| Original research | Peer reviewed |
Cite as: Mallorquí J, Simon-Grifé M, Ferrer-Soler L, et al. Reduced mortality and morbidity associated with verotoxin 2e-induced edema disease in pigs using a recombinant verotoxin 2e vaccine. J Swine Health Prod. 2018;26(5):253-261.
Also available as a PDF.
SummaryObjective: The efficacy of Vepured, a recombinant verotoxin 2e (VT2e) vaccine, against clinical signs and mortality of VT2e-induced toxemia was evaluated in a controlled experimental challenge. Materials and methods: Piglets free of VT2e neutralizing antibodies (NAb) were selected and blocked by weight and litter and randomly allocated between three groups: vaccinated (n = 32); non-vaccinated (n = 32); and non-vaccinated, non-challenged (n = 10). Piglets were vaccinated intramuscularly with 1 mL of Vepured (vaccinated) or phosphate-buffered saline (non-vaccinated) at two days of age. The onset and duration of protection were investigated via intravenous VT2e challenge, using mortality and clinical signs related to VT2e-induced toxemia. Results: Mortality in the non-vaccinated piglets was 92.3% and 68.8% at the onset of immunity and through the experiment duration, respectively, whereas all vaccinated piglets survived the challenge. The total clinical score and percentage of animals with clinical signs were greater (P < .05) in the non-vaccinated group. Also, vaccinated pigs had better growth performance than non-vaccinated pigs. Neutralizing antibodies against VT2e were detected in most (78.6%) vaccinated piglets at 21 days and in all vaccinated piglets at 28 days and mean NAb titers (log2) were 3.9 and 4.3, respectively. Moreover, NAb persisted for at least 112 days in most (94.1%) vaccinated animals (mean NAb titer was 3.8). Implications: In this study, active immunization with Vepured conferred effective protection against VT2e-induced toxemia, reducing the presence and severity of clinical signs and preventing mortality related to VT2e-induced toxemia from 21 to 112 days after vaccination. | ResumenObjetivo: En un desafío experimental controlado, se evaluó la eficiencia del Vepured, una vacuna de verotoxina 2e recombinante (VT2e por sus siglas en inglés), contra los signos clínicos y la mortalidad de la toxemia provocada por la VT2e. Materiales y métodos: Se seleccionaron lechones libres de anticuerpos neutralizantes (NAb por sus siglas en inglés) contra VT2e y se organizaron en bloques por peso y camada, y se distribuyeron al azar en tres grupos: vacunados (n = 32); no vacunados (n = 32); y no vacunados, no desafiados (n = 10). A los dos días de edad, los lechones se vacunaron intramuscularmente con 1 mL de Vepured (vacunado) o con solución salina tamponada con fosfato (no vacunado). Se investigaron el inicio y la duración de la protección por medio de un desafío intravenoso de VT2e, midiendo signos clínicos y mortalidad relacionados con la toxemia inducida por VT2e. Resultados: La mortalidad en los lechones no vacunados fue, respectivamente, de 92.3% y 68.8% durante el inicio de la inmunidad y en el experimento de duración de la inmunidad, respectivamente, mientras que todos los lechones vacunados sobrevivieron a la prueba. La puntuación clínica total y el porcentaje de animales con signos clínicos fue mayor (P < .05) en el grupo no vacunado. Así mismo, los cerdos vacunados tuvieron un mejor desempeño de crecimiento comparado con los cerdos no vacunados. Se detectaron anticuerpos neutralizantes contra la VT2e en la mayoría (78.6%) de los cerdos vacunados a los 21 días y en todos los lechones vacunados a los 28 días, la media de los títulos NAb (log2) fue de 3.9 y 4.3, respectivamente. Además, los NAb persistieron por lo menos 112 días en la mayoría (94.1%) de los animales vacunados (la media de los NAb fue de 3.8). Implicaciones: En este estudio, la inmunización activa con Vepured confirió protección efectiva contra la toxemia inducida por VT2e, reduciendo la presencia y severidad de los signos clínicos y previniendo la mortalidad relacionada con la toxemia inducida por VT2e entre 21 y 112 días después de la vacunación. | ResuméObjectif: Évaluer l’efficacité de Vepured, un vaccin recombinant de la vérotoxine 2e (VT2e), à réduire les signes cliniques et la mortalité associés à une toxémie induite par VT2e dans un challenge expérimental contrôlé. Matériels et Méthodes: Des porcelets exempts d’anticorps neutralisants (AcN) contre VT2e ont été sélectionnés et bloqués en fonction du poids et de la portée et distribués de manière aléatoire en trois groupes: vaccinés (n = 32); non-vaccinés (n = 32); non-vaccinés, non-challengés (n = 10). Les porcelets étaient vaccinés par voie intramusculaire avec 1 mL de Vepured (vacciné) ou de la saline tamponnée (non-vacciné) à 2 jours d’âge. Le début et la durée de la protection étaient étudiés par challenge intraveineux avec VT2e, se servant de la mortalité et des signes cliniques associés à une toxémie induite par VT2e. Résultats: La mortalité chez les porcelets non-vaccinés était de 92.3% et 68.8% au début de l’immunité et au long de la durée de l’expérience, respectivement, alors que les porcelets vaccinés ont survécu au challenge. Le pointage clinique total et le pourcentage des animaux avec des signes cliniques étaient supérieurs (P < .05) dans le groupe des animaux non-vaccinés. De plus, les porcs vaccinés avaient des meilleures performances de croissance que les porcs non-vaccinés. Des AcN contre VT2e ont été détectés dans la majorité (78.6%) des porcelets vaccinés à 21 j d’âge et chez tous les porcelets vaccinés à 28 j d’âge et les titres d’AcN moyens (log2) étaient 3.9 et 4.3, respectivement. De plus, les AcN ont persisté pour au moins 112 j dans la plupart (94.1%) des animaux vaccinés (titre AcN moyen était de 3.8). Implications: Dans la présente étude, l’immunisation active avec Vepured a conféré une protection efficace contre une toxémie induite par VT2e, réduisant la présence et la sévérité des signes cliniques et prévenant la mortalité reliée à la toxémie causée par VT2e entre les jours 21 et 112 après la vaccination. |
Keywords: swine, edema disease, vaccine, verotoxin, Vepured
Search the AASV web site
for pages with similar keywords.
Received: June 27, 2017
Accepted: March 12, 2018
Edema disease (ED) is an enterotoxemia caused by certain Escherichia coli colonizing the small intestine and producing verotoxin (VT2e). The toxin, also known as Stx2e, is absorbed from the intestine into the bloodstream and damages endothelial cells.1 The endothelial cell damage induces an increase in vascular endothelium permeability of the blood vessels resulting in edema in target tissues such as the brain, intestine, eyelids, lungs, kidneys, and spleen.1 Edema disease is mainly observed in recently weaned piglets, although it can also be observed during the growing and finishing phases.1 Clinical signs associated with ED are mainly neurological: ataxia, convulsions, paralysis, and rigidity as a consequence of edema in the nervous system.2 Experimentally, intravenous injection of pigs with VT2e reproduced clinical signs of ED.3,4 Swelling of the eyelids and throat and dyspnea may also appear in pigs affected by ED.3,5,6 Average morbidity of ED is 10% to 30% and the associated mortality ranges from 50% to over 90%.1
Traditional control of ED is mainly the use of antimicrobial therapy however, its efficacy is poor because VT2e has already been absorbed into circulation when clinical signs become apparent.1 Furthermore, with on-going international pressure to decrease antibiotic use in agriculture due to its perceived link with increasing antibiotic resistance,7,8 an efficacious vaccine is required to induce a protective immune response against this disease.
Therefore, immunoprophylaxis seems to be a promising approach. Active immunity against an intravenous challenge with VT2e toxoid, prepared with glutaraldehyde or formaldehyde, was shown to be efficacious in previous studies.9,10 However, these vaccine candidates were not safe because of residual toxicity.9,10 Conversely, recombinant VT2e vaccines have proved to induce protection against ED and also proved safe due to no residual toxicity both in challenge experiments and in field trials.4,11-13 In the present study, we evaluated the protection induced in piglets against VT2e-induced toxemia by a recombinant VT2e vaccine. The onset of immunity (OOI) and the duration of immunity (DOI) against VT2e-induced toxemia were studied.
Materials and methods
All procedures involving animals were conducted in accordance with the European Union Guidelines for Animal Welfare (Directive 2010/63/UE) and approved by the Ethical Committee of HIPRA Scientific SLU and The Department of Agriculture, Livestock, Fisheries and Food of the Catalonia Government (file: 8294).
VT2e toxin production
The VT2e toxin used in the seroneutralization assays and in animal challenges was prepared from E coli strain 107/86 (O139:K12:H1).14,15 Escherichia coli 107/86 was cultured in Bacto Tryptic Soy Broth (Becton Dickinson, Le Pont-de-Claix, France) with Bacto Yeast Extract (Becton Dickinson, Erembodegem, Belgium) medium at 37°C for 3 hours. Bacterial cells were then collected by centrifugation (8000g, 20 minutes) and resuspended in phosphate-buffered saline (PBS; Sigma-Aldrich Company Ltd, Dorset, England) with Polymyxin B (1 × 104 IU/mL; Xellia, Copenhagen, Denmark). After incubation at 37°C for 1 hour, the supernatant was recovered by centrifugation (11,500g, 30 minutes), filtered, and stored at -20°C.
The 50% cytotoxic dose (CD50) of VT2e was 3.1 × 105 CD50/mL, evaluated in vitro using Vero cells according to the procedure published by Gentry and Dalrymple.16
Test product
One milliliter of Vepured vaccine (Laboratorios HIPRA S.A., Amer, Spain) contains 600 ELISA Units of Antigenic Mass of purified recombinant verotoxin 2e adjuvanted with 2117 mg of aluminum hydroxide and 10 mg of diethylaminoethyl-dextran hydrochloride.17
Experimental design
The piglets included in this study were obtained from a commercial farm located in Catalonia, Spain. The farm was considered free of ED because it did not have a history of ED outbreaks, the animals did not present ED clinical signs, and verotoxigenic E coli was not found in the feces as analyzed by bacteriological diagnosis. One week before the trial, blood samples were collected from 15 sows to confirm that they did not have VT2e neutralizing antibodies (NAb). Seventy-four piglets from VT2e NAb-free sows were selected and blocked by weight and litter and assigned into 3 groups: vaccinated (n = 32); non-vaccinated (n = 32); and non-vaccinated, non-challenged (sentinel, n = 10). Between each block group, the average weight was equivalent and piglets from each litter were represented in each group. Blood samples were obtained from these piglets before vaccination to confirm that they did not have VT2e NAb on day 0 of the study.
Piglets were weaned at approximately 21 days of age and raised on the commercial farm until the challenge period when they were moved to the experimental isolation unit of the HIPRA Scientific SLU facilities.
Piglet immunization and challenge
Vaccinated piglets were injected intramuscularly at two days of age with 1 mL of Vepured (batch number: P.86YG). Non-vaccinated piglets received 1 mL of PBS intramuscularly. To evaluate the safety of Vepured vaccine, the piglets were monitored daily during the post-vaccination period for local clinical signs such as inflammation and nodules and general clinical signs including edema and neurological signs related to ED.
The OOI experiment was conducted 21 days after treatment administration, when 14 piglets from the vaccinated group (one piglet died before the challenge) and 13 piglets from the non-vaccinated group (two piglets died before the challenge) were given VT2e toxin (4.7 × 104 CD50/kg) intravenously. The DOI experiment was conducted 112 days after treatment administration, when an additional 17 piglets from the vaccinated group and 16 piglets from the non-vaccinated group (one piglet died before the challenge) were given VT2e toxin (6 × 103 CD50/kg) intravenously. In the DOI experiment, piglets were anaesthetized intramuscularly with 0.2 mL/kg of a mixture of Xilagesic (Calier, Barcelona, Spain) and Zoletil 100 (Virbac, Barcelona, Spain) before the challenge to ensure the correct administration of VT2e toxin intravenously. Piglets from the sentinel group were distributed equally to each experiment (Table 1). All piglets were observed three times the day of the VT2e toxin challenge and twice a day for 7 days thereafter.
Table 1: Experimental design used to assess the onset and duration of immunity conferred by Vepured against VT2e-induced toxemia
| Experiment | Treatment* | Age at vaccination, d | No. of pigs at vaccination | Toxin challenge dose, CD50/kg | Age at challenge, d | No. of pigs on challenge day |
|---|---|---|---|---|---|---|
| OOI | Vaccinated | 2 | 15 | 4.7 × 104 | 23 | 14† |
| Non-vaccinated | 15 | 4.7 × 104 | 23 | 13† | ||
| Sentinel | 5 | None | NA | 5 | ||
| DOI | Vaccinated | 2 | 17 | 6 × 103 | 114 | 17 |
| Non-vaccinated | 17 | 6 × 103 | 114 | 16‡ | ||
| Sentinel | 5 | None | NA | 5 |
* Vaccinated piglets were injected intramuscularly with 1 mL of Vepured, non-vaccinated piglets were injected intramuscularly with 1 mL of phosphate-buffered saline; sentinel pigs were non-injected and non-challenged.
† Three pigs (one from the vaccinated group and two from the non-vaccinated group) from the OOI experiment died post vaccination and prior to the challenge. Necropsy was performed on these animals to establish the cause of death. The deaths were not related to vaccination. The vaccinated piglet died due to severe anorexia and growth retardation. Intestinal lesions such as congestive small intestine with gas-hemorrhagic content were observed in one piglet from the non-vaccinated group. The other piglet from the non-vaccinated group died due to septicemia and paralysis resulting from tail docking.
‡ On day 112, one animal from the non-vaccinated group died before the challenge as a consequence of anesthesia. This animal had a severe cough on the morning of day 112 and the necropsy revealed macroscopic pneumonia lesions.
VT2e = verotoxin 2e; OOI = onset of immunity; DOI = duration of immunity; NA = not applicable.
The experiment was carried out under blinded conditions, as staff involved in the animal experimental phase, specifically those who performed clinical evaluations, were not aware of the treatment received by each individual animal. During the post-challenge period, clinical signs were scored depending on their severity. Palpebral and throat edema, tremors, ataxia, or mild dyspnea were scored as 1 (mild clinical signs). The presence of paralysis, opisthotonos, extensor rigidity, or severe dyspnea were scored as 2 (severe clinical signs). The total clinical signs score was calculated for each animal as the ratio between the summation of the daily clinical signs score and the number of days for which the animal lived. During the post-challenge period, piglets were euthanized for ethical reasons with an intravenous overdose of sodium pentobarbital ante finem after showing paralysis or opisthotonos at two consecutive observations. Before euthanasia, pigs were anaesthetized intramuscularly with 0.2 mL/kg of a mixture of Xilagesic and Zoletil 100.
Blood samples were collected before vaccination (day 0), before the challenge (OOI = day 21; DOI = day 112) and at the end of the study (7 days after challenge) to evaluate the presence of VT2e NAb. In the DOI experiment, blood samples were collected periodically between vaccination and challenge (days 28, 56, 84, 98, and 105 post vaccination) to follow the antibody response evolution. Additionally, pigs from the DOI experiment were weighed immediately before the challenge and at the end of the study.
Bacteriological isolation
To identify verotoxin-producing E coli, rectal swabs were inoculated into 5 mL peptone water and incubated overnight at 37°C. Samples were then cultured on blood agar plates (Becton Dickinson, San Agustin de Guadalix, Spain) and on MacConkey agar plates (Becton Dickinson, Heidelberg, Germany) and incubated overnight at 37°C. The blood agar plates were used to evaluate the presence of hemolytic E coli in the samples. The MacConkey agar plates were used to confirm the presence of virulence factors related to VT2e producing E coli (adhesion F18 and toxin VT2e genes) by polymerase chain reaction.18
Seroneutralization assay
Fresh Vero cells were counted, suspended to 4 × 104 cells/mL in Glasgow Minimum Essential Medium (GMEM; Gibco, Paisley, United Kingdom), and 0.1 mL were pipetted into 96-well microtiter plates. Monolayers were established by 24 h of incubation at 37°C in 5% CO2.
Serial two-fold dilutions of each serum sample, starting with a 1:2 dilution, were prepared using medium with 2% antibiotics (Penicillin 1 × 104 IU/mL and Streptomycin 10mg/mL; Gibco, Paisley, United Kingdom). Sixty microliters of diluted serum samples were added to a 96-well microtiter plate with 60 µL of VT2e toxin solution (adjusted to 20 CD50/mL in GMEM) or with 60 µL of GMEM. Negative (60 µL of GMEM and 60 µL of GMEM with 2% antibiotics) and positive (60 µL of VT2e toxin solution and 60 µL of GMEM with 2% antibiotics) controls were included on each plate. Diluted serum samples and controls were incubated at 37ºC in 5% CO2 for 1 hour.
After incubation, 100 µL of diluted serum samples and controls were added to Vero cell monolayers and incubated at 37°C for 24 hours in 5% CO2. All serum samples and controls were plated in duplicate.
Supernatant was removed and the wells were washed twice with water (0.2 mL/well). The remaining cells were fixed by adding 37% formaldehyde (0.2 mL/well; Merck, Darmstadt, Germany) for 3 min. Formaldehyde was removed and the plates were stained with crystal violet (0.2 mL/well; Merck, Darmstadt, Germany) for 20 min. Excess stain was removed by rinsing with water and the stain was eluted with ethanol (0.1 mL/well; Panreact, Darmstatd, Germany). Absorbance was determined at 595 nm in a spectrophotometer.
Using optical density (OD), the percentage of live cells was calculated for the positive control (average of positive control OD/ average negative control OD × 100). The percentage of live cells was calculated for each diluted serum sample (average OD of toxin-diluted serum sample/average OD of GMEM-diluted serum sample ×100).
The NAb titer was defined as the reciprocal of the highest serum dilution that neutralized 50% of the cytopathic effect of the VT2e toxin used in the assay. A serum dilution neutralized at least 50% of the cytopathic effect of the VT2e when the percentage of live cells was greater than the percentage of live cells in the positive control + ([100 – percentage of live cells in the positive control]/2). Sera with a NAb titer equal or greater than 2 were considered positive for VT2e NAb, while sera with a NAb antibody titer less than 2 were considered negative for VT2e NAb.
Statistical analysis
Data analyses were conducted using the IBM SPSS Statistics 22 software (IBM Corp, Armonk, New York). The total clinical signs score observed after the challenge in the vaccinated groups were compared with the control group using a Kruskall-Wallis test. The percentage of animals with clinical signs after the challenge and mortality observed in the vaccinated groups were compared with the control group using the Chi-square test. Analysis of variance with a post-hoc Scheffé test was used to compare body weight between groups. A significance level of P < .05 was used for all variables evaluated.
The NAb titers were transformed to base 2 logarithms to calculate the means and confidence intervals.
Results
Safety of Vepured
No evidence of clinical signs or local reactions caused by the intramuscular administration of Vepured to two-day old piglets negative for VT2e NAb were observed.
Protection against VT2e-induced toxemia after OOI
Antibody response. On day 21 of the study, the majority (11 of 14) of piglets from the vaccinated group had NAb against VT2e, with a 3.9 mean NAb titer. Seven days after the challenge, all vaccinated piglets were positive for VT2e NAb and the mean NAb titer increased to 8.4. The animals from the non-vaccinated group were negative for VT2e NAb at day 21 and the sole surviving animal was negative for VT2e NAb at the end of the study. Animals from the sentinel group remained negative for VT2e NAb throughout the study (Table 2).
Table 2: Presence of VT2e neutralizing antibodies after vaccination with Vepured*
| Experiment | Day of study | Vaccinated | Non-vaccinated | |
|---|---|---|---|---|
| Pigs positive for NAb, No. (%) | NAb titer, mean (95% CI)† | Pigs positive for NAb, No. (%) | ||
| OOI | 0 | 0 (0) | 0 | 0 (0) |
| 21 | 11 (78.6) | 3.9 (2.5-5.3) | 0 (0) | |
| 28 | 14 (100) | 8.4 (7.6-9.3) | 0 (0) | |
| DOI | 0 | 0 (0) | 0 | 0 (0) |
| 28 | 17 (100) | 4.3 (3.2-5.4) | 0 (0) | |
| 56 | 17 (100) | 4.8 (3.8-5.9) | 0 (0) | |
| 84 | 17 (100) | 4.8 (4.0-5.6) | 0 (0) | |
| 98 | 17 (100) | 4.2 (3.5-5.0) | 0 (0) | |
| 105 | 16 (94.1) | 3.8 (3.1-4.5) | 0 (0) | |
| 112 | 16 (94.1) | 3.8 (3.1-4.5) | 0 (0) | |
| 119 | 17 (100) | 6.0 (4.7-7.3) | 0 (0) | |
* All pigs (5 animals in each experiment) in the sentinel non-vaccinated, non-challenged group remained negative for neutralizing antibodies against VT2e throughout the study.
† Antibody titers are expressed as log2 value of the reciprocal of the highest dilution causing neutralization.
VT2e = verotoxin 2e; NAb = neutralizing antibody; OOI = onset of immunity; DOI = duration of immunity.
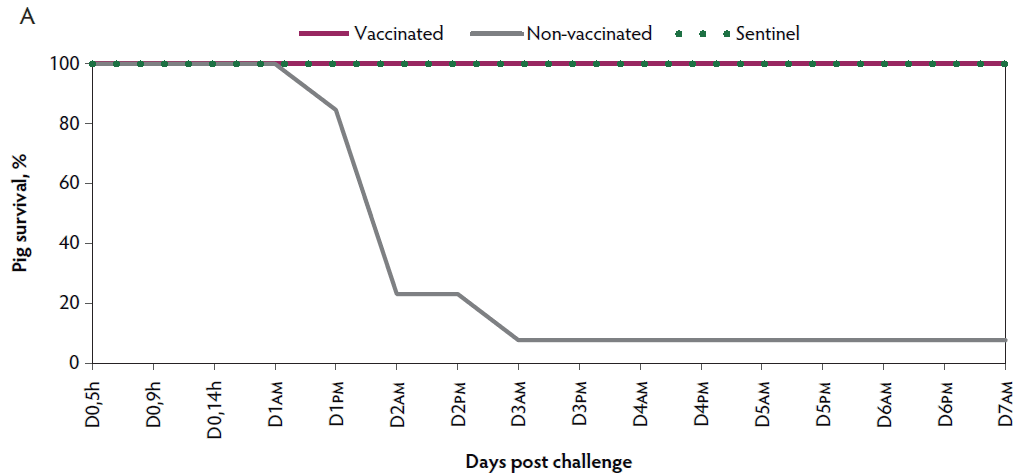
Mortality. In the non-vaccinated group, rapid disease progression was observed, with 92.3% (12 of 13) mortality within three days post challenge. Four of the piglets were euthanized ante finem and the others died after showing mild or severe clinical signs of VT2e-induced toxemia. In contrast, there was 0% mortality observed in vaccinated piglets after the challenge (Figure 1A).
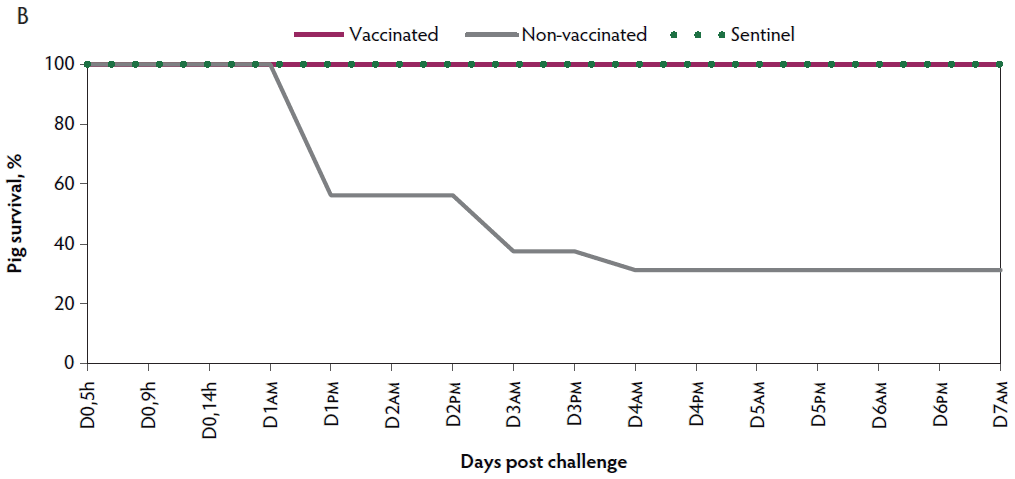
Figure 1: Survival curve after VT2e challenge. Observation of clinical signs occurred at 5, 9, and 14 hours post challenge and twice a day for 7 days thereafter. A, Percentage of piglets alive after a VT2e challenge given 21 days post vaccination (OOI experiment). B, Percentage of piglets alive after a VT2e challenge given 112 days post vaccination (DOI experiment). VT2e = verotoxin 2e; OOI = onset of immunity; DOI = duration of immunity.
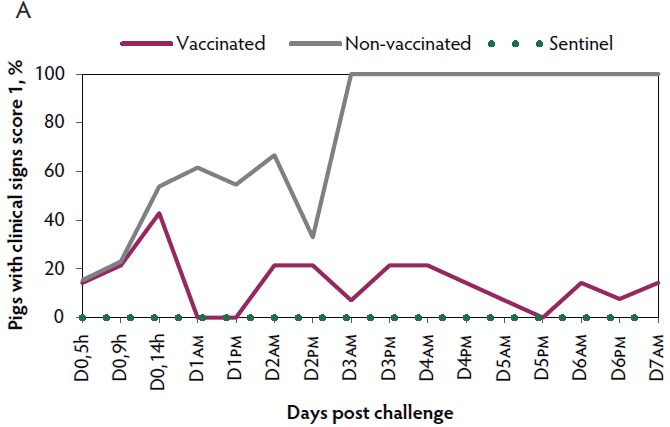
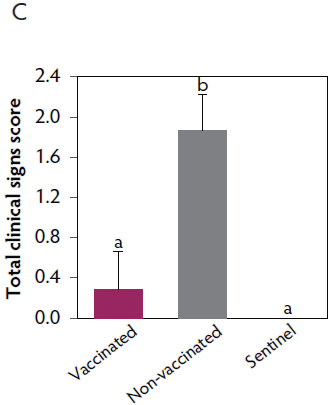
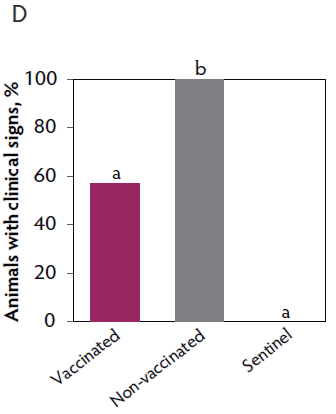
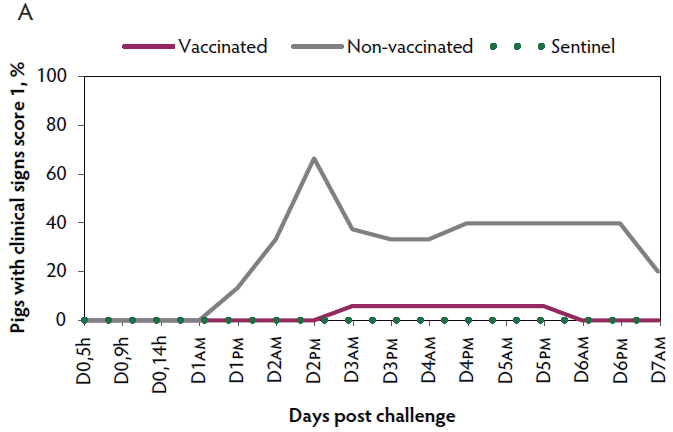
Clinical signs. The onset of clinical signs of VT2e-induced toxemia, such as palpebral and throat edema were observed at 5 hours after challenge in both vaccinated and non-vaccinated groups. During the 7 days post challenge, all piglets from the non-vaccinated group showed clinical signs related to VT2e-induced toxemia and most of them (8 of 13) showed severe clinical signs. In fact, there was 100% mortality of piglets that demonstrated severe clinical signs. In contrast, none of the vaccinated piglets showed severe clinical signs associated with VT2e-induced toxemia. Only 8 of 14 vaccinated piglets showed mild clinical signs related to VT2e-induced toxemia and only 2 of 14 showed clinical signs 7 days post challenge. Clinical signs associated with VT2e-induced toxemia were not observed in piglets from the sentinel group (Figure 2A and B). The total clinical score was greater (P < .05) in the non-vaccinated group than in the vaccinated group (Figure 2C). Additionally, the percentage of animals with clinical signs related to VT2e-induced toxemia was greater (P < .05) in the non-vaccinated group (100%) compared to the vaccinated group (57.1%; Figure 2D).
Figure 2: Clinical signs associated with VT2e-induced toxemia observed in piglets after a VT2e challenge given 21 days post vaccination (OOI experiment). Observation of clinical signs occurred at 5, 9, and 14 hours post challenge and twice a day for 7 days thereafter. A, Percentage of animals with mild clinical signs scored as 1 after challenge. B, Percentage of animals with severe clinical signs scored as 2 after challenge. C, Total clinical signs score from day 0 to day 7 post challenge. D, Percentage of animals with clinical signs from day 0 to day 7 post challenge. Significant differences are represented with different superscript letters (Figure C: Kruskall-Wallis; P < .05 and Figure D: Chi-square statistic; P < .05). VT2e = verotoxin 2e; OOI = onset of immunity.
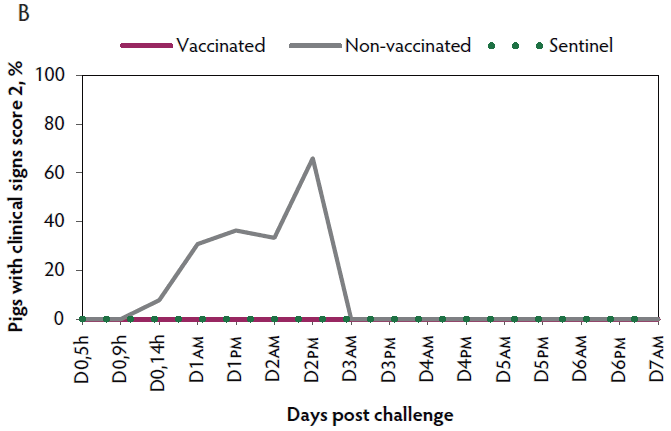
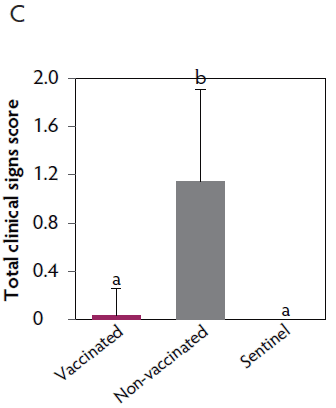
Duration of immunity against VT2e-induced toxemia
Antibody response. At 112 days post vaccination, most piglets (16 of 17) remained positive for VT2e NAb (Table 2). The mean NAb titers remained persistent from day 28 to 112 post vaccination, fluctuating from 3.8 to 4.3. However, after the DOI challenge (119 days after vaccination), the mean NAb titers increased from 3.8 to 6. The animals from the non-vaccinated group and the sentinel group remained negative for VT2e NAb throughout the study (Table 2).
Mortality. Rapid disease progression was observed in the non-vaccinated group, with 68.8% (11 of 16) mortality within 4 days post challenge. Nine pigs from the non-vaccinated group were euthanized ante finem after showing severe clinical signs related to VT2e-induced toxemia during two consecutive observations. In contrast, there was 0% mortality observed in the vaccinated group post challenge (Figure 1B).
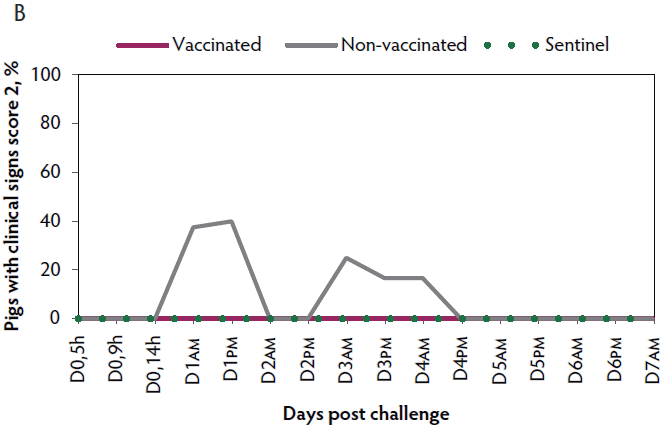
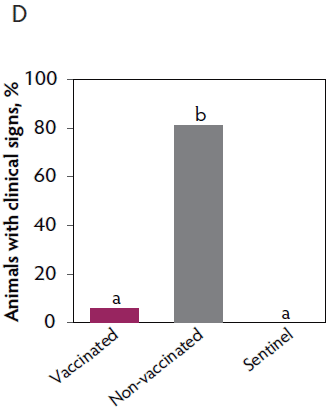
Clinical signs. The onset of clinical signs of VT2e-induced toxemia in the non-vaccinated group was observed 24 hours post challenge. Thirteen of 16 pigs from this group showed clinical signs related to VT2e-induced toxemia during the post-challenge period and in most of them (10 of 13), the clinical signs were severe. In contrast, none of the 17 vaccinated piglets showed severe clinical signs during the 7 days post challenge. Only one of the vaccinated piglets developed mild clinical signs associated with VT2e-induced toxemia from days 3 to 5 post challenge. Clinical signs associated with VT2e-induced toxemia were not observed in piglets from the sentinel group (Figure 3A and B). The total clinical score was greater (P < .05) in the non-vaccinated group than in the vaccinated group (Figure 3C). Additionally, the percentage of animals with clinical signs related to VT2e-induced toxemia was greater (P < .05) in the non-vaccinated group (81.3%) compared to the vaccinated group (5.8%; Figure 3D).
Figure 3: Clinical signs associated with VT2e induced toxemia observed in piglets after a VT2e challenge given 112 days post vaccination (DOI experiment). Observation of clinical signs occurred at 5, 9, and 14 hours post challenge and twice a day for 7 days thereafter. A, Percentage of animals with mild clinical signs scored as 1 after challenge. B, Percentage of animals with severe clinical signs scored as 2 after challenge. C, Total clinical signs score from day 0 to day 7 post challenge. D, Percentage of animals with clinical signs from day 0 to day 7 post challenge. Significant differences are represented with different superscript letters (Figure C: Kruskall-Wallis; P < .05 and Figure D: Chi-square statistic; P < .05). VT2e = verotoxin 2e; DOI = duration of immunity.
Weight gain. At the end of the study, the body weight of vaccinated pigs was greater than that of non-vaccinated pigs (P < .05). Seven days after challenge with VT2e toxin, vaccinated pigs had gained an average of 4.7 kg whereas non-vaccinated pigs that survived the challenge had lost an average of 8.3 kg. No statistical differences were observed between the vaccinated and the sentinel groups (Table 3).
Table 3: Piglet weights after duration of immunity VT2e challenge*
| Day of challenge | End of study | |||
|---|---|---|---|---|
| Treatment | N | Weight, mean (SD), kg | N | Weight, mean (SD), kg |
| Vaccinated | 17 | 34.3 (12.0)a | 17 | 39.0 (12.8)a |
| Non-vaccinated | 16 | 31.8 (12.6)a | 5 | 23.5 (5.6)b |
| Sentinel | 5 | 35.5 (8.4)a | 5 | 40.5 (9.8)a,b |
* In the DOI study, animals were challenged 112 days after vaccination. Seven days after challenge (day 119), at the end of the study, all the animals were euthanized.
a,b Values within a column with different superscripts are significantly different (ANOVA; P < .05).
DOI = duration of immunity; VT2e = verotoxin 2e.
Discussion
In the present study, the efficacy of Vepured was evaluated in laboratory conditions by means of an intravenous VT2e toxin challenge with the intent to demonstrate the reduction of morbidity and mortality attributed to VT2e-induced toxemia. The challenge model used produced clinical signs typically associated with ED. It is expected that this vaccine will protect against outbreaks of ED. However, further large-scale clinical trials on commercial pig farms are needed to confirm the efficacy of Vepured against ED under natural field conditions.
The clinical signs of ED are most commonly observed in the first weeks after weaning. For this reason, early vaccination of piglets and a rapid onset of immunity after weaning are required. In the OOI experiment, two-day old piglets were immunized and were challenged with wild-type VT2e 21 days later. The results support that Vepured vaccination induced an immune response within 21 days that reduced morbidity and mortality associated with VT2e-induced toxemia. In contrast, all but one pig in the non-vaccinated group died after the challenge. Clinical signs of ED can also appear in the fattening herd, resulting in morbidity, mortality, and affecting pig performance.1 The DOI study suggested that one vaccine dose was able to protect piglets in the fattening period, for at least 112 days after vaccination, reducing morbidity and mortality associated with VT2e-induced toxemia. Previous studies have demonstrated that vaccination protected pigs from weight loss associated with VT2e toxemia.10,11,19 In the present DOI experiment, body weight before the challenge was equivalent in both the vaccinated and non-vaccinated groups. However, after the challenge, pigs from the vaccinated group performed better than those from the non-vaccinated group.
The administration protocol of Vepured to two-day old piglets could allow a farmer to incorporate this vaccination with other routine management practices, such as iron administration. Additionally, the small volume of vaccine (1 mL) is desirable for two-day old piglets. In a previous study, Vepured vaccine efficacy was demonstrated even in the presence of maternally derived antibodies (MDA), although MDA to VT2e seems to be atypical in the field.20
In previous studies, VT2e NAb in pigs from farms with and without clinical signs of ED were not detected, suggesting that VT2e does not normally induce detectable levels of antibodies after a natural infection.21 In the present study, VT2e NAb were detected in most of the vaccinated piglets at 21 days after vaccination and in all piglets at 28 days after vaccination (mean neutralization antibody titers of 3.9 and 4.3, respectively). Moreover, NAb persisted for at least 112 days in all the vaccinated animals (mean neutralization antibody titers of 3.8) except for one animal, which was found to be negative for VT2e NAb from day 105. Interestingly, this was the only animal from the vaccinated group affected by mild clinical signs related to VT2e-induced toxemia after the experimental challenge but was still protected from mortality. This specific animal presented VT2e NAb seven days after infection (day 119 after vaccination) indicating that vaccination generated an immune memory, which allowed the immune system to respond more rapidly and effectively to the toxin. This circumstance was also observed with three vaccinated animals that were negative for VT2e NAb at day 21 post vaccination but before the challenge. Neutralizing antibodies were not detected in any of the non-vaccinated animals at any time during the study. The results of this study suggest that the presence of NAb may predict protection against VT2e-induced toxemia. However, this was not evaluated in this study and further research is warranted in this area.
In agreement with previous reports where experimental vaccines were tested,4,11 the results of this study demonstrated a reduction in the presence and severity of clinical signs of VT2e-induced toxemia after experimental challenge in the vaccinated group.
Implications
Under the conditions of this study:
- Mortality associated with VT2e-induced toxemia was reduced in piglets immunized with Vepured vaccine.
- Morbidity and severity of clinical signs associated with VT2e-induced toxemia was reduced in piglets immunized with Vepured vaccine.
Acknowledgments
Drs Mallorquí and Simon-Grifé equally contributed to the studies presented in this paper. The authors wish to thank Alberto Moreno, Cristina Rodríguez, Eva Andreu, Ester Puigvert, Marta Bau, and Marc Guàrdia for their valuable assistance during the development of the Vepured vaccine. We also thank Judit Moreno for her expert assistance in the immunological analysis and Ariadna Pararols for her essential technical assistance in the animal experimental phase.
Conflict of interest
The authors are employees of HIPRA. This study was funded by HIPRA, which provided the animals, the E coli strain, and the Vepured vaccine and carried out the study. The study design and all procedures, data collection, registries, manipulation, and analysis of samples and data were conducted at HIPRA by HIPRA personnel.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Fairbrother JM, Gyles C. Colibacillosis. In: Zimmerman J, Karriker L, Ramirez A, Schwartz K, Stevenson G, eds. Disease of swine. 10th ed. Ames, Iowa: Blackwell Publishing; 2012:723-749.
2. Clugston RE, Nielsen NO, Smith DLT. Experimental edema disease of swine (E. coli enterotoxemia). III. Pathology and pathogenesis. Can J Comp Med. 1974;38:34-43.
3. MacLeod DL, Gyles CL, Wilcock BP. Reproduction of edema disease of swine with purified Shiga-like toxin-II variant. Vet Pathol. 1991;28:66-73.
4. Oanh TK, Nguyen VK, de Greve H, Goddeeris BM. Protection of piglets against edema disease by maternal immunization with Stx2e toxoid. Infect Immun. 2012;80:469-473.
5. Gannon VPJ, Gyles CL, Wilcock BP. Effects of Escherichia coli Shiga-like toxins (verotoxins) in pigs. Can J Vet Res. 1989;53:306-312.
6. Imberechts H, De Greve H, Lintermans P. The pathogenesis of edema disease in pigs. A review. Vet Microbiol. 1992;31:221-233.
7. Teuber M. Veterinary use and antibiotic resistance. Curr Opin Microbiol. 2001;4:493-499.
8. Phillips I, Casewell M, Cox T, de Groot B, Friis C, Jones R, Nightingale C, Preston R,Waddell J. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J Antimicrob Chemother. 2004;53:28-52.
*9. Awad-Masalmeh M, Reitinger H, Willinger H. Efficacy of edema principle toxin as a vaccine against edema disease of weaned piglets. Proc IPVS Congress. Rio De Janeiro, Brazil. 1988;116.
10. Gordon NA, Whipp SC, Moon HW, O’Brien AD, Samuel JE. An enzymatic mutant of Shiga-like toxin-II variant is a vaccine candidate for edema disease of swine. Infect Immun. 1992;60:485-490.
11. Bosworth BT, Samuel JE, Moon HW, O’Brien AD, Gordon VM, Whipp SC. Vaccination with genetically modified Shiga-like toxin IIe prevents edema disease in swine. Infect Immun. 1996;64:55-60.
12. Makino SI, Watarai M, Tabuchi H, Shirahata T, Furuoka H, Kobayashi Y, Takeda Y. Genetically modified Shiga toxin 2e (Stx2e) producing Escherichia coli is a vaccine candidate for porcine edema disease. Microb Pathog. 2001;31:1-8.
13. Fricke R, Bastert O, Gotter V, Brons N, Kamp J, Selbitz HJ. Implementation of a vaccine against Shigatoxin 2e in a piglet producing farm with problems of Oedema disease: case study. Porcine Health Manag. 2015;1:6. doi:10.1186/2055-5660-1-6.
14. Imberechts H, De Greve H, Schlicker C, Bouchet H, Pohl P, Charlier G, Bertschinger H, Wild P, Vandekerckhove J, Van Damme J, Van Montagu M, Lintermans P. Characterization of F107 Fimbriae of Escherichia coli 107/86, which causes edema disease in pigs, and nucleotide sequences of the F107 major fimbrial subunit gene, fedA. Infect Immun. 1992;60:1963-1971.
15. Bertschinger HU, Bachmann M, Mettler C, Pospischil A, Schraner EM, Stamm M, Sydler T, Wild P. Adhesive fimbriae produced in vivo by Escherichia coli O139:K12(B):H1 associated with enterotoxaemia in pigs. Vet Microbiol. 1990;25:267-281.
16. Gentry MK, Dalrymple JM. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980;12:361-366.
*17. European Medicines Agency. CVMP assessment report for VEPURED. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/veterinary/004364/WC500235001.pdf. Published June 2017. Accessed June 15, 2017.
18. Zhang W, Zhao M, Ruesch L, Omot A, Francis D. Prevalence of virulence genes in Escherichia coli strains recently isolated from young pigs with diarrhea in the US. Vet Microbiol. 2007;123:145-152.
19. Johansen M, Andresen LO, Jorsal SE, Thomsen LK, Waddell TE, Gyles CL. Prevention of edema disease in pigs by vaccination with verotoxin 2e toxoid. Can J Vet Res. 1997;61:280-285.
*20. Simon-Grifé M, Mallorquí J, Ferrer-Soler L, Roca M, Saun X, Sitjà M. Maternal antibodies does not interfere with VEPURED efficacy. Proc ESPHM. Prague, Czech Republic. 2017;354.
21. Gannon VPJ, Gyles CL, Friendship RW. Characteristics of verotoxigenic Escherichia coli from pigs. Can J Vet Res. 1988;52:331-337.
* Non-refereed references.
