| Case report | Peer reviewed |
Cite as: Sturos MJ, Robbins RC, Moreno R, et al. Narasin toxicosis in finishing pigs. J Swine Health Prod. 2016;24(4):205–211.
Also available as a PDF.
SummaryThis case report documents a clinical case of narasin toxicosis in a group of 19-week-old finisher pigs caused by accidental overdose of narasin in the feed at concentrations varying from 139 ppm (139 g per tonne) to 645 ppm (645 g per tonne). Affected pigs exhibited anorexia, pain (vocalization), skeletal muscle weakness, ataxia, recumbency, and dyspnea. Pathological lesions in the affected pigs examined were primarily restricted to skeletal muscle degeneration and necrosis. Skeletal muscles that were most severely affected included the diaphragm and outer muscularis layer of the proximal esophagus. Of the 3000 exposed pigs, 86 pigs died and 415 pigs were euthanized for animal welfare reasons. The overdose was caused by a broken load cell allowing undetected continuous leakage of narasin within the micro-ingredient batching system at the feed mill. Corrective action was implemented at the feed mill to prevent further episodes. | ResumenEste reporte documenta un caso clínico de toxicosis de narasina en un grupo de cerdos de finalización de 19 semanas de edad causada por una sobredosis accidental de narasina en el alimento en concentraciones variando de 139 ppm (139 gr por tonelada) a 645 ppm (645 gr por tonelada). Los cerdos afectados presentaron anorexia, dolor (vocalización), debilidad musculo esquelética, ataxia, recumbencia, y disnea. Las lesiones patológicas en los cerdos afectados examinados fueron principalmente restringidas a degeneración musculo esquelética y necrosis. Los músculos esqueléticos que fueron afectados más severamente incluyeron el diafragma y la capa muscular externa del esófago proximal. De los 3000 cerdos expuestos, 86 cerdos murieron y 415 cerdos fueron sacrificados por razones de bienestar animal. La sobredosis fue causada por una fuga continua, no detectada, de narasina dentro del sistema de procesamiento de micro ingredientes en el molino de alimento debido a un compartimiento de descarga roto. Se implementó una acción correctiva en el molino de alimento para prevenir episodios futuros. | ResuméCe rapport de cas fait état d’un cas clinique d’intoxication au narasin chez un groupe de porcs en finition âgés de 19 semaines causée par une surdose de narasin dans l’alimentation à des concentrations variant de 139 ppm (139 g par tonne) à 645 ppm (645 g par tonne). Les porcs affectés présentaient de l’anorexie, de la douleur (vocalisation), faiblesse des muscles squelettiques, ataxie, décubitus, et dyspnée. Les lésions pathologiques chez les porcs affectés examinés étaient principalement limitées à de la dégénérescence des muscles squelettiques et de la nécrose. Les muscles squelettiques les plus sévèrement affectés incluaient le diaphragme et la musculeuse externe de l’œsophage proximal. Sur les 3000 porcs exposés, 86 sont morts et 415 ont été euthanasiés pour des raisons de bien-être animal. La surdose a été causée par un bris d’une cellule de chargement ce qui a entrainé une fuite continue non-détectée de narasin à l’intérieur du système de mélange des micro-ingrédients à la meunerie. Une action corrective a été mise en place à la meunerie afin de prévenir de nouveaux épisodes. |
Keywords: swine, narasin, toxicity, finisher pigs
Search the AASV web site
for pages with similar keywords.
Received: October 28, 2015
Accepted: February 2, 2016
Ionophores are compounds that are capable of transporting charged molecules across biological membranes and have diverse uses in both human and veterinary medicine, to include artificial activation of human oocytes clinically,1 inhibitors of human cancer stem cells experimentally,2 well-established anticoccidial agents in poultry,3 and approved for increased weight gain and feed efficiency in swine in Canada (Monteban 70; Elanco, Division of Eli Lilly Canada, Guelph, Ontario, Canada) and the United States (Skycis 100; Elanco Animal Health, Division of Eli Lilly and Company, Indianapolis, Indiana). Polyether ionophore antibiotics frequently used in veterinary medicine, such as monensin, lasalocid, salinomycin, and narasin, have the potential to cause toxicosis either by administration at levels above the recommended safe dosages4 or by concurrent administration with known potentiating agents such as tiamulin.5 Narasin is a monovalent polyether carboxylic ionophore antibiotic which is produced by a strain of the bacteria Streptomyces aureofaciens.6,7 Narasin is currently used in swine in the United States as a feed additive, with label indications of increased rate of weight gain and improved feed efficiency in growing-finishing swine when fed at 15 g per tonne to 30 g per tonne for at least 4 weeks.8 Narasin toxicosis in swine is a syndrome similar to that described for monensin and other ionophores, which is clinically characterized by anorexia, diarrhea, respiratory distress, ataxia, muscle weakness, lethargy, recumbency, and death.9-12 These clinical signs are not pathognomonic for ionophore toxicosis and may be confused with some acute infectious diseases (eg, disease caused by Streptococcus suis, Hemophilus parasuis, or F18-expressing Escherichia coli), selenium toxicosis, or water deprivation in older pigs. Gross lesions vary from none to pale pink, red, or white areas in either skeletal or cardiac muscle or both, and in cases of cardiac involvement, there have been reports of epicardial hemorrhage, pulmonary edema, pulmonary congestion, hydrothorax, and hydroabdomen. The defining microscopic feature is necrosis of striated muscle. Differential diagnoses to be considered with these gross and histological findings in either skeletal muscle or heart or both should also include gossypol toxicosis, nutritional myopathies, and porcine stress syndrome.4
Previously published reports of accidental narasin toxicosis in swine involve either contamination of pig feeds from unknown sources,11 contamination of pig feeds in a facility also processing poultry feeds,10 and contamination of pig feed containing tiamulin with narasin labeled for swine.13 To the knowledge of the authors, this report describes the first documented case of narasin toxicosis in finishing pigs associated with inadvertent overdose of the ration with narasin labeled for swine, but not associated with concurrent administration of ionophore potentiators.
Herd description
This facility was under veterinary care and certified by Pork Quality Assurance (PQA; National Pork Board). The case herd was an 8000-finisher farm in the Oklahoma panhandle, composed of eight 1000-finisher tunnel-ventilated, curtain-sided barns, with 48 pens per barn. In July 2014, each barn housed 1000 nineteen-week-old, mixed-breed finisher pigs. Pigs were multi-sourced by site, single-sourced by barn, and managed all-in, all-out. In each pen, ad-libitum feed was available in a round feeder, with ad-libitum water available through water nipples mounted on a dual-head swinging water pipe. The pigs and their environment were monitored daily by caretakers.
Pigs were assigned a feed budget that consisted of six finishing rations formulated to meet or exceed the nutritional recommendations reported by the National Research Council.14 The second finishing ration consisted of a corn and soybean-meal base containing 30 g per tonne narasin (Skycis 100; Elanco Animal Health). Pigs were fed 56.8 kg each of the second ration, which would last approximately 4 weeks.
Case description
At the case site, each of the eight barns received a delivery of the second finishing ration on July 17, 2014. It was estimated that pigs in Barn 3 started consuming the overdosed feed around July 25. On July 27, caretakers observed pigs nosing feed out of their feeders. At this time, caretakers started the barn on water-soluble potassium penicillin. On July 28, the caretaker contacted production management to report that the prevalence of gaunt pigs in Barn 3 had increased and now barns 4 and 5 were affected. In addition, the caretaker had noted pigs with signs of dyspnea and ataxia in each barn. Management discontinued administration of potassium penicillin in favor of water-soluble oxytetracycline and liquid aspirin for treatment of suspected pneumonia. Individual pigs exhibiting clinical signs of pneumonia were treated with injectable enrofloxacin (Baytril 100; Bayer HealthCare LLC, Animal Health Division, Shawnee Mission, Kansas). On July 28, two hundred and forty-nine pigs in Barn 3 and 95 pigs in Barn 4 were individually treated for suspected pneumonia. On July 29, the caretaker reported no improvement, and the production manager visited the farm and reported that over 50% of pigs in each barn appeared gaunt, ataxic, or dyspneic, or had tremors.
On July 29, the site veterinarian visited the farm and found clinical signs were confined to three barns, but varied in severity among barns. Barn 3 was most severely affected, with 20% of pigs dog-sitting or in lateral recumbency, while 10.0% and 8.5% of pigs were affected in barns 4 and 5, respectively. The veterinarian described a rapid onset of tremors and incoordination coinciding with vocalization that subsided when an affected pig sat back on its haunches or became recumbent. Affected pigs remained alert and responsive. Paddling, nystagmus, or other neurologic signs were not observed. In addition, all pigs that had received an enrofloxacin injection the day prior had a scab 2.5 cm to 4 cm in diameter at the site of injection. Because clinical signs were consistent with prior reports of ionophore toxicosis, the feed was removed from the affected barns and associated bins that day, and all antibiotic therapy was discontinued.
On July 30, the veterinarian reported improvement in the prevalence of affected pigs: 11.0%, 6.0%, and 2.5% in barns 3, 4, and 5, respectively. However, mortality increased over the next 7 days. In total, 86 pigs died and 415 pigs were euthanized when they became non-ambulatory, in accordance with the producer’s animal welfare policy.
Laboratory findings
Two 19-week-old pigs (pigs #1 and #2) exhibiting ataxia and muscle tremors were euthanized on July 29, two days after the onset of clinical signs, and gross examination by the herd veterinarian found mild pneumonia and empty stomachs, but no other gross lesions or effusions. Feed samples, as well as fresh and formalin-fixed tissues including lung, liver, spleen, kidney, heart (ventricle), tonsil, lymph node, skeletal muscle, tongue, small intestine, and colon, were submitted to the University of Minnesota Veterinary Diagnostic Laboratory (UMN VDL, St Paul, Minnesota) for additional testing. Differential diagnoses included water deprivation, ionophore toxicosis, or unusual presentations of Streptococcus suis, Hemophilus parasuis, or Shiga-toxin-producing F18 E coli infections.
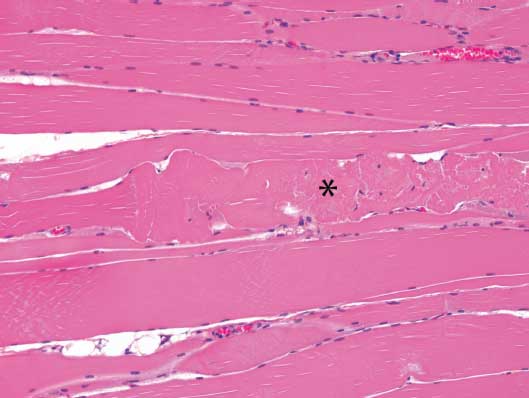
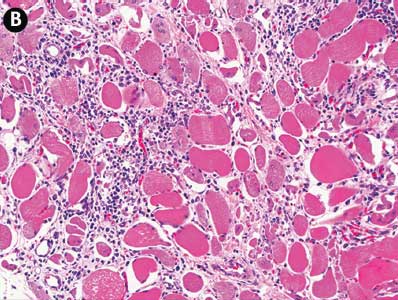
Microscopic examination of tissues revealed marked acute segmental skeletal muscle necrosis characterized by swollen myofibers with loss of cross-striations and myofibril distinction, homogeneous pale to deeply eosinophilic material (hyaline degeneration) or amorphous flocculent eosinophilic material replacing the normal sarcoplasmic tissue, and shrunken pyknotic nuclei at the periphery of the myofibers (Figure 1). In some areas, there was formation of linear bands of eosinophilic material (contraction bands) within the affected fibers. Necrotic myofibers were frequently clustered, and clusters of necrotic fibers were distributed throughout the muscle sections, often near the center of fascicles. In some sections, small numbers of macrophages infiltrated the affected fibers as well as the adjacent endomysium. All skeletal muscles submitted were affected, with the exception of the tongue, but the sectional area affected varied from 10% to 50% between sections. The submitted ventricular myocardium was unaffected. There were no significant microscopic lesions in other tissues.
Figure 1: Tissues from nine finisher pigs were examined histologically as part of the diagnostic investigation of a case of narasin toxicosis in finishing pigs. Pig #1, skeletal muscle, acute ionophore toxicosis. There is segmental acute degeneration and necrosis of a skeletal muscle fiber in the center of the image (*). Section stained with hematoxylin and eosin; ×10 magnification.
Liver mineral values were analyzed by inductively coupled plasma mass spectrometry at Michigan State University Diagnostic Center for Population and Animal Health (MSU DCPAH; Lansing, Michigan) and were within normal limits. Liver samples tested by gas chromatography mass spectrometry performed at MSU DCPAH were negative for toxic organic compounds. Molecular diagnostics performed at UMN VDL were positive for porcine reproductive and respiratory syndrome virus (PRRSV) and Mycoplasma hyorhinis, and negative for porcine circovirus type 2, influenza virus, transmissible gastroenteritis virus, porcine epidemic diarrhea virus, swine delta coronavirus, and Mycoplasma hyopneumoniae.
Feed analysis at Covance Incorporated (Covance, Greenfield, Indiana) and MSU DCPAH indicated that the samples tested were positive for narasin. Covance analysis by liquid chromatography and post-column derivatization detected narasin concentrations of 645, 279, and 242 g per tonne in feed samples obtained from barns 3, 4, and 5, respectively. These samples were 21.5, 9.3, and 8.0 times higher than the expected as-fed, labeled concentration. The narasin-positive feed sample analyzed by MSU DCPAH was quantified by Iowa State University Veterinary Diagnostic Laboratory (ISU VDL; Ames, Iowa) by liquid chromatography and post-column derivatization; narasin was detected at 139 g per tonne, which was 4.6 times higher than the labeled concentration. The findings of marked necrosis of skeletal muscles and demonstration of elevated levels of narasin in the feed were consistent with the clinical diagnosis of suspected ionophore toxicosis.
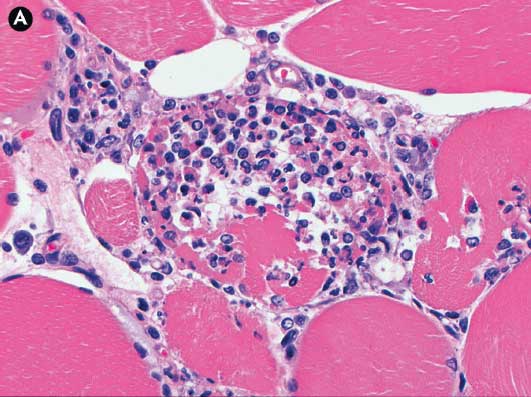
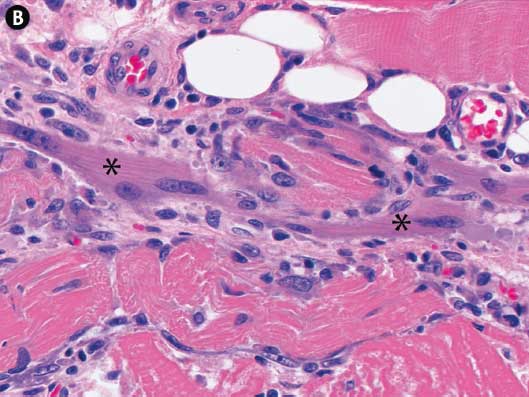
On August 4, eight days after the onset of clinical signs, one 18-week-old pig (Pig #3) with clinical signs of ionophore toxicosis and a skin reaction at the site of the enrofloxacin injection was euthanized, and tissues were submitted to UMN VDL for testing. No gross lesions were noted at necropsy, other than the focal circular erythematous region around the injection site. Tissues submitted included skeletal muscle (loin [m longissimus lumborum], ham [m semimembranosus], tongue [m lingualis proprius]), heart (ventricle), and skin with subcutis. Microscopic evaluation revealed minimal acute skeletal muscle necrosis with numerous regenerating myofibers (small myofibers with increased sarcoplasmic basophilia, indistinct or absent cross-striations, and rows of centrally placed, large vesiculate nuclei) and moderate infiltration by macrophages, eosinophils, and non-degenerate neutrophils in response to the necrotic tissue (Figure 2). The tongue and ventricular myocardium were unaffected. Within the erythematous tissues from the injection site there was moderate localized acute inflammation in the dermis, subcutis, and muscles surrounding a central area of coagulative necrosis (loss of cellular detail with preservation of cellular outlines) in the superficial muscle. Bacteria were not observed within the affected tissues.
Figure 2: Case described in Figure 1. Pig #3, skeletal muscle, subacute ionophore toxicosis. Panel A: Macrophages are infiltrating necrotic muscle fibers and phagocytizing degenerate myofibrils. Panel B: Centrally there are regenerative muscle fibers (*) with large oval centrally placed nuclei and pale basophilic sarcoplasm. There is a normal muscle fiber in the upper right and variably degenerate muscle fibers in lower portions of image. All sections stained with hematoxylin and eosin; ×20 magnification.
On August 7, eleven days after the onset of clinical signs, six 20-week-old pigs were submitted to assess the progression of the ionophore toxicosis. Three pigs (pigs #4, #5, and #6) which were clinically affected (off-feed, trembling, vocalizing), and three pigs (pigs #7, #8, and #9) from the same barn that were clinically unaffected, were euthanized and tissues harvested. At necropsy, all clinically affected pigs exhibited acute multifocal pneumonia. Clinically unaffected pigs exhibited no gross lesions at necropsy. Tissues submitted included formalin-fixed samples of the proximal esophagus, tongue, ham, shoulder (m deltoideus), loin, tenderloin (m psoas major), diaphragm, heart, spleen, kidney, small intestine, lung, liver, and colon in addition to serum and fresh samples of lung, liver, spleen, kidney, heart, small intestine, and colon.
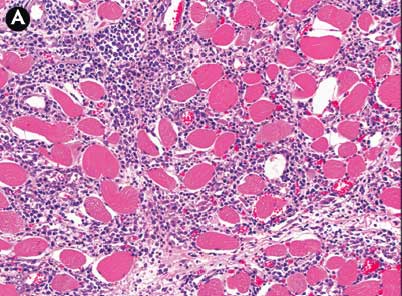
All pigs were positive for PRRSV by polymerase chain reaction. The three clinically affected pigs had mild, multifocal acute pneumonia histologically. Ongoing acute skeletal muscle necrosis, characterized by amorphous to clumped eosinophilic material within the sarcoplasm, was minimal. All pigs had evidence of skeletal muscle regeneration with mild fibrosis in some areas. The clinically affected pigs also had moderate to large numbers of macrophages and small numbers of eosinophils and lymphocytes within the endomysium of some sections.
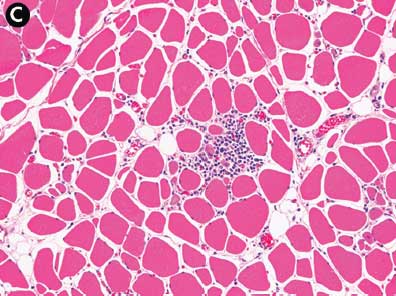
The severity of lesions varied among pigs and among muscles within the same pig. The severity and extent of muscle lesions in the clinically affected pigs was much greater than in the non-clinically affected pigs. Within the same pig, the severity of lesions between muscles followed the pattern of proximal esophagus (outer muscularis) ≥ diaphragm > shoulder > ham > tenderloin > loin (Figure 3). The tongue, inner muscularis of the proximal esophagus, and ventricular myocardium were unaffected.
Figure 3: Case described in Figure 1. Pig #5. Skeletal muscle, subacute ionophore toxicosis. There is wide variation in the severity of lesions between skeletal muscle groups within the same pig. Panel A: esophagus, outer muscularis layer. Extensive infiltration of macrophages within affected myofibers and the endomysium. Panel B: diaphragm. Moderate infiltration by macrophages with occasional regenerative fibers. Panel C: ham. Mild infiltration by macrophages with numerous small regenerative fibers. Panel D: loin. Scant macrophage infiltrates with numerous small regenerative fibers. All sections stained with hematoxylin and eosin; ×10 magnification.

Serum creatinine kinase (CK) concentrations were markedly elevated in clinically affected pigs (10,624 U per L, 20,899 U per L, and 6565 U per L in pigs #4, 5, and 6, respectively; institutional reference interval 24 to 225 U per L), and moderately elevated in non-clinically affected pigs (2270 U per L, 9567 U per L, and 3622 U per L in pigs #7, #8, and #9, respectively).
Serum aspartate transferase (AST) concentrations in clinically affected pigs were 361 U per L, 586 U per L, and 391 U per L for pigs #4, #5, and #6, respectively; reference interval 32 to 84 U per L). Serum AST concentrations in non-clinically affected pigs were 34 U per L, 174 U per L, and 110 U per L for pigs #7, #8, and #9, respectively.
Serum potassium was markedly elevated in clinically affected pigs (12.6 mmol per L, 11.9 mmol per L, and 9.4 mmol per L for pigs #4, #5, and #6, respectively; reference interval 4.4 to 6.7 mmol per L), and within the reference intervals for all non-clinically affected pigs.
In one non-clinically affected pig (pig #8), there was also a mild elevation in serum sorbitol dehydrogenase (9 U per L; reference interval 1 to 6 U per L). There were no other significant changes in serum chemistry or electrolyte values for any of the tested pigs.
Treatment and outcome
Feed was immediately removed from affected barns’ feeders and associated feed bins and disposed of. Non-medicated corn and soybean meal diets were fed throughout the remaining growing-finishing period. Immediately before the pigs had access to the overdosed feed, the average daily feed intake (ADFI) of barns 3, 4, and 5 had been 1.95 kg per day. During the time that the pigs had access to the overdosed feed, ADFI decreased to 0.72 kg per day. At the end of the growing-finishing period, pigs from barns 3, 4, and 5 were on average 4.0 kg lighter and had higher feed conversion (feed-to-gain ratio 2.4 versus 2.8) than pigs from barns 1, 2, 6, and 7. No water-soluble or injectable antibiotics were subsequently administered to pigs in barns that received the overdosed feed. Within 7 days of removing the contaminated feed, no new pigs were being found with clinical signs consistent with ionophore toxicosis, and 14 days after feed removal all pigs appeared clinically normal. In consultation with the Food Animal Residue Avoidance Databank (FARAD) and in light of the resolution of muscle lesions and absence of clinical signs, the three barns were marketed 9 weeks later. Of the 3000 exposed pigs, 86 pigs died and 415 pigs were euthanized for animal welfare reasons. Following an investigation, the manager of the farm-owned feed mill determined that the problem arose in the micro-ingredient batching system, which resulted in the dosing error. The affected portion of the micro-ingredient batching system was dismantled and inspected, revealing that a broken load cell allowed product to continuously leak from the bin without detection. The load cell was repaired and, as an additional precaution, the producer immediately discontinued use of narasin in the affected mill.
Discussion
Multiple ionophores are available and labeled for use in animal feed, and these compounds are different in several ways, including the spectrum of molecules transported, the relative affinity for the ions transported, the comparative toxicity between ionophores, and the target organs affected between species. Monovalent carboxylic ionophores such as monensin and narasin are capable of transporting monovalent cations such as sodium (Na+) and potassium (K+) through biological membranes, including cell membranes and mitochondrial membranes, but do not directly transport divalent cations such as calcium (Ca++). Other ionophores, such as lasalocid, are capable of directly transporting Ca++. Ionophores also have differing affinities within the spectrum of ions transported; for example, narasin has been reported to preferentially transport K+ over Na+, while monensin preferentially transports Na+ over K+.15,16 Each ionophore has varying toxicity, depending on the dosage at which no observable adverse effects occur, and the general relative toxicity between selected ionophores has been reported to be salinomycin < lasalocid ≤ narasin = monensin < maduramicin.17 In general, striated muscles (skeletal and cardiac) are the target organs of ionophore toxicity, but between species the severity of skeletal versus cardiac muscle necrosis is variable. Horses are reported to have greater involvement of cardiac muscle with little skeletal muscle involvement; dogs and pigs are reported to have greater involvement of the skeletal muscle; and cattle, poultry, and rodents have equivalent involvement of skeletal and cardiac muscle.4
The definitive mechanism of ionophore toxicity has not been identified, but appears to converge on increased free cytoplasmic calcium concentrations which activate cellular proteases, phospholipases, and caspases, as well as increasing mitochondrial permeability, resulting in cellular degradation, energy depletion, and cell death.12,18 Free cytosolic calcium is also important in contraction of skeletal and cardiac muscle, which would hasten energy depletion.
Definitive diagnosis of ionophore toxicity requires demonstration of the presence of the compound in feed at an unsafe concentration for the species exposed, expression of clinical signs in the exposed animals, and demonstration of compatible gross and histopathologic lesions.4 In this case, multiple samples of feed were quantitatively analyzed by two different laboratories (ISU VDL and Covance), with all samples markedly elevated beyond the recommended concentration. The marked difference in quantitative values demonstrates the need to collect multiple representative feed samples for toxicology testing, because the distribution of additives in feed may not be homogeneous. Safety studies in pigs demonstrated no adverse effects when narasin was administered at 45 g per tonne,8 which is 1.5 times the recommended concentration. Pigs have been reported to show clinical signs (anorexia, dyspnea, depression, leg weakness, ataxia, and recumbency) when administered narasin at 82.5 and 137.5 g per tonne, or 2.8 and 4.6 times the recommended dosage, respectively.13 The reported median lethal dose in 9-week-old pigs for a single oral dose is 8.9 mg per kg of body weight.13 The estimated intake of narasin by pigs in this case was 1.8 to 8.6 mg per kg of body weight per day, determined by barn feed intake, narasin concentrations demonstrated in the feed, and an estimated average body weight of 52 kg.
Spontaneous narasin toxicosis has been previously reported in several species, which include pigs in South Africa,10 Brazil,11 and Canada,13 rabbits in Brazil,19 and dromedary camels.20 Follow-up experimental reproduction of narasin toxicosis was performed and included in the published rabbit case and one published pig case report.11,19 The pathological investigation in this case demonstrated extensive skeletal muscle involvement without ventricular myocardial involvement, which is similar to the spontaneous toxicity reported in conjunction with tiamulin in growing pigs in Canada13 and in rabbits in Brazil,19 as well as the experimentally induced narasin toxicosis in rabbits19 and pigs11 in Brazil. Interestingly, this is also similar to the reported findings in experimentally induced monensin toxicosis in pigs, in which there was extensive skeletal muscle involvement, but necrosis in the heart was inconsistent and affected only the atria (left atrium more frequently than the right).9 In the cases of spontaneous narasin toxicosis in South African10 and Brazilian pigs,11 myocardial necrosis was reported in the ventricles and unspecified anatomical locations, respectively. The cause of the differences in the presence and distribution of cardiac involvement reported in spontaneous toxicoses, experimental toxicoses, and this case is unknown, but may be related to dose ingested, duration of access to contaminated feed, or other factors. Distribution of lesions in numerous skeletal muscles was reported for experimentally induced narasin toxicosis in Brazilian pigs, and the most severely affected muscle was reported to be the diaphragm, as in this case.
The area of cellulitis and dermatitis surrounding the locally extensive coagulative necrosis in pig #3 appears most consistent with a local ischemic event (eg, arterial infarction), which, given the location, was most likely related to the reported enrofloxacin injection. The relationship between the documented narasin toxicosis and the suspected injection-site reaction is unknown.
Implications
• Narasin toxicity should be considered in cases of sudden-onset anorexia or feed refusal with sudden death, painful or weak pigs, and muscle necrosis.
• Multiple samples of feed should be submitted for quantitative ionophore analysis and suspect feed should be replaced while test results are pending,
• Numerous skeletal muscle samples, including diaphragm and proximal esophagus, should be submitted for histopathologic evaluation in suspected cases. Pigs have been reported to occasionally have atrial-selective myocardial necrosis with ionophore toxicosis and both atrial and ventricular myocardium should be sampled.
• Serum CK, AST, and potassium concentrations are indirect measurements of muscle damage and may be useful in monitoring resolution of cases of ionophore toxicosis.
• Feed-mill management and maintenance are of utmost importance to ensure safe and accurate administration of feed additives or micronutrients with narrow safety margins.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
Conflict of interest
Dr Roman Moreno and Dr Rebecca C. Robbins are employed by Seaboard Foods, which owned the pigs described in this case report.
References
1. Ebner T, Montag M, on behalf of the Oocyte Activation Study Group. Live birth after artificial oocyte activation using a ready-to-use ionophore: A prospective multicentre study. Repro BioMed Online. 2015;30:359–365. doi:10.1016/j.rbmo.2014.11.012.
2. Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034.
3. Chapman HD, Jeffers TK, Williams RB. Forty years of monensin for the control of coccidiosis in poultry. Poult Sci. 2010;89:1788–1801. doi: 10.3382/ps.2010-00931.
4. Novilla MN. The veterinary importance of the toxic syndrome induced by ionophores. Vet Hum Tox. 1992;34:66–70.
5. Van Vleet JF, Runnels LJ, Cook JR Jr, Scheidt AB. Monensin toxicosis in swine: Potentiation by tiamulin administration and ameliorative effect of treatment with selenium and/or vitamin E. Am J Vet Res. 1987;48:1520–1524.
6. Berg DH, Hamill RL. The isolation and characterization of narasin, a new polyether antibiotic. J Antibiot. 1978;31:1–6.
7. Pressman BC, Fahim M. Pharmacology and toxicology of the monovalent carboxylic ionophores. Annu Rev Pharmacol Toxicol. 1982;22:465–490.
8. US Department of Health and Human Services, Food and Drug Administration. Freedom of information summary, Original new drug application, NADA 141-340, Skycis 100, Narasin. 2012. Available at www.fda.gov/downloads/animalveterinary/ products/approvedanimaldrugproducts/ foiadrugsummaries/ucm326896.pdf. Accessed 17 April 2016.
9. Van Vleet JF, Amstutz HE, Weirich WE, Rebar AH, Ferrans VJ. Clinical, clinicopathologic, and pathologic alterations of monensin toxicosis in swine. Am J Vet Res. 1983;44:1469–1475.
10. Van Halderen A, Bastianello SS, Fourie N, Zumpt IF. An outbreak of narasin poisoning in swine. J S Afr Vet Assoc. 1993;64:43–46.
11. Armién AG, Peixoto PV, Döbereiner J, Tokarnia CH. Surto de intoxicação por narasina em suínos [An outbreak of narasin poisoning in swine (in Portugese)]. Pesq Vet Bras [Braz Vet Res]. 1997;17:63–68.
12. Novilla MN. Ionophores. In: Gupta RC, ed. Veterinary Toxicology: Basic and Clinical Principles. 2nd ed. Waltham, Massachusetts: Academic Press; 2012:1281–1299.
13. Carpenter JA, Charbonneau G, Josephson G. Tiamulin and narasin toxicosis in nursery pigs. J Swine Health Prod. 2005;13:333–336.
14. Committee on Nutrient Requirements of Swine, Board on Agriculture and Natural Resources, Division on Earth and Life Studies, National Research Council. Nutrient Requirements of Swine. 11th rev ed. Washington, DC: National Academies Press; 2012.
15. Riddell FG, Arumugam S, Cox BG. The monensin-mediated transport of Na+ and K+ through phospholipid bilayers studied by 23Na- and 39K-NMR. Biochim Biophys Acta - Biomembranes. 1988;944:279–284.
16. Riddell FG, Tompsett SJ. The transport of Na+ and K+ ions through phospholipid bilayers mediated by the antibiotics salinomycin and narasin studied by 23Na- and 39K-NMR spectroscopy. Biochim Biophys Acta - Biomembranes. 1990;1024:193–197.
17. Oehme FW, Pickrell JA. An analysis of the chronic oral toxicity of polyether ionophore antibiotics in animals. Vet Hum Toxicol. 1999;41:251–257.
18. Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. Calcium in cell injury and death*. Annu Rev Pathol Mech Dis. 2006;1:405–434. doi: 10.1146/annurev.pathol.1.110304.100218.
19. Salles MS, Lombardo de Barros CS, Barros SS. Ionophore antibiotic (narasin) poisoning in rabbits. Vet Hum Toxicol. 1994;36:437–444.
20. Abu Damir H, Ali MA, Abbas T, Omer E, Al Fihail A. Narasin poisoning in the dromedary camel (Camelus dromedarius). Comp Clin Path. 2012:1–7.
