3. Gagnon CA, del Castillo JR, Music N, Fontaine G, Harel J, Tremblay D. Development and use of a multiplex real-time quantitative polymerase chain reaction assay for detection and differentiation of Porcine circovirus-2 genotypes 2a and 2b in an epidemiological survey. J Vet Diagn Invest. 2008;20:545–558.
4. Wasilk A, Callahan JD, Christopher-Hennings J, Gay TA, Fang Y, Dammen M, Reos ME, Torremorell M, Polson D, Mellencamp M, Nelson E, Nelson WM. Detection of U.S., Lelystad, and European-like porcine reproductive and respiratory syndrome viruses and relative quantitation in boar semen and serum samples by real-time PCR. J Clin Microbiol. 2004;42:4453–4461.
5. Chow SC, Wang H, Shao J. Comparing means. In: Chow SC, Wang H, Shao J, eds. Sample Size Calculations in Clinical Research. 2nd ed. New York, New York: Chapman and Hall/CRC; 2008:60–92.
6. Park C, Oh Y, Seo HW, Han K, Chae C. Comparative effects of vaccination against porcine circovirus type 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) in a PCV2-PRRSV challenge model. Clin Vaccine Immunol. 2013;20:369–376.
7. Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng X-J, Lum MA, Andrews JJ, Rathje JA. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32:648–660.
8. Pogranichniy RM, Yoon K-J, Harms PA, Swenson SL, Zimmerman JJ, Sorden SD. Characterization of immune response of young pigs to porcine circovirus type 2 infection. Viral Immunol. 2000;13:143–153.
9. Yoon IJ, Joo HS, Goyal SM, Molitor TW. A modified serum neutralization test for the detection of antibody to porcine reproductive and respiratory syndrome virus in swine sera. J Vet Diagn Invest. 1994;6:289–292.
10. Park C, Seo HW, Han K, Kang I, Chae C. Evaluation of the efficacy of a new modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine (Fostera PRRS) against heterologous PRRSV challenge. Vet Microbiol. 2014;172:432–442.
11. Fenaux M, Halbur PG, Haqshenas G, Royer P, Thomas P, Nawagitgul P, Gill M, Toth TE, Meng XJ. Cloned genomic DNA of type 2 porcine circovirus is infectious when injected directly into the liver and lymph nodes of pigs: Characterization of clinical disease, virus distribution, and pathologic lesions. J Virol. 2002;76:541–551.
12. Kim D, Ha Y, Lee Y-H, Chae S, Lee K, Han K, Kim J, Lee J-H, Kim S-H, Hwang K-K, Chae C. Comparative study of in situ hybridization and immunohistochemistry for the detection of porcine circovirus 2 in formalin-fixed, paraffin-embedded tissues. J Vet Med Sci. 2009;71:1001–1004.
13. Seo HW, Han K, Oh Y, Kang I, Park C, Joo HE, Kim S-H, Lee B-H, Chae C. Evaluation of commercial polyclonal- and monoclonal-antibody-based immunohistochemical tests for 2 genotypes of Porcine circovirus type 2 and comparison with in-situ hybridization assays. Can J Vet Res. 2014;78:233–236.
14. Han K, Seo HW, Oh Y, Kang I, Park C, Kang SH, Kim S-H, Lee B-H, Kwon B, Chae C. Evaluation of monoclonal antibody-based immunohistochemistry for the detection of European and North American Porcine reproductive and respiratory syndrome virus and a comparison with in situ hybridization and reverse transcription polymerase chain reaction. J Vet Diagn Invest. 2012;24:719–724.
15. Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng X-J, Andrews JJ, Lum MA, Rathje JA. Comparison of the antigen distribution of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1996;33:159–170.
16. Chae C. Commercial porcine circovirus type 2 vaccines: Efficacy and clinical application. Vet J. 2012;194:151–157.
17. Meerts P, Misinzo G, Lefebvre D, Nielsen J, Bøtner A, Kristensen CS, Nauwynck HJ. Correlation between the presence of neutralizing antibodies against porcine circovirus 2 (PCV2) and protection against replication of the virus and development of PCV2-associated disease. BMC Vet Res. 2006;2:6. doi:10.1186/1746-6148-2-6.
18. Meerts P, Van Gucht S, Cox E, Vandebosch A, Nauwynck HJ. Correlation between type of adaptive immune response against porcine circovirus type 2 and level of virus replication. Viral Immunol. 2005;18:333–341.
19. Fort M, Olvera A, Sibila M, Segalés J, Mateu E. Detection of neutralizing antibodies in postweaning multisystemic wasting syndrome (PMWS)-affected and non-PMWS-affected pigs. Vet Microbiol. 2007;125:244–255.
20. Fort M, Fernandes LT, Nofrarias M, Diaz I, Sibila M, Pujols J, Mateu E, Segales J. Development of cell-mediated immunity to porcine circovirus type 2 (PCV2) in caesarean-derived, colostrum-deprived piglets. Vet Immunol Immunopathol. 2009;129:101–107.
21. Seo HW, Han K, Park C, Chae C. Clinical, virological, immunological and pathological evaluation of four porcine circovirus type 2 vaccine. Vet J. 2014;200:65–70.
22. Labarque G, Van Gucht S, van Reeth K, Nauwynck H, Pensaert M. Respiratory tract protection upon challenge of pigs vaccinated with attenuated porcine reproductive and respiratory syndrome virus vaccines. Vet Microbiol. 2003;95:187–197.
23. Kimman TG, Cornelissen LA, Noormann RJ, Rebel JMJ, Stockhofe-Zurwieden N. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine. 2009;27:3704–3718.
24. Mateu E, Diaz I. The challenge of PRRS immunology. Vet J. 2008;177:345–351.
25. Lopez OJ, Osorio FA. Role of neutralizing antibodies in PRRSV protective immunity. Vet Immunol Immunopathol. 2014;102:155–163.
26. Chand RJ, Tribe BR, Rowland RR. Pathogenesis of porcine reproductive and respiratory syndrome virus. Curr Opin Virol. 2012;2:256–265.
27. Harms PA, Sorden SD, Halbur PG, Bolin SR, Lager KM, Morozov I, Paul PS. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet Pathol. 2001;38:528–539.
28. Diaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Different European-type vaccines against porcine reproductive and respiratory syndrome virus have different immunological properties and confer different protection to pigs. Virology. 2006;351:249–259.
29. Conti P, Kempuraj D, Kandere K, Di Gioacchino M, Barbacane RC, Castellani ML, Felaco M, Boucher W, Letourneau R, Theoharides TC. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol Lett. 2003;86:123–129.
