| Case study | Peer reviewed |
Cite as: Clement T, Singrey A, Lawson S, et al. Measurement of neutralizing antibodies against porcine epidemic diarrhea virus in sow serum, colostrum, and milk samples and in piglet serum samples after feedback. J Swine Health Prod. 2016;24(3):147–153.
Also available as a PDF.
SummaryThe introduction of porcine epidemic diarrhea virus (PEDV) into the naive US swine population in April 2013 resulted in significant mortality. The high mortality rates observed indicated the need to boost herd immunity to PEDV. To optimize feedback protocols or other future control measures used to increase immunity, a fluorescent focus neutralization (FFN) assay was developed and used to determine the titers of neutralizing antibodies in sow serum, milk, and colostrum samples and in piglet serum samples. Sow serum samples from two farm sites within different production systems (A, B) were tested. At least 24 sows per site were screened for neutralizing antibodies at 0, 3, 6, 7, and 24 weeks post feedback (PF). These functional antibodies were detected in sow serum samples at both sites 3, 6, 7, and 24 weeks PF and in milk and colostrum samples by 7 weeks PF. At 6 weeks PF, neutralizing antibodies were detected in 27 of 30 Site A piglets (90%), compared to 15 of 29 Site B piglets (52%). Piglets at both sites had detectable neutralizing antibodies, and sentinel pigs were successfully introduced into both systems without re-infection with PEDV by 24 weeks PF. | ResumenLa introducción del virus de la diarrea epidémica porcina (PEDV por sus siglas en inglés) en la población porcina libre del virus en EUA en Abril del 2013 resultó en mortalidad significativa. Los altos índices de mortalidad observados señalaron la necesidad de aumentar la inmunidad del hato contra PEDV. Para optimizar los protocolos de retroalimentación u otras medidas futuras de control utilizadas para incrementar la inmunidad, se desarrolló un ensayo de neutralización de focos fluorescentes (FFN por sus siglas en inglés) y se utilizó para determinar los títulos de anticuerpos neutralizantes en muestras de suero de hembra, leche, calostro y suero de lechones. Se analizaron muestras de suero de hembras de dos sitios porcinos en de dos sistemas (A, B). Se muestrearon por lo menos 24 hembras por sitio en busca de anticuerpos neutralizantes a las 0, 3, 6, 7, y 24 semanas post retroalimentación (PF por sus siglas en inglés). Estos anticuerpos funcionales se detectaron en muestras de suero de hembras en ambos sitios a las 3, 6, 7, y 24 semanas PF y en muestras de leche y calostro a las 7 semanas PF. A las 6 semanas PF, se detectaron anticuerpos neutralizantes en 27 de 30 lechones del Sitio A (90%), comparado con 15 de 29 lechones del Sitio B (52%). Los lechones en ambos sitios tuvieron anticuerpos neutralizantes detectables, y se introdujeron cerdos centinelas exitosamente en ambos sistemas sin reinfección con PEDV a las 24 semanas PF. | ResuméL’introduction du virus de la diarrhée épidémique porcine (VDEP) dans la population porcine naive des États-Unis en avril 2013 a entrainé de nombreuses mortalités. Les taux de mortalité élevés observés indiquaient le besoin de stimuler l’immunité des troupeaux envers le VDEP. Afin d’optimiser les protocoles de rétroaction ou autres mesures de contrôle utilisées pour augmenter l’immunité, une épreuve de neutralisation de fluorescence a été développée et utilisée pour déterminer les titres d’anticorps neutralisants dans des échantillons de sérum, de lait, et de colostrum de truies et dans des échantillons de sérum de porcelets. Des échantillons de sérum de truie de deux sites de ferme différents de deux systèmes de production différents (A, B) ont été testés. Au moins 24 truies par site ont été testées pour des anticorps neutralisants à 0, 3, 6, 7, et 24 semaines post-rétroaction (PR). Des anticorps fonctionnels ont été détectés dans les échantillons de sérum des truies aux deux sites à 3, 6, 7, et 24 semaines PR et dans les échantillons de lait et de colostrum à la 7e semaine PR. À 6 semaines PR, des anticorps neutralisants ont été détectés chez 27 des 30 porcelets du Site A (90%), comparativement à 15 des 29 porcelets du Site B (52%). Aux deux sites, les porcelets avaient des anticorps neutralisants détectables, et des porcs sentinelles ont été introduits de manière réussie dans les deux systèmes sans ré-infection avec le VDEP à 24 semaines PR. |
Keywords: swine, porcine epidemic diarrhea virus, neutralizing antibody, feedback, fluorescent focus neutralization, PEDV
Search the AASV web site
for pages with similar keywords.
Received: August 28, 2015
Accepted: December 2, 2015
Porcine epidemic diarrhea virus (PEDV) is a highly contagious, enveloped, single-stranded positive-sense RNA virus belonging to the Coronaviridae family. The virus was first identified in Europe in 1971 and later in the United States in April 2013.1 Porcine epidemic diarrhea virus had also been reported in Korea, China, Japan, the Philippines, and Thailand prior to 2013.2 Infection with PEDV results in severe diarrhea and dehydration, which is followed by high mortality in suckling piglets.3 In addition to high mortality rates in young piglets, PEDV infection also contributes to significant production losses in older animals.4
The lack of effective PEDV vaccines capable of eliciting lactogenic protective immunity led multiple production systems in the United States to adopt feedback exposure protocols. Experimental infection using feedback of PEDV-infected intestinal material given to pigs by oral dosing was previously demonstrated in England.5 Feedback of intestines infected with transmissible gastroenteritis virus (TGEV), another coronavirus, had been used previously to protect piglets from TGEV-induced mortality.6,7 The protective mechanism underlying the immunity provided by feedback exposure to infected intestinal material has not been fully established, but is likely a result of virus replication in the mucosal epithelium and subsequent development of mucosal effectors of protective immunity (antibodies or cell-mediated responses) that are transferred from the exposed sow to the piglet through milk or colostrum. Neutralizing antibodies may help prevent binding of virus to receptors, block uptake into cells, prevent uncoating of the viral genomes in endosomes, or cause aggregation of virus particles, or the enveloped virus may be lysed by antiviral antibodies and complement.8
The overall goal of this study was to evaluate humoral immune responses, mainly focusing on neutralizing antibody responses, elicited by feedback exposure to PEDV-infected intestinal material. Additionally, we assessed the titers and duration of PEDV neutralizing antibodies in the serum of exposed sows and newborn piglets, and in the milk and colostrum of exposed sows. This was an observational case study conducted in two distinct farm sites within different production systems (A, B) that were naturally infected with PEDV in 2014. We compared titers of neutralizing antibodies after initial PEDV infection and feedback exposure in both study sites.
All samples used in this study were derived from routine diagnostic submissions to the South Dakota Animal Disease Research and Diagnostic Laboratory (SD ADRDL). Therefore, institutional animal care and use committee approval was not required for the specific purposes of this study.
Farm sites (A and B) and feedback protocols
Two commercial units performed whole-herd feedback protocols. Site A was a 4000-sow farrow-to-wean farm and Site B was a 4300-sow breed-to-wean site. After first detection of PEDV by polymerase chain reaction (PCR) testing, both sites stopped all traffic to and from the units and performed herd closure. Each site humanely euthanized PEDV-infected piglets according to standard farm practices. The intestines and intestinal contents were collected and homogenized with water using a blender. Site A fed 4 fluid ounces (approximately 118 mL) of the intestinal homogenate to all sows and gilts in the herd, while site B provided 4 to 8 fluid ounces (approximately 118 to 236 mL) of the feedback preparation per animal. The frequency and duration of the feedback exposure protocol adopted by sites A and B were different. Site A used higher frequency and duration of feedback than did Site B. During the first 2 weeks, Site A administered feedback three times a week to all pigs, while Site B administered feedback only once a week during this time period. After 2 weeks, Site B discontinued feedback, whereas Site A continued feedback during weeks 3 to 6, administering feedback twice a week to the gilts and once a week to the open sows.
Serum, milk, and colostrum sample collection
Sow serum samples were collected at 0, 3, 6, 7, and 24 weeks post feedback (PF) exposure. Milk samples (Site A, n = 7) (Site B, n = 29) were collected after farrowing from the same group of sows. Colostrum samples from Site B (n = 34) were also collected from the same group of sows. Limited milk and no colostrum samples were obtained from Site A due to the difficulty of obtaining these samples after farrowing. Serum samples from piglets farrowed from these sows were collected and evaluated for neutralizing antibodies at 9 weeks PF (Site A, 12 to 14 days of age) and 10 weeks PF (Site B, 18 days of age).
Fluorescent focus neutralization assay
Anti-PEDV neutralizing antibody titers were determined by fluorescent focus neutralization (FFN) assays as previously described.9 Endpoints were interpreted as the highest serum dilution resulting in 90% fewer fluorescent foci than in negative controls. Titers of < 1:20 were considered negative and ≥ 1:20 were indicative of the presence of neutralizing antibodies.
Statistical analysis
Statistical analysis was performed using GraphPad InStat version 3.06 (GraphPad Software, Inc, La Jolla, California). Intracomparison and intercomparison of means were calculated between sites and at different collection times post inoculation using one-way analysis of variance with Tukey’s HSD multiple comparisons test to determine mean significance.10 Differences between groups were considered statistically significant at P < .05 for all analyses.
Neutralizing antibodies in sow serum samples
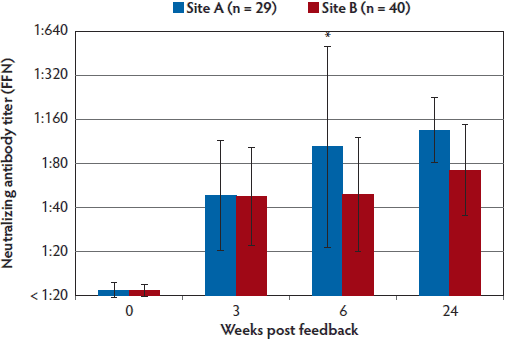
The FFN assay detected neutralizing antibodies in sow serum samples at both sites by the third week after initiation of the feedback protocol (Figure 1). The majority of the samples collected when the feedback protocol was initiated had FFN titers of < 1:20, indicating the whole herd had just been introduced to PEDV and had not developed PEDV neutralizing antibody previously. Six weeks after feedback exposure, Site A sow serum titers were significantly higher than those from Site B (P < .01) (Figure 1).
Figure 1: Following feedback exposure, this longitudinal case study measured PEDV neutralizing antibodies in serum samples from sows in two separate production systems. Both sites (A and B) housed at least 4000 breeding animals given a top dressing on feed of at least 4 ounces (approximately 118 mL) of homogenized intestinal contents from PEDV-infected piglets. The frequency and duration of feedback were greater in Site A than in Site B. This figure shows the mean PEDV FFN assay titers with standard deviation in sow serum samples from sites A and B at 0, 3, 6, and 24 weeks PF exposure. A significant difference (*) was observed by pairwise analysis between site A (n = 29) and B (n = 40) at week 6 (P < .01; one-way analysis of variance with Tukey’s HSD multiple comparisons test). When significance is not indicated, values are to be interpreted as not significantly different. Animals in both sites had detectable PEDV-neutralizing antibody, and sentinel pigs were successfully introduced into their systems without re-infection of PEDV by 24 weeks PF. PEDV = porcine epidemic diarrhea virus; FFN = fluorescent focus neutralization; PF = post feedback.
Indirect fluorescent antibody assay and comparison with FFN
At the time of the study, a commercial ELISA was not available, so an “in-house” indirect fluorescent antibody assay (IFA) was performed on sow serum samples at 6 weeks and 24 weeks PF (Figure 2). This assay has been previously described.9 A positive sample was indicated if a PEDV-specific fluorescent signal was observed at a serum dilution of 1:40 or greater.
Figure 2: Percent positive PEDV titers using IFA and FFN tests performed on sow serum samples from 6 weeks PF (Panel A), and 24 weeks PF (Panel B) in the case study described in Figure 1. Titers to FFN were detected for the duration of 24 weeks in 100% of animals tested. PEDV = porcine epidemic diarrhea virus; IFA = indirect fluorescent antibody; FFN = fluorescent focus neutralization; PF = post feedback.
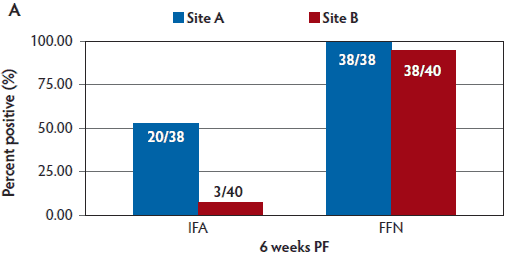
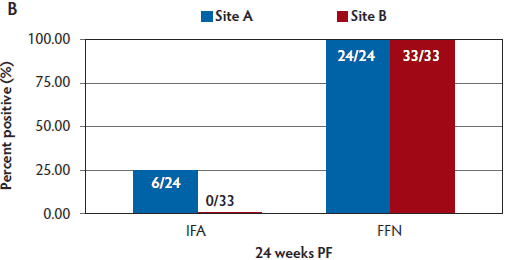
By 6 weeks PF, a greater percentage of sows were seropositive via FFN testing than via IFA testing. In Site A, 100% of sows were seropositive by FFN at 6 weeks PF, and in Site B, 95% of sows were seropositive by FFN. By 24 weeks PF, 100% of sows in both sites were seropositive by FFN (Figure 2).
PCR and sequencing
Intestinal homogenates used for feedback exposure were sent to the SD ADRDL and real-time multiplex PCR for PEDV, porcine deltacoronavirus (PDCoV), and TGEV (EZ-PED/TGE/PDCoV MPX 1.0; Tetracore Inc, Rockville, Maryland) was performed to obtain the semi-quantitative cycle threshold (Ct) values for the presence of PEDV nucleic acid. The feedback material had low Ct values, indicating a large amount of PEDV nucleic acid. For Site A, the feedback material Ct = 16.57, and for Site B, the feedback material Ct = 17.97. Deoxyribonucleic acid sequencing of the S1 region of the spike gene was performed on the intestinal homogenate for reference.
Clinical signs
Piglet loss during the initial outbreaks at both sites was reported as 100% for 2 to 3 weeks. Approximately 6 weeks after initial infection, clinical signs at Site A were reported as “clinically insignificant,” but clinical signs at Site B were reported as “clinically significant,” with the request to perform additional PCR and DNA sequencing to rule out a variant PEDV as the cause of continued clinical signs. Polymerase chain reaction testing indicated that shedding of the PEDV at Site B was continuing, and S1 PEDV sequencing confirmed that the virus was the same PEDV strain that was originally introduced into the herd prior to initiation of the feedback exposure protocol. Polymerase chain reaction was also performed to rule out introduction of other enteric coronaviruses, such as PDCoV and TGEV, which were not detected.
Neutralizing antibodies in serum, milk, and colostrum samples
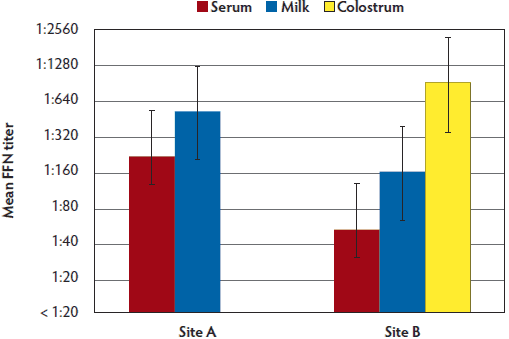
Neutralizing antibodies were detected in milk and serum samples collected on Site A from individual sows at the time of farrowing. Interestingly, neutralizing antibody titers in milk were similar to those detected in serum, with titers ranging from 1:160 to 1:640 in serum samples and 1:160 to 1:1280 in milk samples (Figure 3). Neutralizing antibody titers in colostrum samples collected on Site B were higher than titers in milk and serum samples collected at this site (Figure 4, Figure 5). Additionally on Site B, mean antibody titers detected in milk samples were higher than titers detected in serum samples.
Figure 3: Milk and serum PEDV FFN titers from seven sows in Site A in the case study described in Figure 1. Limited milk and no colostrum samples were obtained from Site A due to the difficulty of obtaining these samples from multiple sows after farrowing. PEDV = porcine epidemic diarrhea virus; FFN = fluorescent focus neutralization.
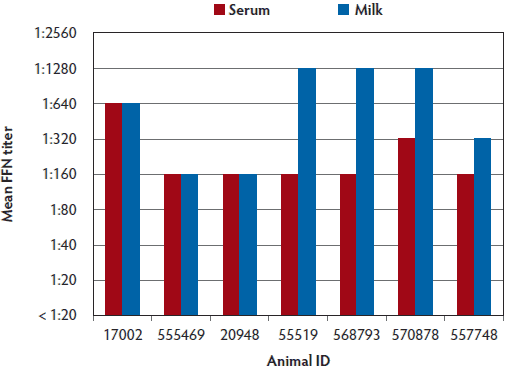
Figure 4: Mean PEDV FFN titers in sow serum (n = 38), colostrum (n = 34), and milk (n = 29) collected at 7 weeks PF from Site B in the case study described in Figure 1. PEDV = porcine epidemic diarrhea virus; FFN = fluorescent focus neutralization; PF = post feedback.
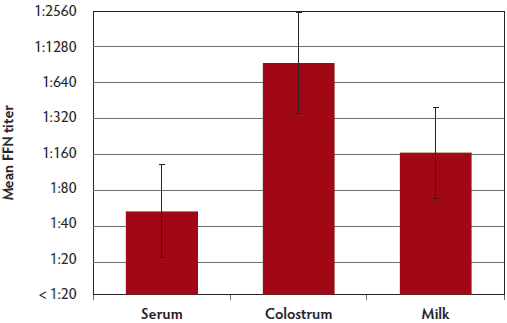
Figure 5: Comparison of PEDV mean FFN titers for site A (serum and milk samples) and B (serum, milk, and colostrum samples) at 7 weeks PF. Case study described in Figure 1. PEDV = porcine epidemic diarrhea virus; FFN = fluorescent focus neutralization; PF = post feedback.
Neutralizing antibodies in piglet serum samples
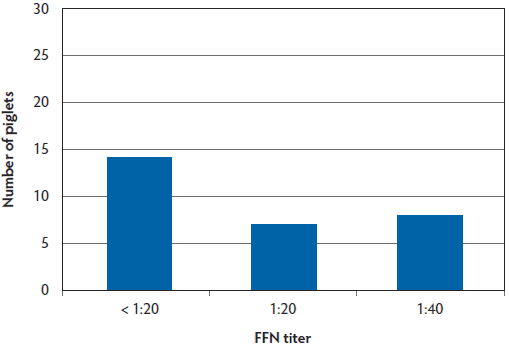
To assess passive transfer of neutralizing antibodies to piglets following feedback exposure of sows, serum samples were collected from piglets. These samples were collected and selected for convenience from piglets farrowed from sows that were monitored throughout the 24-week study. At 9 to 10 weeks PF, neutralizing antibodies were detected in samples from 27 of 30 Site A piglets tested (90%) and in only 15 of 29 samples from Site B piglets tested (52%) (Figure 6, Figure 7).
Figure 6: Site A piglet serum PEDV FFN titers at ages 12-14 days of age (9 weeks PF in the case study described in Figure 1) demonstrating 27 of 30 piglets (90%) with positive FFN titers. PEDV = porcine epidemic diarrhea virus; FFN = fluorescent focus neutralization; PF = post feedback.
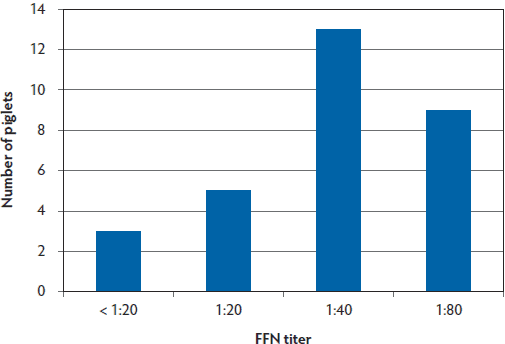
Figure 7: Site B piglet serum PEDV FFN titers at 18 days of age (10 weeks PF in the case study described in Figure 1) demonstrating 15 of 29 of piglets (52%) with positive FFN titers. PEDV = porcine epidemic diarrhea virus; FFN = fluorescent focus neutralization; PF = post feedback.
Discussion
In this observational case study, we have determined that neutralizing antibodies were detectable in sow serum samples within 3 weeks after the introduction of PEDV and subsequent feedback of infected material. In addition, neutralizing antibodies were detected in colostrum and milk samples of exposed sows and in serum samples of suckling piglets, suggesting that colostrum and milk are sources of neutralizing antibodies for piglets. The differences in the feedback protocols adopted by sites A and B (higher frequency and duration at Site A than at Site B) may have resulted in milder clinical signs and higher neutralizing antibody titers against PEDV for Site A versus Site B sows at 6 weeks post exposure. Subsequently, piglets in Site A had higher titers than piglets in Site B. However, other factors besides “frequency of the feedback” could have contributed to this difference, such as the homogeneity of the feedback between the two sites for consistent exposure of more sows, management practices for ensuring adequate feedback to all sows, loss of virus viability during mixing or administering the feedback, host genetic background, whether all piglets were able to nurse in order to obtain lactogenic antibodies, or other unknown factors.
Feedback of PEDV-infected intestinal homogenates was used to induce herd immunity when PEDV was first introduced into the United States. Relative success in controlling PEDV outbreaks was observed in production systems that adopted feedback exposure protocols. However, controlled experimental studies are needed to more definitively determine “success.” In addition, some drawbacks related to administering PEDV-infected feedback material have to be considered, including the potential for transmission of other pathogens within the herd, the maintenance of high PEDV viral load in the environment (which could lead to co-infections with other PEDV strains circulating in the field), or increased potential for spread of PEDV to uninfected farms.11 Therefore, it will be important to continue research on the best vaccine candidates for enteric protection against PEDV.
This case study was a comparison of two sow herds after initial PEDV infection and subsequent feedback. The titers of neutralizing antibodies in sow serum samples were compared to those in milk and colostrum samples. Results show that titers of PEDV-neutralizing antibodies in milk were at least as high as those in serum samples of feedback-exposed sows, whereas neutralizing antibody titers in colostrum samples were higher than those in serum and milk samples. The relationship between neutralizing antibody titers in serum and milk suggests that serum antibody can be used as an indicator of herd immunity. This specimen also requires less processing than milk or colostrum for higher-throughput laboratory testing. It has been determined that the major antibody isotype in sow serum and colostrum is IgG, whereas IgA is the major antibody isotype in milk.12 In addition, using radiolabeled immunoglobulin, it was determined that all colostral IgG and most of IgM antibodies are derived from serum, suggesting that serum is a good indicator of the antibodies that are transferred to colostrum.13 To date, the specific antibody isotype that is responsible for PEDV neutralization is unknown; however, most likely all isotypes may exert neutralizing functions.
There is a PEDV-specific S1 ELISA that measures IgA and IgG antibodies in serum and colostrum, and it is suggested that these measurements might be useful in determining passive immunity.14 However, the FFN assay would provide a “functional” assessment of these antibodies and not just a quantitative, indirect measure. By comparison, serum IFA appears to have a lower diagnostic sensitivity, and results do not necessarily correlate with the functional antibody response indicated by the FFN assay. While the IFA appears to have reasonable diagnostic sensitivity in the weeks immediately following PEDV exposure, titers of antibody detected by the IFA assay format appear to drop below detectable levels more quickly than functional neutralizing antibodies detected by FFN. In general terms, the IFA is detecting different specific types of antibodies than the FFN and appears to have a lower diagnostic sensitivity when evaluating samples collected well after PEDV exposure. Practitioners can use the knowledge gained from this study to understand that these two different test platforms, IFA and FFN, are both very useful in health management, but one should use a degree of caution when interpreting the results.
Serum samples from all sows tested from both sites in this study presented detectable neutralizing antibodies by 24 weeks PF. By this time point, both production systems had incorporated sentinel pigs into their farms and did not observe recurrence of PED, indicating that protective levels of herd immunity were reached. In an independent study conducted in approximately 800 swine herds, it was determined that the time to stability (no detectable PEDV shedding), ranged from 7 to 64 weeks, with an average time of 28 weeks.15 These observations corroborate those in this case study. Various factors, such as feedback consistency, frequency, and coverage of the herd, are likely to contribute to the time to stability. Interestingly, in this study, we observed a correlation between the titers of PEDV-neutralizing antibodies and time to stability. The authors recognize that a limitation of the experimental design of this observational case study is the limited number of sites tested due to the extravagant cost to accomplish a broad study of this type for greater statistical power. Nonetheless, this case study provides important information on the kinetics and titers of PEDV-neutralizing antibodies developed after different feedback protocols. This information will serve as a guide that will help in the design of future studies on PEDV immunobiology conducted to elucidate the contribution of neutralizing antibody for protection and the effectiveness of feedback protocols in the control of the disease.
Implications
• Under the conditions of this study, introduction of PEDV in sow farms with subsequent feedback of PEDV-infected material is associated with increased PEDV-specific neutralizing antibodies.
• Under the conditions of this study, neutralizing antibodies to PEDV are transferred from sow milk and colostrum to piglets.
• After PEDV introduction and feedback in a herd, PEDV-neutralizing antibodies may be detected in serum samples from pigs up to 24 weeks post feedback.
• Functional neutralizing antibody titers, as detected by the FFN, are detectable for a longer duration than are IFA titers. Practitioners should exercise caution when interpreting results between these two different testing platforms.
Acknowledgements
We would like to thank the companies, staff, and veterinarians that contributed to this project. Their dedication and persistence in providing the samples and information was greatly appreciated. This project was funded through National Pork Board Research Grant # 13-263, the South Dakota Agricultural Experimental Station, USDA Hatch Program, and the Animal Disease Research and Diagnostic Laboratory.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Stevenson GW, Hoang H, Schwartz KJ, Burrough ER, Sun D, Madson D, Cooper VL, Pillatzki A, Gauger P, Schmitt BJ, Koster LG, Killian ML, Yoon KJ. Emergence of porcine epidemic diarrhea virus in the United States; clinical signs, lesions, and viral genomic sequences. J Vet Diag Invest. 2013;25:649–654.
2. Song D, Park B. Porcine epidemic diarrhea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012;44:167–175.
3. Saif L, Pensaert MB, Sestak K, Yeo S-G, Jung K. Coronaviruses. In: Zimmerman J, Karriker L, Ramirez A, Schwartz K, Stevenson G, eds. Diseases of Swine. 10th ed. Hoboken, New Jersey: Wiley-Blackwell. 2012:501–524.
4. Alvarez J, Sarradell J, Morrison R, Perez A. Impact of porcine epidemic diarrhea on performance of growing pigs. PLoS ONE 2015:10(3):e0120532. doi:10.1371/journal.pone.0120532.
5. Chasey D, Cartwright SF. Virus-like particles associated with porcine epidemic diarrhea. Res Vet Sci. 1978;25:255–256.
6. Yanga ST, Gardner IA, Hurd HS, Eernisse KA, Willeberg P. Management and demographic factors associated with seropositivity to transmissible gastroenteritis virus in US swine herds, 1989-1990. Prev Vet Med. 1995;24:213–228.
7. Carpenter J, Templeton C. Evaluation of a transmissible gastroenteritis virus eradication program in a breeding stock supply herd. Swine Health Prod. 1996;4:239–246.
8. Klasse PJ, Sattentau QJ. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J Gen Virol. 2002;83:2091–2108.
9. Okda F, Liu X, Singrey A, Clement T, Nelson J, Christopher-Hennings J, Nelson EA, Lawson S. Development of an indirect ELISA, blocking ELISA, fluorescent microsphere immunoassay and fluorescent focus neutralization assay for serologic evaluation of exposure to North American strains of Porcine Epidemic Diarrhea Virus. BMC Vet Res. 2015:1:180. doi:10.1186/s12917-015-0500-z.
10. Lin C, Gao X, Oka T, Vlasova AN, Esseili MA, Wang Q, Saif LJ. Antigenic relationships among porcine epidemic diarrhea virus and transmissible gastroenteritis virus strains. J Virol. 2015;89:3332–3342. doi:10.1128/JVI.03196-14.
11. Kuldeep S, Chatth KS, Roth JA, Saif LJ. Strategies for design and application of enteric viral vaccines. Annl Rev Animal Bios. 2015:3:375–395. doi:10.1146/annurev-animal-022114-111038.
12. Curtis J, Bourne FJ. Immunoglobulin quantitation in sow serum, colostrum and milk and the serum of young pigs. Biochimica et Biophysica Acta (BBA)-Protein Structure. 1971;236:319–332.
*13. Bourne FJ, Curtis J. The transfer of immunoglobulins IgG, IgA and IgM from serum to colostrum and milk in the sow [abstract]. Immunology. 1973;24:157.
14. Gerber PF, Gong Q, Huang Y-W, Holtkamp D, Opriessnig T. Detection of antibodies against porcine epidemic diarrhea virus in serum and colostrum by indirect ELISA. Vet J. 2014;202:33–36.
15. University of Minnesota College of Veterinary Medicine. Swine Health Monitoring Project: Time to stability for PED virus in SHMP sow herds. 2014. Available at http://www.cvm.umn.edu/sdec/prod/groups/cvm/@pub/@cvm/@sdec/ documents/content/cvm_content_498631.pdf. Accessed 24 January 2016.
* Non-referred reference.
