| Original research | Peer reviewed |
Cite as: Ježek J, Starič J, Nemec M, et al.. The influence of age, farm, and physiological status on pig hematological profiles. J Swine Health Prod. 2018;26(2):72–78.
Also available as a PDF.
SummaryObjectives: To evaluate influence of age, farm, and physiological status on pig hematological profiles. Materials and methods: This study was carried out on five 1-site, farrow-to-finish pig farms in Slovenia, where a total of 382 clinically normal pigs were sampled. All farms were free of Aujeszky’s disease (pseudorabies), classical swine fever, and porcine reproductive and respiratory syndrome. Blood samples were taken from the anterior vena cava. Hematological analyses were performed with an automated hematological analyser. The following hematological variables were measured: red blood cell count (RBC), white blood cell count (WBC), hematocrit (Hct), hemoglobin concentration (Hb), erythrocyte indices, and platelet count (PLT). Differential WBC counts were determined manually using stained smears. Results: The farms themselves influenced all of the investigated variables except RBC and WBC differential (ie, lymphocytes, monocytes, and band neutrophils). A trend of lower values of RBC, Hb, and Hct, higher WBC numbers, and a higher percentage of segmented granulocytes were observed in lactating sows when compared to pregnant sows. Age significantly influenced hematological values and differential WBC counts except basophils, monocytes, and band neutrophils. Values of mean corpuscular volume (MCV) increased with age, the highest values being found in sows. Numbers of WBC and PLT decreased with age, the lowest number being observed in sows. Implications: Hematological examination may be an important diagnostic tool in the assessment of pig health status, but to interpret the results properly, it is important to consider pig age, health history, and clinical data. | ResumenObjetivos: Evaluar la influencia de la edad, granja, y estado fisiológico en el perfil hematológico del cerdo. Materiales y métodos: Este estudio fue realizado en cinco granjas de un sitio, nacimiento a venta, en Eslovenia, donde se tomaron 382 muestras totales de cerdos clínicamente normales. Todas las granjas estaban libres de la enfermedad de Auyesky (pseudorabia), fiebre porcina clásica, y síndrome reproductivo y respiratorio porcino. Las muestras de sangre se tomaron de la vena cava anterior. Se realizaron análisis hematológicos con un analizador hematológico automatizado. Se midieron las siguientes variables hematológicas: conteo de glóbulos rojos (RBC por sus siglas en inglés), conteo de glóbulos blancos (WBC por sus siglas en inglés), hematocrito (Hct por sus siglas en inglés), concentración de hemoglobina (Hb por sus siglas en inglés) índices de eritrocitos, y conteo de plaquetas (PLT por sus siglas en inglés). Los conteos diferenciales de WBC se determinaron manualmente utilizando laminillas teñidas. Resultados: Las granjas mismas influenciaron todas las variables investigadas excepto el diferencial de RBC y WBC (ie, linfocitos, monocitos, y neutrófilos en banda). Se observó una tendencia de valores inferiores de RBC, Hb, Hct, con números más altos de WBC, y un porcentaje más alto de granulocitos segmentados en hembras lactantes al compararlos con los de hembras gestantes. La edad influyó significativamente los valores hematológicos y los conteos diferenciales de WBC excepto basófilos, monocitos y neutrófilos en banda. Los valores de volumen corpuscular medio (MCV) aumentaron con la edad, encontrándose los valores más altos en hembras. Los números de WBC y PLT disminuyeron con la edad, observándose el número más bajo en hembras. Implicaciones: El examen hematológico puede ser una herramienta de diagnóstico importante en la valoración del estado de salud del cerdo, pero para interpretar los resultados correctamente, es importante considerar la edad del cerdo, la historia de salud y los datos clínicos. | ResuméObjectifs: Évaluer l’influence de l’âge, de la ferme d’élevage, et du statut physiologique sur les profils hématologiques du porc. Matériels et méthodes: Cette étude a été menée sur cinq sites uniques de fermes porcines de type naisseur-finisseur en Slovénie, où un total de 382 porcs cliniquement normaux ont été échantillonnés. Toutes les fermes étaient exemptes de pseudorage, de peste porcine classique, et du syndrome reproducteur et respiratoire porcin. Des échantillons sanguins ont été pris dans la veine cave antérieure. Les analyses hématologiques ont été réalisées à l’aide d’un analyseur hématologique automatisé. Les variables hématologiques suivantes ont été mesurées: comptage des globules rouges (GR), comptage des globules blancs (GB), hématocrite (Hct), concentration en hémoglobine (Hb), indices érythrocytaires, et comptage des plaquettes (PLT). Des dénombrements différentiels des GB ont été réalisés manuellement sur des frottis colorés. Résultats: Les fermes elles-mêmes influençaient toutes les variables étudiées sauf les GR et les différentiels des GB (ie, lymphocytes, monocytes et neutrophiles immatures). Une tendance à des valeurs inférieures pour les GR, Hb, et Hct, et un nombre plus élevé de GB, et un pourcentage plus grand de granulocytes segmentés ont été observés chez les truies en lactation comparativement aux truies gestantes. L’âge influençait de manière significative les valeurs hématologiques et les comptes différentiels de GB sauf pour les basophiles, les monocytes et neutrophiles immatures. Les valeurs du volume corpusculaire moyen (VCM) augmentaient avec l’âge, les valeurs les plus élevées étant retrouvées chez les truies. Le nombre de GB et de PLT diminuait avec l’âge, les valeurs les plus basses étant observées chez les truies. Implications: L’examen hématologique peut être un outil diagnostique important dans l’évaluation de l’état de santé des porcs, mais afin d’interpréter les résultats de manière adéquate, il est important de considérer l’âge des porcs, l’historique de santé, et les données cliniques. |
Keywords: swine, pig, hematology, pig health status
Search the AASV web site
for pages with similar keywords.
Received: June 22, 2017
Accepted: October 20, 2017
Despite the common availability of hematological tests in veterinary medicine, they are still rarely performed in the routine evaluation of pig health status. Low individual animal intrinsic value, blood sample collection difficulty, different husbandry techniques, large variations between hematologic values in a given population, and the reality that reference value ranges are relatively wide are the main reasons for the low utility of swine hematology.1 Measuring animal hematological parameters can provide important information on animal health and is a practical tool for assessing pathological conditions in individual animals and for monitoring the health status of groups of animals. Additionally, hematological values reflect the response of the animal to its environment and may reveal adverse conditions, even though animals may not be displaying clinical signs of disease.2
A variety of factors should to be taken into account when evaluating individual animal results, collating data, and drawing whole herd or population conclusions. The interpretation of hematological data is often limited pursuant to the broad animal-to-animal variations occurring in normal populations. Friendship et al3 placed emphasis on the division of animals in different production groups, as some parameters vary greatly, even on a daily basis. Furthermore, gender, breed, and stage of gestation are factors influencing variability of blood values. However, very few recent studies have investigated the influence of age,4,5 reproductive status (gestating versus lactating sows),6-8 or individual farm effects9 in relation to pig hematological parameters.
Diet and disease are important external influencers of certain hematological values. An increase in tannic acid concentration (125 mg to 1000 mg per kg) in the diet of weanling pigs linearly reduced total red blood cell count (RBC), hemoglobin (Hb), and hematocrit (Hct) on days 21 and 28 of treatment.10 The long-term dietary use of clinoptiolite-rich tuff at the inclusion rate of 2% was not associated with adverse effects on growing and finishing pigs’ overall condition and health status or significant changes of their hematological profiles.11 Infectious agent health challenge often results in changes in white blood cell count (WBC), as well as differences in RBC and plasma parameters depending on temporal occurrence, severity of clinical signs, and magnitude of immune response. Increased sedimentation rate, as well as decreased Hb and Hct values, are most commonly present1,12 with disease challenges. Anemia is a cardinal clinical sign of Mycoplasma suis infection in pigs.13 Mycoplasma suis blood loads had significant negative correlation with RBC, Hct, and Hb.14 Hematological alterations appear in bacterial septic response and viral diseases. Leukocytosis, a reflection of the initial inflammatory response primarily mediated by neutrophils, was evident 12 hours after induction of septic injury of the abdomen in female pigs (27 to 37 kg), and was followed by a steady decrease.15 The number and percentage of neutrophils were both twofold higher in septic pigs 24 hours after Escherichia coli injection than their own basal values on day 0.16 Porcine reproductive and respiratory syndrome virus (PRRSV)-positive young pigs (age 28 to 160 days) had significantly lower WBC than PRRSV-negative pigs.17 Unthrifty nursery pigs had higher Hb concentrations and Hct values when compared to those of healthy pigs, indicating dehydration and (or) malnourishment in unthrifty pigs.18 In pigs aged 22 to 26 days fasted for 72 hours, Hct values started to increase at the onset of the fasting period and continued to increase (33.5% to 40.1%) throughout the fasting period.19 In diseased pigs, appetite and water intake are often decreased, and with diarrhea they lose more fluid, which may contribute to hemoconcentration.
The aim of this study was to analyze the blood samples of pigs from five small one-site farms to evaluate influence of age, farm, and physiological status (pregnant versus lactating sows) on the hematological profiles to acquire orientational reference values for different categories of pigs to then serve as an additional diagnostic tool in the assessment of pig health status.
Materials and methods
Farms and animals
The blood samples were taken from pigs on five small, one-site farms included in a serological study of selected pathogens. All procedures complied with the relevant Slovenian legislation (Animal Protection Act, Official Gazette of the republic of Slovenia, No 43/2007).
The study involved five small one-site farrow-to-finish pig farms in Slovenia between January 2014 and September 2014. All farms were free of pseudorabies, classical swine fever, and porcine reproductive and respiratory syndrome (PRRS). Age groups were established (Table 1) and animals from each farm were sampled. A total of 382 blood samples were taken. All pigs included in the study were clinically healthy at the time of blood sampling.
Table 1: Number of blood samples taken per age group from each of five 1-site, farrow-to-finish Slovenia farms
| Age group/farm | Sows | Boars | Growers (age 7-14 weeks) | Finishers | Total/farm |
|---|---|---|---|---|---|
| 1 | 42 | 1 | 10 | 7 | 60 |
| 2 | 39 | 1 | 15 | 5 | 60 |
| 3 | 45 | 3 | 10 | 9 | 67 |
| 4 | 61 | 1 | 20 | 5 | 87 |
| 5 | 92 | 1 | 10 | 5 | 108 |
| Total/age group | 279 | 7 | 65 | 31 | 382 |
Previous treatment
The pigs from all five farms were vaccinated against Mycoplasma hyopneumoniae. Additionally, sows from Farm 1 were vaccinated against atrophic rhinitis, weaners from Farm 2 were vaccinated against porcine circovirus 2 (PCV2), sows from Farm 4 were vaccinated against E coli, and sows and weaners from Farm 5 were vaccinated against E coli and PCV2, respectively. Treatments for endo- and ectoparasites were performed on all sows on the 100th day of pregnancy, as well as on all growing pigs at approximately 25 to 30 kg of body weight. Group samples of feces were collected on each farm and examined for internal parasites using flotation and sedimentation methods; low, clinically insignificant numbers of the protozoan Balantidium coli were found in all five group samples of feces.
Nutrition
Each of the five farm owners provided information about the feeding regimes on their farm, including feeding intervals, composition of category-dependent diets, and feed consumption. Breeding animals were fed twice daily, between 6 and 7 am and 4 and 7 pm in the afternoon-evening, and growers and finishers were fed ad libitum. Diets were composed of corn, barley, wheat, and soybeans, supplemented with complementary feed and mineral and vitamin mixtures according to NRC20 category recommendations. No other additives (eg, therapeutics or nostrums) were added to the feed on any of the five farms.
Blood sample collection
All blood samples were collected from the anterior vena cava; animals were restrained using wire-noose snares. Blood samples for hematological analysis were collected in evacuated blood collection tubes containing the anticoagulant K3EDTA; the tubes were gently vibrated by hand for 30 seconds to assure the proper contact and mixing of the blood and anticoagulant. Blood samples were collected at 9 am on all farms, approximately 2 hours after the morning feeding. One blood sample was obtained from every animal in this study.
Hematological analysis
All hematological analyses were performed on the day of sampling, utilising the Scil Vet abc Plus (Horiba, Japan) automated hematological analyzer. The following hematological variables were measured: RBC, WBC, Hct, Hb, and erythrocyte indices (mean corpuscular volume [MCV], mean corpuscular hemoglobin [MCH], mean corpuscular hemoglobin concentration [MCHC]), and platelet count (PLT). Differential WBC was determined according to the standard procedure: smears stained with Hemacolor (Merck, Darmstadt, Germany) and manually counted via microscopic examination. The laboratory where the analyses were performed participates in the Randox International Quality Assessment Scheme (RIQAS) hematology program.
Statistical analysis
Statistical analysis of hematological data was performed using the SPSS software package (SPSS 22.0 for Windows, Chicago, Illinois). For hematologic variables, descriptive statistics were calculated with regard to farm, physiological status, and age. Data were checked for normality, and reference intervals were calculated in accordance with Farver.21 Reference values are presented as mean (median) values, and the range between the 2.5th and 97.5th percentiles. Ninety-five percent confidence intervals were not used because individual pig data were not normally distributed. Physiological state and farm effects were assessed using the GLM variance analysis procedure, with physiological state and farm being fixed factors, in accordance with the following model:
Yij = μ + Fi + Sj + eij
where F means effect of herd (herd 1/…/ herd 5) and S means physiological state (pregnant or lactating).
The effect of age was assessed using GLM variance analysis procedure in a separate model, which only includes the influence of age on hematological variable values,
Yi = μ + Vi + ei
where V means effect of age (breeding sows or finishers or growers [11 to 14 weeks] or weaners [7 to 10 weeks]).
The level of significance was set at P < .05.
Results
Table 2 presents sow hematological values from all farms and a comparison with reference values from the existing literature.
Table 2: Hematological values for sows (n = 272) from five Slovenian farrow-to-finish pig farms and a comparison with reference values from the existing literature
| Parameter (unit) | Mean (median) | Range* | Reference values |
|---|---|---|---|
| RBC (1012/L) | 5.62 (5.63) | 4.3-7.0 | 5.8-8.122 5.0-8.01,23 |
| WBC (109/L) | 15.21 (14.90) | 9.78-22.67 | 10.0-20.023 10-2222 11.0-22.01 |
| Hb (g/dL) | 11.6 (11.5) | 9.3-13.8 | 10.0-15.523 10.8-14.822 10.0-16.01 |
| Hct (%) | 34.9 (34.6) | 27.1-42.9 | 32-4723 33-4522 32-501 |
| MCV (fL) | 62.2 (62.0) | 56.0-68.0 | 50-681,23 50-6522 |
| MCH (pg) | 20.6 (20.6) | 18.4-23.2 | 17-211,22,23 |
| MCHC (g/dL) | 33.2 (33.2) | 31.0-35.4 | 30-341,23 30-3522 |
| PLT (109/L) | 274 (273) | 134-429 | 250-60023 220-62022 325-7151 |
| Seg (%) | 48 (48) | 25-72 | 28-5223 10-3922 28-471 |
| Eos (%) | 7 (6) | 1-17 | 0.5-111 1-823 0-622 |
| Baso (%) | 0 (0) | 0-2 | 0-21,22,23 |
| Lymph (%) | 43 (43) | 23-66 | 40-6423 49-8522 39-621 |
| Mono (%) | 1 (1) | 0-5 | 2-823 0-522 2-101 |
| Band (%) | 0 (0) | 0-1 | 0-41,23 0-722 |
* 2.5th to 97.5th inter-percentile range.
RBC = red blood cell count; WBC = white blood cell count; Hb = hemoglobin; Hct = hematocrit; MCV = mean corpuscular volume; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; PLT = platelets; Seg = segmented neutrophils; Eos = eosinophils; Baso = basophils; Lymph = lymphocytes; Mono = monocytes; Band = band neutrophils
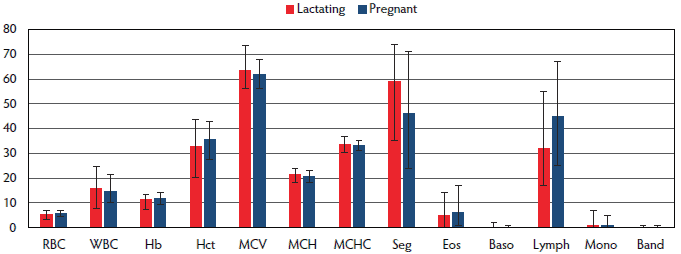
Figure 1 presents hematological values for pregnant (n = 224) and lactating (n = 48) sows. Lower RBC, Hb, and Hct values were observed in lactating sows when compared to pregnant sows. A higher number of WBC and a higher percentage of segmented granulocytes were found in lactating sows.
Figure 1: Hematological values of sows from five 1-site, farrow-to-finish pig farms in Slovenia are presented (median, range) in terms of physiological status. RBC = red blood cell count; WBC = white blood cell count; Hb = hemoglobin; Hct = hematocrit; MCV = mean corpuscular volume; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; PLT = platelets; Seg = segmented neutrophils; Eos = eosinophils; Baso = basophils; Lymph = lymphocytes; Mono = monocytes; Band = band neutrophils.
The results of hematological variables for sows at the herd level are presented in Table 3. Herd significantly influenced Hb, Hct, MCV, MCH, MCHC, PLT, and differential WBC values except lymphocytes, monocytes, and band neutrophils.
Table 3: Sow hematological values in terms of herd*
| Parameter | Farm 1 N = 39 | Farm 2 N = 39 | Farm 3 N = 45 | Farm 4 N = 61 | Farm 5 N = 88 | P† |
|---|---|---|---|---|---|---|
| Mean (median) Range | Mean (median) Range | Mean (median) Range | Mean (median) Range | Mean (median) Range | ||
| RBC (1012/L) | 5.76 (5.78) 4.49-7.15 | 5.93 (5.92) 4.39-6.99 | 5.40 (5.37) 3.20-6.62 | 5.56 (5.60) 3.52-7.36 | 5.60 (5.65) 3.74-7.03 | .102 |
| WBC (109/L) | 16.29 (16.24) 8.90-24.50 | 16.16 (15.80) 11.20-24.86 | 14.77 (14.10) 8.23-26.77 | 14.82 (14.20) 8.66-23.34 | 14.80 (14.75) 9.72-19.63 | .436 |
| Hb (g/dL) | 12.2 (12.5) 9.5-14.6 | 11.9 (12.0) 9.7-14.2 | 11.0 (10.8) 6.9-13.7 | 11.5 (11.4) 7.8-13.6 | 11.5 (11.6) 7.7-14.0 | .003 |
| Hct (%) | 36.6 (36.3) 27.8-44.1 | 37.9 (37.8) 30.0-44.8 | 33.2 (32.9) 20.0-42.3 | 34.0 (33.8) 22.5-41.4 | 34.4 (34.3) 21.9-42.3 | < .001 |
| MCV (fL) | 63.6 (63.0) 58.0-71.9 | 64.0 (63.0) 59.0-73.8 | 61.6 (62.0) 55.1-67.8 | 61.5 (62.0) 54.5-66.4 | 61.7 (61.0) 56.0-68.8 | < .001 |
| MCH (pg) | 21.2 (21.1) 19.2-23.9 | 20.2 (20.0) 18.4-23.0 | 20.4 (20.4) 18.1-23.2 | 20.8 (20.8) 18.2-23.2 | 20.7 (20.7) 18.3-23.3 | .012 |
| MCHC (g/dL) | 33.3 (33.2) 32.0-34.9 | 31.5 (31.5) 30.1-32.8 | 33.1 (33.0) 32.0-34.7 | 33.8 (33.6) 32.3-36.7 | 33.7 (33.5) 32.3-36.1 | < .001 |
| PLT (109/L) | 208 (210) 80-343 | 284 (294) 149-383 | 310 (316) 103-459 | 281 (282) 160-424 | 276 (268) 157-466 | < .001 |
| Seg (%) | 58 (59) 31-81 | 45 (46) 29-72 | 45 (44) 19-68 | 48 (49) 21-71 | 47 (46) 23-71 | .014 |
| Eos (%) | 5 (5) 0-10 | 7 (6) 1-18 | 9 (9) 3-22 | 7 (6) 0-17 | 6 (6) 1-14 | .005 |
| Baso (%) | 0 (0) 0-1 | 0 (0) 0-1 | 0 (0) 0-1 | 0 (0) 0-3 | 0 (0) 0-1 | .020 |
| Lymph (%) | 35 (35) 16-66 | 45 (48) 25-62 | 44 (44) 26-64 | 43 (44) 21-63 | 45 (45) 22-70 | .062 |
| Mono (%) | 1 (1) 0-7 | 2 (2) 0-6 | 2 (1) 0-6 | 1 (1) 0-4 | 1 (1) 0-5 | .065 |
| Band (%) | 0 (0) 0-1 | 0 (0) 0-1 | 0 (0) 0-1 | 0 (0) 0 | 0 (0) 0-1 | .370 |
* 2.5th to 97.5th inter-percentile range.
† Data were analyzed by general linear model (GLM) analysis of variance.
RBC = red blood cell count; WBC = white blood cell count; Hb = hemoglobin; Hct = hematocrit; MCV = mean corpuscular volume; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; PLT = platelets; Seg = segmented neutrophils; Eos = eosinophils; Baso = basophils; Lymph = lymphocytes; Mono = monocytes; Band = band neutrophils.
The hematological values and reference intervals of growers and finishers are presented in Table 4. Age significantly influenced values of RBC (P < .001), WBC (P < .001), Hb (P < .001), Hct (P < .001), MCV (P < .001), MCH (P < .001), MCHC (P < .001), PLT (P < .001) and WBC differential, except basophils, monocytes, and band neutrophils.
Table 4: Hematological values of growers (7-14 weeks) (n = 54), and finishers (n = 31) from five Slovenian farrow-to-finish pig farms
| Parameter | Age | Mean (median) | Range* |
|---|---|---|---|
| RBC (1012/L) | Growers | 6.43 (6.46) | 5.40-7.28 |
| Finishers | 6.92 (6.89) | 5.74-8.63 | |
| WBC (109/L) | Growers | 22.44 (22.60) | 13.70-34.12 |
| Finishers | 20.97 (20.30) | 14.10-32.10 | |
| Hb (g/dL) | Growers | 10.9 (11.0) | 9.2-12.5 |
| Finishers | 12.6 (12.6) | 11.1-14.4 | |
| Hct (%) | Growers | 35.3 (35.2) | 28.0-41.7 |
| Finishers | 39.9 (39.3) | 34.1-48.7 | |
| MCV (fL) | Growers | 54.9 (55.0) | 47.7-63.0 |
| Finishers | 57.8 (58.0) | 50.0-64.8 | |
| MCH (pg) | Growers | 17.1 (17.3) | 14.0-18.5 |
| Finishers | 18.4 (18.5) | 16.1-20.9 | |
| MCHC (g/dL) | Growers | 31.1 (31.1) | 28.8-33.5 |
| Finishers | 31.7 (31.8) | 29.2-33.7 | |
| PLT (109/L) | Growers | 483 (486) | 273-730 |
| Finishers | 336 (329) | 134-584 | |
| Seg (%) | Growers | 48 (48) | 30-71 |
| Finishers | 41 (41) | 15-67 | |
| Eos (%) | Growers | 2 (1) | 0-9 |
| Finishers | 5 (4) | 0-22 | |
| Baso (%) | Growers | 0 (0) | 0-2 |
| Finishers | 0 (0) | 0-1 | |
| Lymph ( %) | Growers | 49 (47) | 27-69 |
| Finishers | 52 (55) | 27-77 | |
| Mono (%) | Growers | 1 (1) | 0-7 |
| Finishers | 1 (0) | 0-4 | |
| Band (%) | Growers | 0 (0) | 0-1 |
| Finishers | 0 (0) | 0-0 |
* 2.5th to 97.5th inter-percentile range.
RBC = red blood cell count; WBC = white blood cell count; Hb = hemoglobin; Hct = hematocrit; MCV = mean corpuscular volume; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; PLT = platelets; Seg = segmented neutrophils; Eos = eosinophils; Baso = basophils; Lymph = lymphocytes; Mono = monocytes; Band = band neutrophils.
Significantly lower RBC numbers were observed in sows than in young pigs. The highest values of Hct were found in finishers compared to other age groups, and the highest values of MCV were found in sows. Numbers of WBCs and PLTs decreased with age, the lowest numbers being observed in sows. A significantly higher percentage of eosinophils was found in sows than in younger pigs.
Discussion
Hematological reference values for sows were established in this study. When comparing with the reference values from the literature,1,22,23 clinically relevant differences were found for the hematological parameters such as RBC, Hb, and Hct. These values may have differed because published reference values in the literature are not specified by age, or perhaps in part because laboratory methods differed.
Physiological status influenced the values of hematological parameters in our study. The lower values of Hb and Hct established in lactating sows may be related to physiological appearance.1 A higher number of WBCs and a higher percentage of neutrophils were observed in lactating sows than in pregnant sows, consistent with Elbers et al,7 who reported a higher leukocyte count, mostly accounted for by a higher segmented neutrophil count, in the blood samples of sows obtained at weaning when compared with those obtained between 4 and 5 weeks of gestation. During lactation, a physiologic leukocytosis caused by lymphocytosis with or without neutrophilia may occur.24 Increased WBC can be associated with inflammatory response to uterine involution or responses to infections, such as uterine infection. Interpretation of results of lactating sows should take into account that the values of RBC, Hb, and Hct may be slightly lower and WBC values slightly higher than in the reference intervals for sows.
Farm was a significant source of variation in our statistical model for most of the investigated hematological parameters. The influence of farm on biochemical parameter values was evident in a study performed on eight farms in the Netherlands and Belgium.25 No publication about influence of farm on hematological profiles in sows were found. Farm also significantly influenced the blood profiles of pigs from 40 finishing herds in the Netherlands.9 The authors suspect that differences in health status between farms could account for some of the variance in the values of blood parameters, and that the magnitude of between-herd variation in some of the blood parameters can be a useful parameter for herd-health control, as it may reflect herd-health status.9 For example, PRRSV-positive pigs had significantly lower values of WBC in comparison to PRRSV-negative pigs.17 Nutrition may also contribute to some differences in hematological profile.8 Alterations in hematological profile found in a single herd may indicate subclinical disease or other disturbances. Monitoring the levels in individual animals compared to other herd data and clinical examination of suspicious animals with deviating values may facilitate the early detection of disease or conditions that may be subclinical.
Age significantly influenced most hematology variables in our study, which is in accordance with the findings of other studies4,26 and is related to physiological changes.1,24 Reference intervals for growers and finishers were calculated because differences in values of some parameters (RBC, Hb, Hct, MCV, PLT) between these age groups seem to be clinically relevant. Reference intervals for growers and finishers differ from reference intervals for sows, and this should be considered when interpreting the results.
Implications
The hematological data in this study originates from clinically healthy pigs on small commercial farms. The data suggest the following:
- Farm and physiologic state significantly influence the majority of hematological variables;
- Age-related changes in hematological values occur;
- Sources of normal variation must be considered to allow for an appropriate interpretation of the values;
- Reference values serve as a guideline for interpreting the results from individual pigs and can also be used to assess health status in herds when the values of groups of pigs are considered;
- Herd anamnestic and clinical data should be considered when making interpretations.
Acknowledgements
The authors acknowledge the financial support from the Slovenian Research Agency (research core funding No.P4-0092). The authors would like to thank Professor Mary Christopher, University of California, Davis, for useful suggestions with regard to the manuscript, and Brigita Grecs Smole for checking the citations of references, and to native speaker Shawn Thomson for proofreading.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medication, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Thorn CE. Hematology of the pig. In: Weiss DJ, Wardrop KJ, eds. Schalm’s Veterinary Hematology. 6th ed. Ames, Iowa: Wiley Blackwell; 2010:843–851.
2. Eze JI, Onunkwo JI, Shoyinka SVO, Chah FK, Ngene AA, Okolinta N, Nwanta JA, Onyenwe IW. Hematological profiles of pigs raised under intensive management system in south-eastern Nigeria. Niger Vet J. 2010;31:115–123.
3. Friendship RM, Lumdsen JH, McMillan I, Wilson MR. Hematology and biochemistry reference values for Ontario swine. Can J Comp Med. 1984;48:390–393.
4. Yeom SC, Cho SY, Park CG, Lee WJ. Analysis of reference interval and age-related changes in serum biochemistry and hematology in the specific pathogen free miniature pig. Lab Anim Res. 2012;28:245–253.
5. Ventrella D, Dondi F, Barone F, Serafini F, Elmi A, Giunti M, Romagnoli N, Forni M, Bacci ML. The biomedical piglet: establishing reference intervals for haematology and clinical chemistry parameters of two age groups with and without iron supplementation. BMC Veterinary Research. 2017;13:23.
6. Žvorc Z, Mrljak V, Sušić V, Pompe Gotal J. Hematological and biochemical parameters during pregnancy and lactation in sows. Vet Archiv. 2006;76:245–253.
7. Elbers ARW, Geudeke MJ, Kroon MC, Counotte GHM. Haematology and biochemistry reference values for sows kept under modern management conditions. Vet Quat. 1994;16:127–130.
8. Czech A, Grela ER. Biochemical and haematological blood parameters of sows during pregnancy and lactation fed the diet with different source and activity of phytase. Anim Feed Sci Technol. 2004;116:211–223.
9. Elbers ARW, Counotte GHM, Tielen MJM. Haematological and clinicochemical blood profiles in slaughter pigs. Vet Quat. 1992;14:57–62.
10. Lee SH, Shinde PL, Choi JY, Kwon IK, Lee JK, Pak SI, Cho WT, Chae BJ. Effect of tannic acid supplementation on growth performance, blood hematology, iron status and faecal microflora in weanling pigs. Livest Sci. 2010;131:281–286.
11. Alexopoulos C, Papaioannou DS, Fortomaris P, Kyriakis CS, Tserveni-Goussi A, Yannakopoulos A, Kyriakis SC. Experimental study on the effect of in-feed administration of a clinoptilolite-rich tuff on certain biochemical and hematological parameters of growing and fattening pigs. Livest Sci. 2007;111:230–241.
12. Nielsen J, Bøtner A. Hematological and immunological parameters of four and half-month old pigs infected with PRRS virus. Vet Microbiol. 1997;55:289–294.
13. Hoelzle LE, Zeder M, Felder KM, Hoelzle K. Pathobiology of Mycoplasma suis. Vet J. 2014;202:20–25.
14. Ritzmann M, Grimm J, Heinritzi K, Hoelzle K, Hoelzle LE. Prevalence of Mycoplasma suis in slaughter pigs, with correlation of PCR results to hematological findings. Vet Microbiol. 2009;133:84–91.
15. Norbury KC, Moyer MP. Effect of negative pressure therapy on the inflammatory response of the intestinal microenvironment in a porcine septic model. Mediators of Inflammation. 2015, Article ID 419841, 12 pages.
16. Palombo JD, Liu K, Greif WM, Rawn JD, Boyce PJ, Forse RA. Effects of laparoscopic vs laparatomy treatment of E coli peritonitis on hemodynamic responses in a porcine model. Surg Endosc. 1999;13:1001–1006.
17. Štukelj M, Toplak I, Nemec Svete A. Blood antioxidant enzymes (SOD, GPX), biochemical and haematological parameters in pigs naturally infected with porcine reproductive and respiratory syndrome virus. Polish J Vet Sci. 2013;16:369–376.
18. Buzzard BL, Edwards-Callaway LN, Engle TE, Rozell TG, Dritz SS. Evaluation of blood parameters as an early assessment of health status in nursery pigs. J Swine Health Prod. 2013;21:148–151.
19. Xin H, Harmon JD, Dong H, Harris DL, Chepete HJ, Ewan RC, Gramer ML. Effects of post-weaning nutritional conditions on isowean pigs. Trans ASAE. 1999;42:1463–1469.
20. National Research Council US, Committee on Nutrient Requirements of Swine. Nutrient Requirements of Swine. 11th rev ed. Washington, DC: National Academies Press; US; 2012.
21. Farver TB. Concepts of normality in clinical biochemistry. II. Reference interval determination and use. In: Kaneko J, Harvey J, Bruss M, eds. Clinical Biochemistry of Domestic Animals. 5th ed. San Diego: Academic Press; 1997:2–9.
22. Kraft W, Dürr UM, Fürll M, Bostedt H, Heinritzi K. Hämatologie [Hematology]. In: Kraft W, Dürr UM. Klinische Labordiagnostik in der Tiermedizin [Clinical Laboratory Diagnostics in Veterinary Medicine]. 3. Aufl. Stuttgart, Germany: Schattauer; 1999:43–77.
23. Jazbec I. Klinično laboratorijska diagnostika (Clinical laboratory diagnostics). Ljubljana, Slovenija: Veterinarska fakulteta; 1990:82–106.
24. Evans EW. Interpretation of porcine leucocyte responses. In: Feldman BF, Zinkl JG, Jain NC, eds. Schalm’s Veterinary Hematology. 5th ed. Ames, Iowa: Blackwell Publishing; 2006:411–416.
25. Verheyen AJM, Maes DGD, Mateusen B, Deprez P, Janssens GPJ, de Lange L, Counotte G. Serum biochemical reference values for gestating and lactating sows. Vet J. 2007;174:92–98.
26. Štukelj M, Valenčak Z, Krsnik M, Nemec Svete A. The effect of the combination of acids and tannin in diet on the performance and selected biochemical, haematological and antioxidant enzyme parameters in grower pigs. Acta Vet Scand. 2010;52:e19. https://doi.org/10.1186/1751-0147-52-19.
