| Original research | Peer reviewed |
Cite as: Morales J, Manso A, Martín-Jiménez T, et al. Comparison of the pharmacokinetics and efficacy of two different iron supplementation products in suckling piglets. J Swine Health Prod. 2018;26(4):200-207.
Also available as a PDF.
SummaryObjective: To evaluate and compare the efficacy and pharmacokinetics of two iron sources, gleptoferron (GLF) and iron dextran (DXT) in two-day old piglets. Materials and methods: A total of 32 piglets from four litters were used in the study. On the second day of life, eight piglets were selected per litter and injected with one of two sources of iron, GLF or DXT (four piglets per treatment group in each litter). Blood samples were collected prior to treatment and 1, 2, 6, 10, and 12 hours after treatment. Additional samples were collected on days 1, 2, 3, 4, 7, 14, 19, and 24. Serum iron and ferritin concentrations were analyzed in all samples and the following pharmacokinetic parameters of iron were calculated: the peak concentration, time to peak concentration, half time, and extent of absorption. Hematological parameters were also analyzed to assess the iron status: hematocrit, hemoglobin, red blood cells, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration. Piglets were individually weighed weekly. Results: No significant differences in growth performance were observed between groups. Both products were efficient to prevent iron deficiency and anemia in the suckling period. The absorption and the bioavailability of iron were higher with GLF than DXT (overall iron serum concentration, P < .001). Implications: Under the conditions of this study, both iron products are efficient to prevent iron deficiency and anemia in the suckling period. Absorption and bioavailability of GLF are significantly higher and have a confirmed different pharmacokinetic profile to DXT. | ResumenObjetivo: Evaluar y comparar la eficacia y farmacocinética de dos fuentes de hierro, gleptoferron (GLF) y hierro dextrano (DXT) en lechones de dos días de edad. Materiales y métodos: En el estudio se utilizaron un total de 32 lechones de cuatro camadas. En el segundo día de vida, se seleccionaron ocho lechones por camada y se inyectaron con una de dos fuentes de hierro, GLF o DXT (cuatro lechones por grupo de tratamiento en cada camada). Se tomaron muestras de sangre antes del tratamiento, y 1, 2, 6, 10, y 12 horas después del tratamiento. Se tomaron muestras adicionales en el día 1, 2, 3, 4, 7, 14, 19, y 24. Se analizaron concentraciones de ferritina y hierro sérico en todas las muestras, y se calcularon los siguientes parámetros farmacocinéticos de hierro: la concentración pico, tiempo para alcanzar la concentración pico, vida media, y extensión de absorción. También se analizaron los parámetros hematológicos para valorar el status del hierro: hematocrito, hemoglobina, glóbulos rojos, volumen corpuscular medio, hemoglobina corpuscular media, y concentraciones de hemoglobina corpuscular media. Se pesaron los lechones individualmente cada semana. Resultados: No se observaron diferencias significativas en el desempeño del crecimiento entre grupos. Ambos productos fueron eficientes para prevenir la deficiencia de hierro y anemia durante el periodo de lactancia. La absorción y la biodisponibilidad de hierro fueron más altos con el GLF que con el DXT (concentración total de hierro sérico, P < .001). Implicaciones: Bajo las condiciones de este estudio, ambos productos de hierro son eficientes para prevenir la deficiencia de hierro y anemia en el periodo de lactancia. La absorción y la biodisponibilidad de GLF son significativamente más altas y tiene un perfil confirmado farmacocinético diferente al DXT. | ResuméObjectif: Évaluer et comparer l’efficacité et la pharmacocinétique de deux sources de fer, le gleptoferron (GLF) et le fer dextran (DXT) chez des porcelets de deux jours d’âge. Matériels et méthodes: Un total de 32 porcelets provenant de quatre portées a été utilisé dans la présente étude. À leur deuxième jour d’âge, huit porcelets ont été sélectionnés par portée et injectés avec une de deux sources de fer, GLF ou DXT (quatre porcelets par groupe de traitement dans chaque portée). Des échantillons de sang ont été prélevés avant le traitement et 1, 2, 6, 10 et 12 heures après le traitement. Des échantillons supplémentaires ont été prélevés aux jours 1, 2, 3, 4, 7, 14, 19, et 24. Les concentrations sériques de fer et de ferritine ont été analysées dans tous les échantillons et les paramètres pharmacocinétiques du fer suivants ont été calculés: le pic de concentration, le temps requis pour atteindre le pic de concentration, le demi-temps, et la quantité absorbée. Des paramètres hématologiques ont également été analysés pour évaluer le statut du fer: l’hématocrite, l’hémoglobine, la quantité de globules rouges, le volume corpusculaire moyen, l’hémoglobine corpusculaire moyenne, et la concentration moyenne d’hémoglobine corpusculaire. Les porcelets étaient pesés individuellement à chaque semaine. Résultats: Aucune différence significative dans les performances de croissance n’a été observée entre les groupes. Les deux produits étaient efficaces pour la prévention d’une déficience en fer et d’anémie durant la période d’allaitement. L’absorption et la biodisponibilité du fer étaient supérieurs avec le GLF comparativement au DXT (concentration sérique globale du fer (P < 0,001). Implications: Dans les conditions de la présente étude, les deux produits de fer sont efficaces pour prévenir une déficience en fer et l’anémie durant la période d’allaitement. L’absorption et la biodisponibilité de GLF sont significativement plus élevés et ont un profile pharmacocinétique confirmé comme différent par rapport au DXT. |
Keywords: swine, iron, suckling pigs, anemia, pharmacokinetics
Search the AASV web site
for pages with similar keywords.
Received: May 25, 2017
Accepted: December 20, 2017
It is well established that insufficient iron intake in suckling pigs results in iron deficiency or anemia. The pig is born with limited iron stores and the sow’s milk is a poor source of iron, providing piglets with only 1 mg of iron a day.1 This amount of iron is not sufficient to support the rapid growth and expansion of blood volume during the first days of life. Therefore, neonatal piglets require exogenous iron supplementation.2 The practice most commonly used in field conditions is an intramuscular (IM) injection of 200 mg iron dextran (DXT) or gleptoferron (GLF) within the first 3 days of life. Gleptoferron is a macromolecular complex of beta-ferric oxyhydroxide and dextran glucoheptonic acid. It has been postulated that gleptoferron, is superior to iron dextran in preventing anemia for young pigs.3 However, other authors concluded that iron dextran and gleptoferron can be used with similar effect.4,5
The growth potential of current genetic lines has improved in the last decades, while iron dosage remains the same. Therefore, it is important to verify if the routine iron supplementation protocols used today on commercial swine farms are still adequate to prevent iron deficiency and anemia in modern pigs.
In the present study, two iron products were compared: DXT and GLF. Serum iron and ferritin concentrations were measured after a single IM administration during the suckling period. Pharmacokinetic profiles and parameters of iron were evaluated to compare absorption and bioavailability of iron from both compounds. The poor responsiveness of neonatal piglets to oral iron therapy is now well documented. The immaturity of the duodenum to iron absorption may be the main cause6 but studies about absorption and bioavailability of different injectable forms of iron are limited. The product should be rapidly and significantly absorbed from the IM injection site, otherwise iron is not available for hemoglobin synthesis and replenishment of iron stores in the liver. There is also the danger that the non-absorbed iron will deposit in the connective tissue stroma and associated macrophages, resulting in unacceptable muscle staining.7 It is now well accepted that 90% of the injected iron should be absorbed within 72 hours post dosing to be effective.8 Differences in absorption were reported for parenteral iron preparations.7
Materials and methods
Prior to the commencement of the study, the protocol was reviewed and approved by the investigators of PigCHAMP Pro Europa, a swine veterinary consultancy company. Animals were handled in compliance with both Spanish regulations and guidelines for the protection of animals in scientific research (Real Decreto Español 223/88 BOE 67: 8509-8511) and applicable European regulation.
Study facilities
The present study was conducted in Segovia, Spain on a commercial farrow-to-finish farm with a capacity of 500 sows. It involved one lactation room containing 12 farrowing pens. Each farrowing pen measured 2.5 × 2.0 m2 (sow area: 2.0 × 0.6 m2), and had a plastic slatted floor including a heated section for the piglets.
Study animals
Four litters were selected for the study. Only parity 3 to 5 sows (Danbred) were used. Within 24 h after birth, piglets were individually identified with ear tags and weighed. Litters were equalized at 12 piglets per litter by cross-fostering and no more changes in piglet allocation were then allowed. In each litter, eight piglets were randomly selected and allocated to two experimental groups. High quality digestible creep feed (2890 kcal NE/kg; 20.0% crude protein; 1.45% digestible lysine; 9.7% ether extract; 6.3% ash content) was offered from 10 days of age. Piglets were weaned at 28 days of age.
Experimental products
Two commercial iron supplements were evaluated: gleptoferron (Gleptosil, Ceva Santé Animale, Libourne, France) and an iron dextran product (Uniferon, Pharmacosmos A/S, Holbaek, Denmark). In both cases, 1 mL per piglet (200 mg of active compound) was administered by IM injection in the neck.
Experimental design
This study was a monocentric, blinded, randomized, 2-arm study, comparing two commercial iron preparations for prevention of anemia in neonatal piglets. In each litter, GLF was administered to four piglets and DXT was administered to another four piglets. Selection and allocation of piglets to treatment groups was done at random in each litter (computer-generated random allocation). Four different random lists were used in the study, one per litter. The sample size was calculated by power analysis with a power (1 – β) higher than 80% for iron serum concentration, the main analysis variable.
Measurements and samples
Blood samples (3 mL) were collected from the vena cava prior to treatment (day 0) and 1, 2, 6, and 10 hours after treatment. Additional blood samples were collected on days 1, 2, 3, 4, 7, 14, 17, and 21. On day 0, each piglet was sampled only twice (0 and 6 hours, 1 and 10 hours, or 2 and 12 hours). Serum was collected immediately after centrifugation at 3500g for 5 min, and sent to a laboratory (Facsis Consulting SL, Segovia, Spain). Serum iron and ferritin concentrations were measured spectrophotometrically with a Technicon RA-1000 automated system (Bayer, Tarrytown, New York).
Four additional piglets per treatment (1 per litter) were also sampled and blood collected into EDTA tubes for the determination of hematological parameters. Hematocrit (Hct), hemoglobin (Hb), red blood cells (RBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were measured using an automatic blood analyzer, Sysmex TX-1800i (Sysmex Corporation, Kobe, Japan). Blood samples for hematology were collected on day 0 before iron administration, and on days 1, 2, 3, 4, 14, 17, and 21 after treatment. Change in hematological variables between the baseline and the evaluation period were also calculated and compared.9-12
All piglets were individually weighed weekly from study day 0 until day 21. All deaths or clinical incidences were recorded.
Pharmacokinetic analysis
The serum pharmacokinetic parameters were determined using noncompartmental analysis with PK Software (Phoenix 6.0, Certara Inc, Princeton, New Jersey). The maximum serum concentration (Cmax) and the time to reach Cmax (tmax) for each animal were determined directly from the serum concentration data. The area under the curve (AUC) was calculated using the log-linear trapezoidal method. The decay phase of the iron concentration curve (T½) was calculated by a linear regression after logarithmic transformation of these concentrations.
Statistical analyses
SAS 9.0 (SAS Institute Inc, Cary, North Carolina) was used for statistical analysis. All treatment differences were assessed at the 2-sided .05 α level of significance and trends were reported for α = .10.
For parameters measured only once (Cmax, tmax, T½, and AUC), group values were tabulated. Statistical analysis of iron and ferritin serum concentration and measures in plasma samples were conducted with a linear mixed effects model. The fixed effects were treatment group, litter (blocking variable), and body weight on day 0 (covariate), while time was the random effect. Average body weights on day 21 were analyzed using a general linear model (PROC GLM) including treatment, litter (blocking variable), and body weight on day 0 as covariates. The piglet was the experimental unit.
Results
No differences in growth performance were observed between groups during the suckling period, 6.1 kg versus 6.4 kg body weight on study day 21 in the GLF and in the DXT groups, respectively (P = .29). Only two deaths (crushed by the sow) occurred during the study, both were in the DXT group and were observed on days 1 and 3.
Serum iron and ferritin concentrations are presented in Table 1. The linear plots of the serum iron concentration-time profiles after IM administration of the two iron complexes are shown in Figure 1. The main pharmacokinetic parameters are summarized in Table 2. Serum iron concentration reached a peak at 12 h after administration of GLF and at 10 h after administration of DXT. The significantly different parameters between GLF and DXT were the Cmax (4695 µg/dL versus 2118 µg/dL respectively, P < .001) and the serum AUC (197.55 h ∙ µg/dL versus 43.03 h ∙ µg/dL respectively, P < .001). Overall, serum iron concentrations in the experimental period were higher in the GLF than in the DXT group (P < .001). The pharmacokinetic profile shows that serum iron concentrations were significantly higher in GLF piglets from 2 h to 72 h post treatment. Thereafter, no significant differences were observed between groups until weaning, when iron serum content in GLF piglets tended to be higher (P < .10). Ferritin serum concentration did not differ among treatment groups, except at 10 h post treatment when it was higher in GLF than in DXT piglets (9.94 ng/mL versus 3.31 ng/mL respectively; P < .05).
Table 1: Least Square Means (standard deviation) of iron and ferritin serum concentrations in piglets at different time-points after treatment with gleptoferron or iron dextran*
| Iron (μg/dL) | Ferritin (ng/mL) | |||||
|---|---|---|---|---|---|---|
| Time | GLF | DXT | P† | GLF | DXT | P† |
| 0 h‡ | 44.2 (13.6) | 49.8 (20.1) | .62 | 8.31 (3.08) | 9.69 (1.22) | .37 |
| 1 h‡ | 1294.8 (684.7) | 1904.9 (818.0) | .15 | 9.79 (2.86) | 8.71 (1.48) | .59 |
| 2 h‡ | 1881.0 (578.5) | 1313.0 (331.4) | .05 | 9.03 (2.24) | 12.72 (4.02) | .46 |
| 6 h‡ | 4212.8 (776.5) | 1386.9 (695.1) | .02 | 10.46 (2.17) | 9.54 (1.48) | .51 |
| 10 h‡ | 4204.7 (868.3) | 2022.6 (237.0) | .13 | 9.94 (4.76) | 3.31 (0.87) | .02 |
| 12 h‡ | 4677.0 (1471.0) | 1306.7 (316.2) | .004 | 6.26 (2.77) | 6.99 (1.41) | .82 |
| 24 h | 3729.7 (842.8) | 294.9 (132.1) | < .001 | 7.47 (1.85) | 8.64 (3.96) | .31 |
| 48 h | 1955.0 (534.4) | 120.7 (89.7) | < .001 | 11.10 (2.98) | 9.92 (2.59) | .38 |
| 72 h | 684.3 (347.7) | 121.7 (55.7) | < .001 | 12.76 (1.80) | 12.21 (1.89) | .53 |
| 96 h | 150.3 (61.1) | 129.2 (38.1) | .30 | 14.42 (3.17) | 15.27 (3.54) | .62 |
| Day 14 | 161.8 (55.9) | 143.1 (52.6) | .47 | 16.54 (6.08) | 26.42 (43.07) | .49 |
| Day 17 | 140.1 (40.7) | 115.5 (50.2) | .27 | 13.45 (8.99) | 11.55 (4.32) | .60 |
| Day 21 | 146.7 (73.9) | 100.2 (56.4) | .069 | 11.07 (4.96) | 10.93 (3.26) | .95 |
| Average | 1783.1 (1625.3) | 684.02 (672.1) | < .001 | 10.68 (5.52) | 11.36 (15.15) | < .001 |
* A total of 24 two-day old piglets (day 0) from 4 litters (6 piglets per litter) were randomly allocated to two treatment groups resulting in 12 piglets per treatment (3 piglet per litter and treatment).
† A linear mixed effects model was used including the effects of treatment, litter (blocking variable) and time (random effect). Treatment × day interaction effect was P < .001 in iron serum concentration and P = .15 in ferritin serum concentration.
‡ On day 0, each piglet was sampled only twice (0h and 6h, 1h and 10h, or 2h and 12h) resulting in a total of 8 piglets sampled (1 piglet per litter and treatment) at each of these time points.
GLF = gleptoferron; DXT = iron dextran.
Figure 1: Mean concentration-time profiles (with standard error) of iron in serum after single intramuscular administration of 200 mg per piglet of gleptoferron or iron dextran. Data were analyzed using a linear mixed-effects model with treatment, group, and litter (blocking variable) as fixed effects and time as random effect. Treatment × day interaction effect was significant (P < .001).
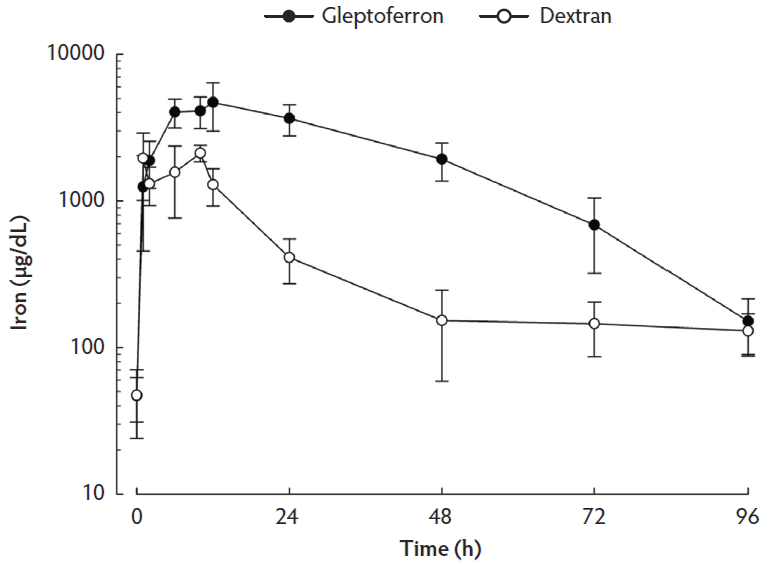
Table 2: Mean pharmacokinetic parameters of iron in serum after single intramuscular administration of 200 mg per piglet of gleptoferron or iron dextran.
| Pharmacokinetic parameter | GLF | DXT |
|---|---|---|
| Cmax (µg/dL) | 4695 | 2118 |
| Tmax (h) | 12.0 | 10.0 |
| T½ (h) | 17.3 | 10.7 |
| AUC0-96h (h ∙ µg/dL) | 197.55 | 43.03 |
| Relative bioavailability* | 4.6 | 1 |
* Relative bioavailability of GLF = AUC0-96h GLF/AUC0-96h DXT, assuming bioavailability of DXT = 1.
GLF = gleptoferron; DXT = iron dextran; Cmax = maximum serum concentration; Tmax = time to reach Cmax; T½ = decay phase of the iron concentration curve; AUC0-96h = area under the curve.
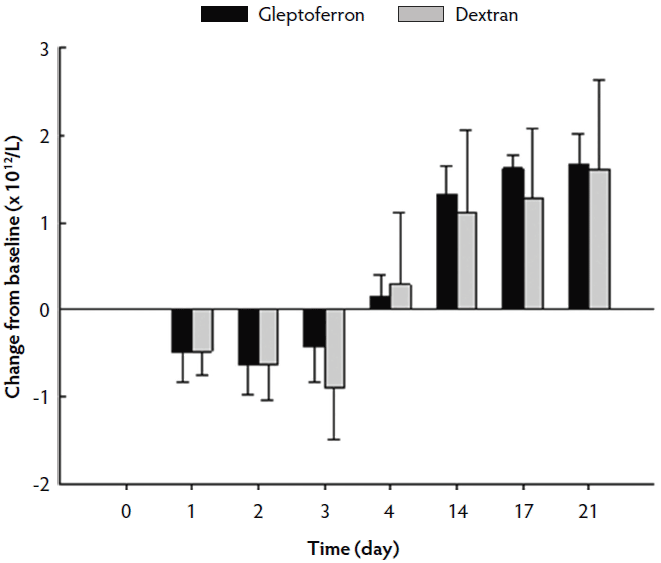
The hematological parameters are presented in Table 3. The Hct, Hb, and RBC decreased up to day 2 or 3 post treatment, before they again increased at day 4 to reach or supersede the day 0 level (Figures 2, 3, and 4). The Hct did not differ between groups except on day 17, when it was higher in GLF than in DXT piglets (45.1% versus 41.7%; P < .05). There was no significant difference between groups for Hb values (9.7 g/dL versus 9.4 g/dL; P = .11). The increase in Hb and Hct levels occurred sooner and were higher and more homogeneous in the GLF group (Figures 2 and 3). Two weeks after treatment, the mean increase in Hb from baseline was 3.10 g/dL for GLF and 2.25 g/dL for DXT (Figure 2). The mean increase in Hct two weeks after treatment was 12.9% for GLF and 7.45% for DXT (Figure 3). However, these differences were not statistically significant (P > .05). No differences were observed in RBC concentrations over time (Figure 4). On day 17, Hct was higher (P < .05) in the GLF than in the DXT group, indicating higher percentage of red blood cells around weaning age. In this sense, MCV was also higher in GLF than in DXT piglets on days 4 and 14 (P < .05), and numerically higher on day 17 (P = .12), which indicates more erythrocytes are being produced, as those new and immature erythrocytes are greater in size. The MCH, also associated with iron deficiencies, tended to be higher on days 4 and 14 (P < .10) and numerically higher on day 17 (P = .17) in GLF than in DXT piglets.
Table 3: Least Square Means (standard deviation) of hematological values in piglets treated with gleptoferron or iron dextran at different time points during the suckling period*
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 14 | Day 17 | Day 21 | Average | |
|---|---|---|---|---|---|---|---|---|---|
| Hct (volume % of red blood cells in blood) | |||||||||
| GLF | 29.47 (0.68) | 28.28 (1.18) | 27.26 (1.18) | 31.82 (2.75) | 37.99 (1.43) | 42.91 (2.59) | 45.13 (1.13) | 41.48 (3.09) | 35.54 (6.79) |
| DXT | 32.80 (3.48) | 31.02 (1.43) | 29.34 (1.31) | 31.38 (1.13) | 38.94 (3.23) | 39.74 (2.67) | 41.69 (1.14) | 41.60 (3.19) | 35.81 (5.58) |
| P† | .097 | .14 | .13 | .84 | .71 | .33 | .04 | .97 | .69 |
| Hb (g/dL) | |||||||||
| GLF | 8.27 (0.27) | 7.47 (0.33) | 7.32 (0.32) | 8.00 (0.80) | 9.53 (0.41) | 11.57 (0.46) | 11.75 (0.28) | 11.35 (0.56) | 9.41 (1.77) |
| DXT | 9.13 (0.94) | 8.30 (0.66) | 8.26 (0.40) | 8.00 (0.21) | 10.19 (0.85) | 11.18 (0.72) | 11.20 (0.42) | 11.35 (0.78) | 9.70 (1.57) |
| P† | .15 | .07 | .04 | .99 | .34 | .52 | .13 | .99 | .11 |
| RBC (× 1012/L) | |||||||||
| GLF | 4.54 (0.18) | 4.07 (0.15) | 3.93 (0.27) | 4.17 (0.45) | 4.79 (0.17) | 5.97 (0.17) | 6.28 (0.13) | 6.31 (0.30) | 5.01 (0.96) |
| DXT | 5.30 (0.57) | 4.79 (0.48) | 4.65 (0.29) | 4.35 (0.23) | 5.49 (0.48) | 6.31 (0.37) | 6.49 (0.21) | 6.81 (0.41) | 5.52 (0.97) |
| P† | .01 | .14 | .03 | .53 | .13 | .32 | .41 | .26 | < .001 |
| MCV (fL) | |||||||||
| GLF | 64.96 (1.55) | 69.37 (0.42) | 69.34 (3.13) | 76.58 (4.92) | 79.33 (1.63) | 71.90 (2.68) | 71.92 (2.89) | 65.85 (4.91) | 71.15 (5.70) |
| DXT | 62.04 (3.20) | 65.03 (3.88) | 55.69 (14.70) | 72.35 (3.14) | 70.90 (1.03) | 62.88 (0.77) | 64.31 (2.73) | 61.03 (2.43) | 64.28 (7.58) |
| P† | .14 | .15 | .29 | .51 | .01 | .046 | .12 | .30 | < .001 |
| MCH (pg) | |||||||||
| GLF | 18.20 (0.33) | 18.33 (0.59) | 18.65 (0.57) | 19.20 (0.90) | 19.91 (0.40) | 19.40 (0.53) | 18.72 (0.81) | 18.02 (1.12) | 18.80 (0.95) |
| DXT | 17.32 (0.84) | 17.37 (1.01) | 17.77 (0.71) | 18.45 (0.61) | 18.56 (0.65) | 17.67 (0.47) | 17.26 (0.59) | 16.65 (0.61) | 17.63 (0.90) |
| P† | .29 | .30 | .23 | .48 | .06 | .06 | .17 | .29 | < .001 |
| MCHC (%) | |||||||||
| GLF | 28.07 (0.74) | 26.43 (0.78) | 26.83 (0.66) | 25.09 (0.94) | 25.12 (0.19) | 26.99 (0.58) | 26.03 (0.39) | 27.43 (0.86) | 26.50 (1.21) |
| DXT | 27.83 (0.36) | 26.72 (1.08) | 27.97 (0.54) | 25.53 (0.53) | 26.18 (0.59) | 28.16 (0.54) | 26.84 (0.54) | 27.29 (0.23) | 27.07 (1.05) |
| P† | .49 | .61 | .25 | .67 | .08 | .15 | .099 | .86 | .005 |
* A total of eight 2-day old piglets (day 0) from 4 litters (2 piglets per litter) were randomly allocated to two treatment groups resulting in 4 piglets per treatment (1 piglet per litter and treatment).
† A linear mixed effects model was used including the effects of treatment, litter (blocking variable), and time (random effect). In all variables (Hct, Hb, RBC, MCV, MCH, and MCHC), treatment × day interaction effect was P < .001.
Hct = hematocrit; Hb = hemoglobin; RBC = Red blood cells; MCV = Mean corpuscular volume; MCH = Mean corpuscular hemoglobin; MCHC = Mean corpuscular hemoglobin concentration; GLF = gleptoferron; DXT = iron dextran.
Figure 2: Mean change in hemoglobin (g/dL) from the baseline after single intramuscular administration of 200 mg per piglet of gleptoferron or iron dextran. Error bars represent the standard deviation.
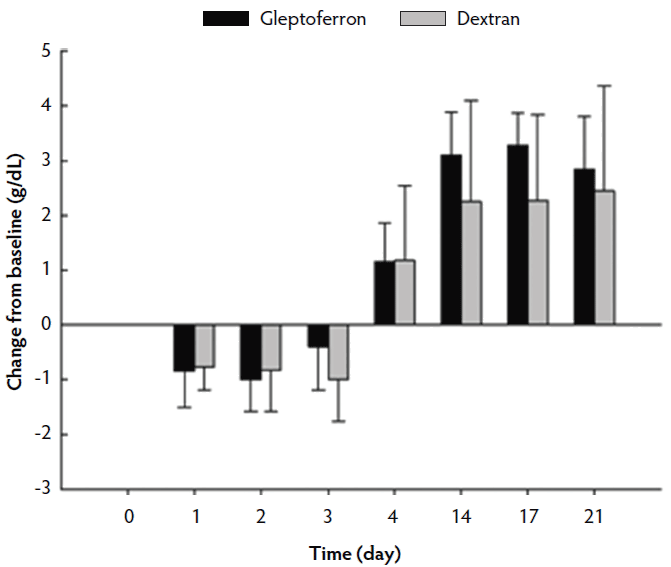
Figure 3: Mean change in hematocrit (%) from the baseline after single intramuscular administration of 200 mg per piglet of gleptoferron or iron dextran. Error bars represent the standard deviation.
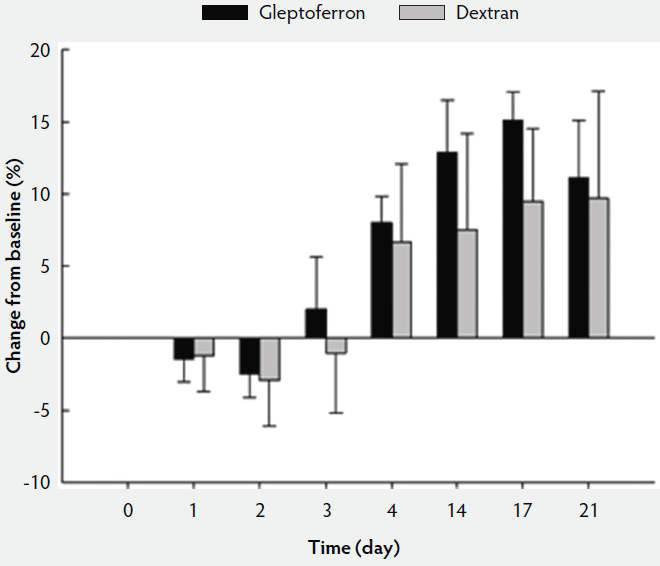
Figure 4: Mean change in red blood cell count (× 1012/L) from the baseline after single intramuscular administration of 200 mg per piglet of gleptoferron or iron dextran. Error bars represent the standard deviation.
Discussion
The small number of the animals and samples tested in the present study needs to be taken into consideration when interpreting the results.
Pigs raised indoors lack access to soil, a rich source of iron, and therefore require exogenous supplementation within the first week of life to prevent iron deficiency and anemia. For many years on commercial farms, administration of 200 mg IM injection of DXT within the first 3 days of life has been performed on a routine basis.2 However, iron requirements might be higher under current swine production conditions including higher prolificacy, lower birth weight, large variation of birth weight within litter, and higher growth performance.13 Therefore, modern pigs likely require a higher dosage of iron or an exogenous source providing for higher absorption and bioavailability.
In the present study, Cmax was 2.2 times higher in the GLF than in the DXT group, resulting in a much higher AUC in the GLF group. The AUC represents total iron exposure over time and consequently iron bioavailability. Assuming linear pharmacodynamics with a constant elimination rate, AUC is proportional to the total amount of iron absorbed by the body. Therefore, the present study confirms that GLF allows 4.6 times higher total iron absorption by the piglet than does DXT. Other authors did not observe differences in iron serum concentration from either iron sources, confirming that both are efficient for anemia prevention in young pigs compared with a negative control group.4 However, in that study, blood samples were collected only at 10, 21, and 50 days post treatment. Considering that the peak iron concentrations are observed at 10 to 12 h post treatment, the absorption phase was missed.
Serum ferritin has been also used to evaluate iron levels in tissues of neonatal pigs, since it responds quickly to iron treatment or iron deficiency.14 Smith et al15 observed a marked increase in serum ferritin 10 to 21 days after treatment with GLF or DXT compared with untreated pigs. Similarly in the present study, no differences in serum ferritin were observed between GLF and DXT. Serum ferritin increased 2.0 to 2.7 times its concentration 14 days after iron treatment, confirming that both iron sources were efficient in preventing iron deficiency.
Despite iron treatment on day 0, Hct, Hb, and RBC decreased from day 0 to day 2 or 3 in both groups. This physiological anemia is explained by the rapid growth of the piglet and subsequent hemodilution.16-18 Synthesis of new erythrocytes cannot occur fast enough to match the rapid increase in blood volume.
Hemoglobin concentration has been used to evaluate iron deficiency and anemia in the literature. Normal iron status is defined as a Hb concentration > 11 g/dL, iron deficiency as a Hb concentration > 9 g/dL but ≤ 11 g/dL, and anemia as a Hb concentration ≤ 9 g/dL.13 Based on this Hb status classification, only two piglets (one in the GLF group and one in the DXT group) showed iron deficiency after day 14 with a Hb concentration of 10.4 g/dL. Iron status was normal in all other pigs (> 11 g/dL) indicating both iron products, GLF and DXT, were efficient in preventing iron deficiency and anemia. However, the increase in Hb levels occurred sooner and was higher and more homogeneous in the GLF group. In a similar study, piglets that received GLF also had a higher level of Hb at weaning than the group receiving DXT indicating better bioavailability of GLF.3 Efficacy of an exogenous iron source to prevent anemia was previously demonstrated by other authors,4,19 who reported a decrease in Hb concentration and Hct percentage at 10 to 13 days of age in pigs receiving no supplemental iron compared with pigs receiving 200 mg IM injection of DXT at 1 d of age. However, these results are not in accordance with other studies where iron supplementation protocols used by participating farms, mainly 200 mg IM of DXT administration, were not sufficient to meet iron requirements in the suckling period.20 The small number of pigs used in the present study and its main objective focusing on pharmacokinetics did not allow evaluation of individual effects which might affect iron deficiency such as birth weight.
No differences in Hb concentration were observed in the study of Bhattarai and Nielsen13, concluding that using Hb as a diagnostic tool may underestimate the iron requirements for young piglets. Therefore, RBC, Hct, MCV, MCH and MCHC were used in this study as additional iron indicators. At the end of the suckling period, normal values of RBC, Hct, MCV, MCH, and MCHC are 5.4 × 1012/L (± 0.50), 34% (± 3), 63.6 fL (± 6.4), 19.2 pg/cell (± 1.9), and 30.2% (± 0.9), respectively.21 In anemic piglets, RBC, Hct, MCV, and MCH are significantly lower and MCHC is significantly higher than normal.21 In the present study, all hematological parameters did not differ in pigs from both treatment groups, but RBC and Hct were higher and MCHC was lower compared with normal values observed by Egeli et al.21
Implications
- Under the conditions of this study, higher Cmax and AUC values are observed with GLF versus DXT.
- Both iron products are efficient to prevent iron deficiency and anemia in the suckling period.
Acknowledgments
Conflict of interest
Dr Daniel Sperling and Dr Hamadi Karembe are employees of Ceva Santé Animale, and Gleptosil is a product offered by Ceva.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Venn JAJ, McCance RA, Widdowson EM. Iron metabolism in piglet anaemia. J Comp Pathol Therap. 1947;57:314-325.
2. Zimmerman DR, Speer VC, Hays VW, Catron DV. Injectable iron-dextran and several oral iron treatments for the prevention of iron-deficiency anemia of baby pigs. J Anim Sci. 1959;18:1409-1415.
*3. Salle E, Auvigne V. Comparative study of the efficacy of gleptoferron and iron dextran in anaemia prevention in piglets. Proc IPVS. Copenhagen, Denmark. 2006;2:567.
4. Pollmann DS, Smith JE, Stevenson JS, Schoneweis DA, Hines RH. Comparison of gleptoferron with iron dextran for anemia prevention in young pigs. J Anim Sci. 1983;56:640-644.
5. Vermeer JE, Kuijpers AH, Elbers AR. Comparison of the efficacy of two different iron supplements for anemia prevention in piglets. Tijdschr Diergeneeskd. 2002;127:110-114.
6. Starzynski RR, Laarakkers CMM, Tjalsma H, Swinkels DW, Pieszka M, Stys A, Mickiewicz M, Lipinski P. Iron supplementation in suckling piglets: how to correct iron deficiency anemia without affecting plasma hepcidin levels. PLoS One. 2013;8:e64022.
7. Miller ER, Ullrey DE, Brent BE, Merkel RA, Laidlaw VA, Hoefer JA. Iron retention and ham discoloration: a comparison of five injectable iron preparations. J Am Vet Med Assoc. 1965;146:331-336.
8. Beresford CR, Golberg L, Smith JP. Local effects and mechanism of absorption of iron preparations administered intramuscularly. Br J Pharmacol Chemother. 1957;12:107-114.
9. Henry DH, Dahl NV, Auerbach M, Tchekmedyian S, Laufman LR. Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist. 2007;12:231-242.
10. Gupta A, Lin V, Guss C, Pratt R, Ikizler TA, Besarab A. Ferric pyrophosphate citrate administered via dialysate reduces erythropoiesis-stimulating agent use and maintains hemoglobin in hemodialysis patients. Kidney Int. 2015;88:1187-1194.
11. Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
12. Madar AA, Stene LC, Meyer HE, Brekke M, Lagerløv P, Knutsen KV. Effect of vitamin D3 supplementation on iron status: a randomized, double-blind, placebo-controlled trial among ethnic minorities living in Norway. Nutr J. 2016;15:74.
13. Bhattarai S, Nielsen JP. Early indicators of iron deficiency in large piglets at weaning. J Swine Health Prod. 2015;23:10-17.
14. Pollock AS, Lipschitz DA, Cook JD. The kinetics of serum ferritin. Proc Soc Exp Biol Med. 1978;157:481-485.
15. Smith JE, Moore K, Boyington D, Pollmann DS, Schoneweis D. Serum ferritin and total iron-binding capacity to estimate iron storage in pigs. Vet Pathol. 1984;21:597-600.
16. Furugouri K. Characteristic aspects of iron metabolism in piglets. Jpn Agr Res Q. 1975;9:171-176.
17. Egeli AK, Framstad T. Evaluation of the efficacy of perorally administered glutamic acid-chelated iron and iron-dextran injected subcutaneously in Duroc and Norwegian Landrace piglets. Zentralbl Veterinarmed A. 1998;45:53-61.
18. Rincker MJ, Hill GM, Link JE, Rowntree JE. Effects of dietary iron supplementation on growth performance, hematological status, and whole-body mineral concentrations of nursery pigs. J Anim Sci. 2004;82:3189-3197.
19. Rincker MJ, Clarke SL, Eisenstein RS, Link JE, Hill GM. Effects of iron supplementation on binding activity of iron regulatory proteins and the subsequent effect on growth performance and indices of hematological and mineral status of young pigs. J Anim Sci. 2005;83:2137-2145.
20. Perri AM, Friendship RM, Harding JCS, O’Sullivan TL. An investigation of iron deficiency and anemia in piglets and the effect of iron status at weaning on post-weaning performance. J Swine Health Prod. 2016;24:10-20.
21. Egeli AK, Framstad T, Morberg H. Clinical biochemistry, haematology and body weight in piglets. Acta Vet Scand. 1998;39:381-393.
* Non-refereed reference.
