| Diagnostic notes | Peer reviewed |
Cite as: Rawal G, Arruda P, Rademacher C, et al. General overview of the detection of Mycoplasma hyopneumoniae DNA by quantitative polymerase chain reaction in diagnostic cases submitted to the Iowa State University Veterinary Diagnostic Laboratory from 2004 to 2016. J Swine Health Prod. 2018;26(6):309-315.
Also available as a PDF.
SummaryMycoplasma hyopneumoniae (Mhp) is the etiologic agent of enzootic pneumonia and a major causative agent of the porcine respiratory disease complex. This study summarizes and describes the general diagnostic trends on Mhp detection by quantitative polymerase chain reaction (qPCR) in cases submitted to the Iowa State University Veterinary Diagnostic Laboratory from 2004 to 2016. The following variables were included in the analysis: animal age, geographic location, sample type, season, and submission year. The overall frequency of detection found was 27.04% and ranged from 17.9% to 40.7%. Lung homogenate and bronchial swabs had a greater Mhp qPCR detection rate than other sample types (P < .001) followed by bronchoalveolar lavage (P < .001), while oral fluids had the lowest Mhp detection rate (P < .001). The fall season had a greater percentage of positive Mhp qPCR results when compared to other seasons (P < .001), while spring had the lowest percentage. Finishing-age pigs had a greater percentage of Mhp qPCR detection when compared to other age groups (P < .001), while suckling pigs had the lowest percentage (P < .001). | ResumenEl Mycoplasma hyopneumoniae (Mhp por sus siglas en inglés) es el agente etiológico de la neumonía enzoótica, y un agente causante mayor del complejo respiratorio porcino. Este estudio resume y describe las tendencias de diagnóstico generales para la detección del Mhp por medio de la reacción en cadena de la polimerasa cuantitativa (qPCR por sus siglas en inglés) en casos enviados al Laboratorio de Diagnóstico Veterinario de la Universidad Estatal de Iowa del 2004 al 2016. El análisis incluyó las siguientes variables: edad del animal, localización geográfica, tipo de muestra, estación, y año de presentación. La frecuencia general de detección fue de 27.04% con un rango de 17.9% a 40.7%. Los homogenizados de pulmón y los hisopos bronquiales tuvieron un mayor índice de detección a la qPCR Mhp que otros tipos de muestra (P < .001) seguidos por el lavado bronquioalveolar (P < .001), mientras que los fluidos orales tuvieron el índice más bajo de detección de Mhp (P < .001). El otoño tuvo el mayor porcentaje de resultados positivos a la Mhp qPCR comparado con otras estaciones (P < .001), mientras que la primavera tuvo el porcentaje más bajo. Los cerdos en edad de finalización tuvieron un porcentaje mayor de detección a la Mhp qPCR al compararlos con grupos de otra edad (P < .001), mientras que los cerdos en lactancia tuvieron el porcentaje más bajo (P < .001). | ResuméMycoplasma hyopneumoniae (Mhp) est l’agent étiologique de la pneumonie enzootique et un agent causal majeur du complexe des maladies respiratoires porcines. La présente étude résume et décrit les tendances diagnostiques générales de la détection de Mhp par réaction d’amplification en chaine par la polymérase quantitative (qPCR) dans les cas soumis au Iowa State University Veterinary Diagnostic Laboratory entre 2004 et 2016. Les variables suivantes étaient incluses dans l’analyse: âge de l’animal, localisation géographique, type d’échantillon, saison, et année de soumission. La fréquence globale de détection trouvée était de 27.04% et variait entre 17.9% et 40.7%. Un homogénat de poumon et des écouvillons bronchiaux avaient un taux de détection de Mhp par qPCR plus grand que les autres types d’échantillons (P < .001) suivi par le lavage broncho-alvéolaire (P < .001), alors que les fluides oraux avaient le taux de détection de Mhp le plus bas (P < .001). Le plus grand pourcentage de résultats qPCR positifs pour Mhp a été obtenu à l’automne comparativement aux autres saisons (P < .001), et le pourcentage le plus bas a été trouvé au printemps. Les porcs en période de finition avaient un pourcentage de détection de Mhp par qPCR plus grand comparativement aux autres groupes d’âge (P < .001), alors que les porcelets à la mamelle avaient le pourcentage le plus faible (P < .001). |
Keywords: swine, Mycoplasma hyopneumoniae, polymerase chain reactio
Search the AASV web site
for pages with similar keywords.
Received: November 14, 2017
Accepted: April 25, 2018
Mycoplasma hyopneumoniae (Mhp) is the causative agent of enzootic pig pneumonia,1,2 a respiratory disease affecting pigs worldwide, characterized by significant economic losses due to slower growth, poor feed conversion, and death.3 The losses associated with Mhp were estimated in the range of $375 to $400 million annually in the United States.4 Mycoplasma hyopneumoniae increased the cost of production by $2.50 per pig in the grow-finish phase with an additional increase in the cost of therapeutics by $0.75 to $0.90 per pig at weaning in the United States.5 The isolation of Mhp is challenging due to the fastidious culture requirements and the relatively slow Mhp growth, often resulting in overgrowth by other mycoplasmas including M flocculare and M hyorhinis.6 Therefore, detection of Mhp for diagnostic purposes is typically performed by quantitative polymerase chain reaction (qPCR), which offers rapid turnaround at a high sensitivity and specificity.7-9 Multiple specimens including bronchial swabs, bronchoalveolar lavages, laryngeal swabs, lung homogenates, lungs, nasal swabs, oral fluids, and tracheal swabs have been used to detect Mhp in pigs.
The Iowa State University Veterinary Diagnostic Laboratory (ISU VDL) is one of the largest in the United States receiving over 80,000 cases annually, of which over 75% are swine related. The objective of this study was to summarize and describe the patterns of Mhp DNA detection by qPCR over time from cases received at the ISU VDL from 2004 to 2016. Mycoplasma hyopneumoniae detection rate was reported by year, age group, specimen, season, and US geographic location.
Materials and methods
This study was based on data derived from diagnostic laboratory submissions, so an Institutional Animal Care and Use Committee approval was not needed.
Eligibility and exclusion criteria
All porcine cases where Mhp qPCR was performed were gathered. For this study, a case was defined as one accession identification number with at least one sample. A case was considered positive when there was at least one sample testing positive for Mhp by qPCR. The data were screened and cases with non-conventional sample types for Mhp diagnostics, including cell cultures, conjunctival swab, environmental, extract, inoculum, liver, semen, and vaccine, were excluded from the analysis. Infrequent sample types (those comprising less than 1% of submissions) such as fibrin, fibrin swab, fluid, fresh tissue, lymph node, tonsil, and tonsil scrapings were also excluded from the analysis, as were cases with inconclusive or suspect results. Submissions were classified by season (December through February as winter, March through May as spring, June through August as summer, and September through November as fall), age group (pigs less than 3 weeks of age were defined as suckling, 3 to 6 weeks as nursery, 7 to 11 weeks as growing, 12 weeks to 200 days as finishing, and greater than 200 days as adult), and sample type, which included bronchial swab, bronchoalveolar lavage, laryngeal swab, lung, lung homogenate, nasal swab, oral fluid, swab, tracheal swab, and other. All specimens are described using the same terminology reported in the VDL submission form. Samples labeled as saliva were included under the oral fluid category and samples labeled as lung swab, multiple, oropharyngeal swab, pharyngeal swab, pleural swab, serum, and tonsil swab were identified as other.
Microsoft Excel (Microsoft, Redmond, Washington) was used to organize the data and Tableau (Tableau, Seattle, Washington) was used to produce plots.
Statistical analysis
Statistical analysis was performed with SAS 9.4 software (SAS Institute Inc, Cary, North Carolina). For statistical analysis, the frequency of positive results was compared across groups (age, season, and sample type) with Pearson’s Chi-square test. The significance level was determined at α = .05.
The K-means clustering method was implemented to categorize the Mhp detection rate as low, medium, or high for specimen types. The aim of the K-means algorithm is to divide the set of observations into K groups, minimizing the within-cluster sum of squares.10 The K-means procedure searched for 3 partitions with locally-optimal, within-cluster sum of squares by moving points from one cluster to another.
Results
A total of 94,986 qPCR results from 37,574 cases were used in the analysis. In total, 1128 qPCR results were excluded from the data analysis due to non-conventional or infrequent sample types and 178 qPCR samples were excluded due to inconclusive status of results. Analysis were conducted with 93,680 samples, from which 25,339 (27%) tested positive for Mhp qPCR, ranging from 17.9% to 40.7% (Table 1).
Table 1: Mycoplasma hyopneumoniae DNA detection by qPCR at the ISU VDL from 2004 to 2016
| Year | Total porcine case submissions, No. | Porcine case submissions for Mhp qPCR testing, No. (%) | Cases positive for Mhp, No. |
|---|---|---|---|
| 2004 | 17,744 | 1175 (6.6) | 263a |
| 2005 | 20,916 | 2402 (11.5) | 680b |
| 2006 | 28,112 | 4391 (15.6) | 1235b |
| 2007 | 27,846 | 4241 (15.2) | 1195b |
| 2008 | 25,254 | 4456 (17.6) | 1499c |
| 2009 | 22,992 | 6291 (27.4) | 1187d |
| 2010 | 25,216 | 5014 (19.9) | 1646c |
| 2011 | 28,938 | 8948 (30.9) | 2789e |
| 2012 | 33,892 | 8667 (25.6) | 3527f |
| 2013 | 38,656 | 9022 (23.3) | 2133a |
| 2014 | 51,146 | 9163 (17.9) | 2513b |
| 2015 | 53,382 | 13,609 (25.5) | 3756b |
| 2016 | 55,275 | 16,301 (29.5) | 2916d |
abcdef Different letters indicate significant differences (P < .005; Chi-square analysis for percent of Mhp positive cases over time).
qPCR = quantitative polymerase chain reaction; ISU VDL = Iowa State University Veterinary Diagnostic Laboratory; Mhp = Mycoplasma hyopneumoniae.
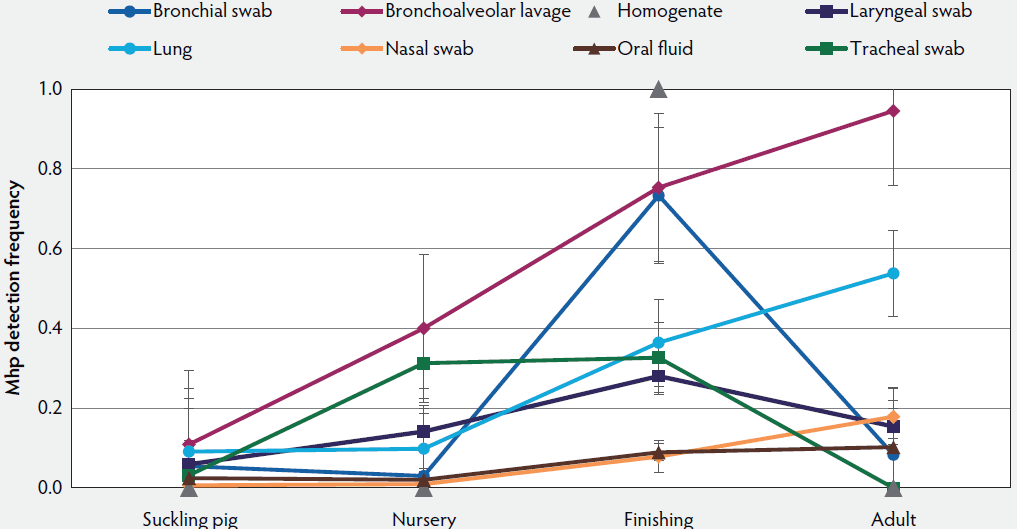
The most common sample types submitted for Mhp qPCR at the ISU VDL were lung, followed by oral fluids, nasal swab, and bronchoalveolar lavage (Figure 1). The Mhp qPCR detection rate was 64% in bronchial swab (2503 of 3909), 49% in bronchoalveolar lavage (3370 of 6872), 18% in laryngeal swab (313 of 1777), 33% in lung (13517 of 40633), 66% in lung homogenate (962 of 1455), 10% in nasal swab (808 of 7892), 8% in oral fluids (1850 of 23804), 18% in swab (341 of 1922), 34% in tracheal swab (606 of 1777), and 27% in other (965 of 3574). Lung homogenate and bronchial swab had a greater Mhp qPCR detection rate than other sample types (P < .001), followed by bronchoalveolar lavage (P < .001), while oral fluids had the lowest Mhp detection rate (P < .001). Using the K-mean clustering method, bronchial swab, bronchoalveolar lavage, and lung homogenate had a high detection rate; lung, tracheal swab, and other had a medium detection rate; and laryngeal swab, nasal swab, oral fluid, and swab had a relatively low detection rate. The fall season had a greater percentage of positive Mhp qPCR results when compared to other seasons (P < .001), while spring had the lowest percentage (P < .001) (Figure 2). Finishing-age pigs had a greater percentage of Mhp qPCR detection when compared to other age groups (P < .001), while suckling pigs had the lowest percentage (P < .001) (Figure 3). The majority (30%) of cases submitted to the ISU VDL for Mhp testing by qPCR were from Iowa, but there were cases from 33 other states (Figure 4). Bronchoalveolar lavage had a greater detection rate of Mhp by qPCR, as compared to other specimens, for all age groups except growing pigs (Figure 5). In growing pigs, lung homogenate had the highest detection rate among the specimens. Nasal swab had the lowest Mhp detection rate for the suckling- and finishing-age groups. In growing pigs, oral fluid had a relatively low Mhp detection rate.
Figure 1: Mycoplasma hyopneumoniae DNA detection by specimen type using qPCR on cases submitted to the ISU VDL from 2004 to 2016. For each bar, blue indicates cases that tested negative and red represents cases with at least 1 positive sample. The number at the top of each bar indicates the percentage of positive cases within each specimen. Different letters indicate significant differences (P < .05; Chi-square analysis). qPCR = quantitative polymerase chain reaction; ISU VDL = Iowa State University Veterinary Diagnostic Laboratory; Mhp = Mycoplasma hyopneumoniae.
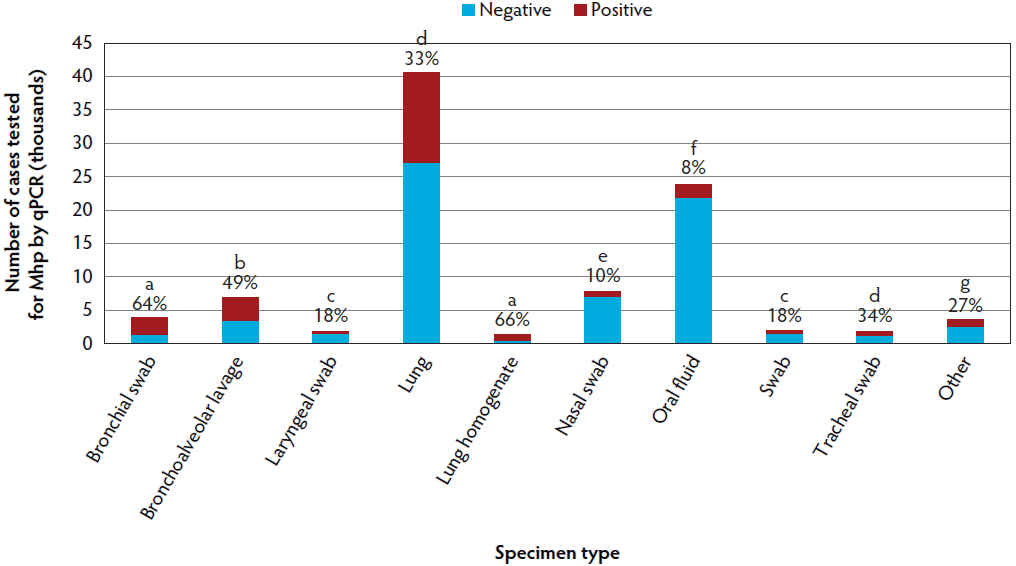
Figure 2: Detection of Mycoplasma hyopneumoniae DNA by season using qPCR on cases submitted to the ISU VDL from 2004 to 2016. Seasons are defined as March through May as spring, June through August as summer, September through November as fall, and December through February as winter. A, the number of Mhp cases submitted in each season per year. B, the percentage of positive cases in each season. qPCR = quantitative polymerase chain reaction; ISU VDL = Iowa State University Veterinary Diagnostic Laboratory; Mhp = Mycoplasma hyopneumoniae.
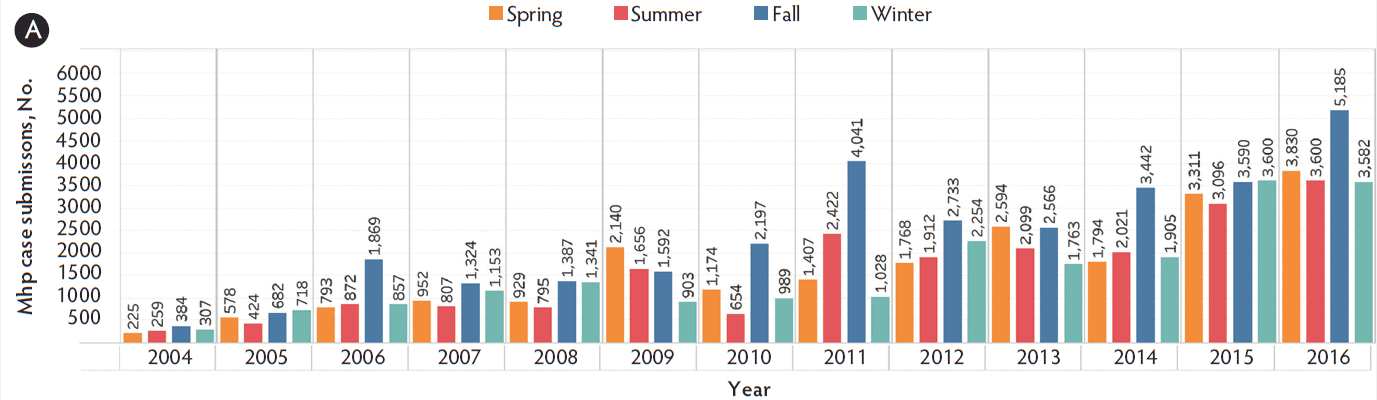
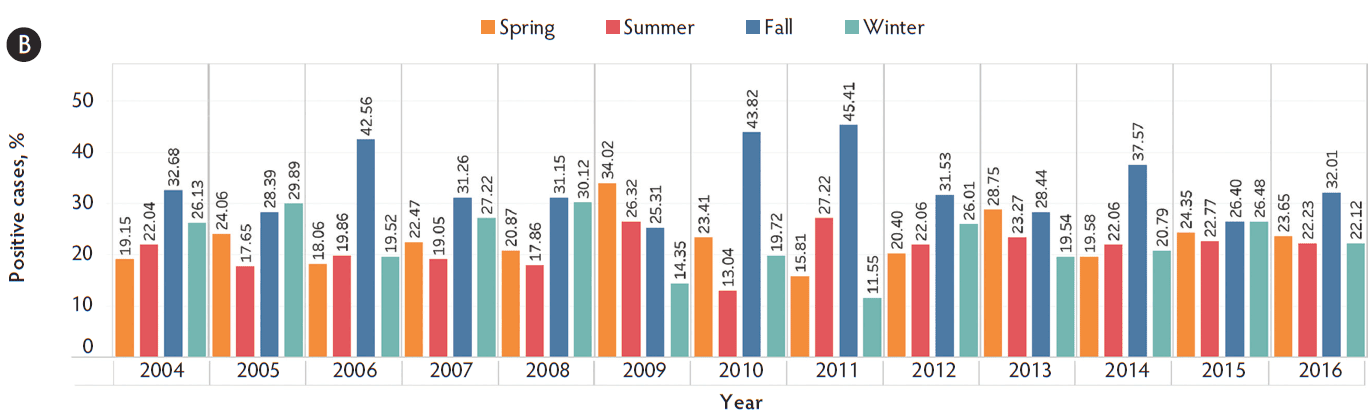
Figure 3: Detection of Mycoplasma hyopneumoniae DNA by age group and season using qPCR on cases submitted to the ISU VDL from 2004 to 2016. Seasons were defined as March through May as spring, June through August as summer, September through November as fall, and December through February as winter. Pigs less than 3 weeks of age were defined as suckling, 3 to 6 weeks as nursery, 7 to 11 weeks as growing, 12 weeks to 200 days as finishing, and greater than 200 days as adult. qPCR = quantitative polymerase chain reaction; ISU VDL = Iowa State University Veterinary Diagnostic Laboratory; Mhp = Mycoplasma hyopneumoniae.
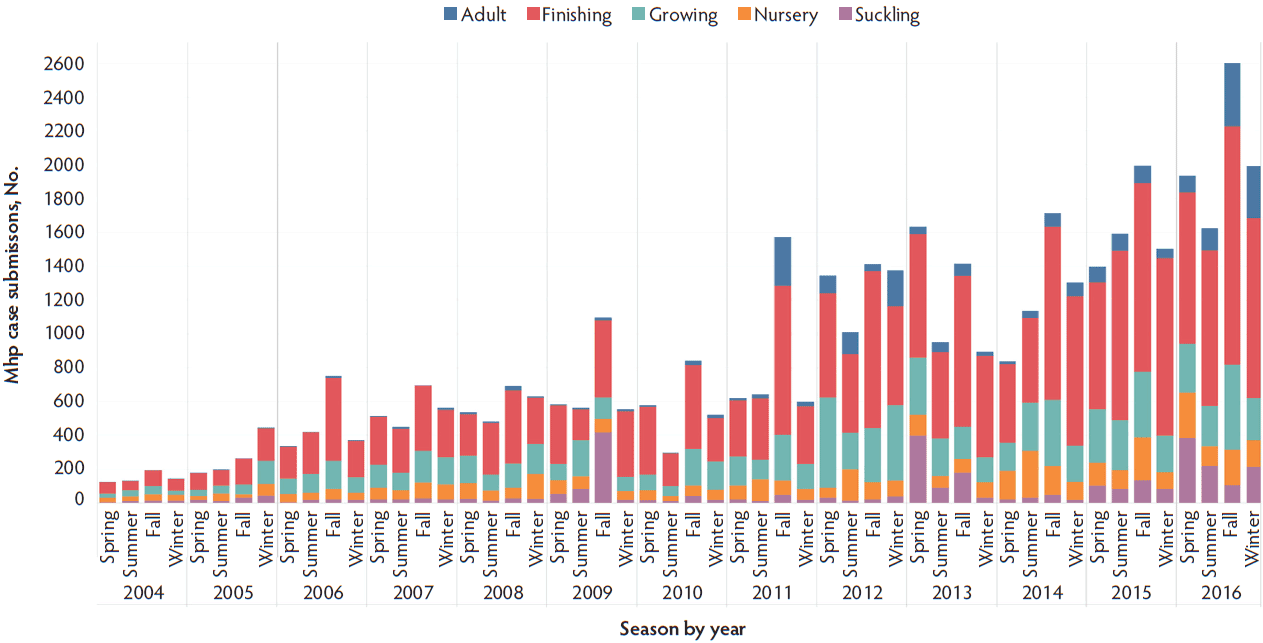
Figure 4: State of origin for cases submitted to the ISU VDL for Mycoplasma hyopneumoniae DNA testing by qPCR from 2004 to 2016. The intensity of the blue color represents the number of cases submitted for Mhp testing to the ISU VDL. ISU VDL = Iowa State University Veterinary Diagnostic Laboratory; qPCR = quantitative polymerase chain reaction; Mhp = Mycoplasma hyopneumoniae.
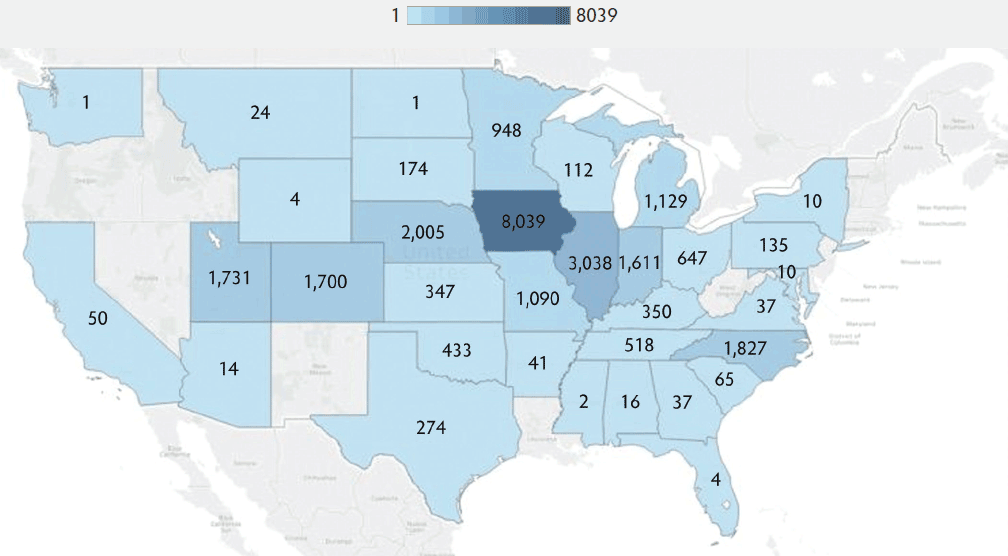
Figure 5: LS Means (with standard deviation) of Mhp DNA detection frequency by age group and specimen type using qPCR on cases submitted to the ISU VDL from 2004 to 2016. Pigs less than 3 weeks of age were defined as suckling, 3 to 6 weeks as nursery, 7 to 11 weeks as growing, 12 weeks to 200 days as finishing, and greater than 200 days as adult. Mhp = Mycoplasma hyopneumoniae; qPCR = quantitative polymerase chain reaction; ISU VDL = Iowa State University Veterinary Diagnostic Laboratory.
Discussion
Mycoplasma hyopneumoniae is one of the most economically important respiratory pathogens in the US swine industry. This study provides valuable information regarding the distribution of Mhp qPCR results over age group, geographic location, season, specimen, and time. The relatively low positivity rate of Mhp detection by qPCR in 2016 (17.9%) was associated with increased testing from adult- and suckling-age groups and from oral fluids. There was a relative and consistent increase in the total Mhp qPCR performed from 2004 to 2016 at ISU VDL but the percentage of the positive results remained relatively similar, which may be due to the endemicity of Mhp. Finishing-age pigs had a greater number of total cases and greater prevalence of Mhp detection compared to other age groups, which was expected as Mhp is a pathogen known to cause clinical disease in that age group of pigs.11 The trend of increasing submissions from adult and suckling pigs, as compared to growing pigs, may reflect the implementation of monitoring programs within breeding herds as part of disease control or elimination efforts.12 Due to a relatively long incubation period and low transmission rate,13 enzootic pneumonia is usually not reported in pigs younger than 6 weeks of age.14 Therefore, in this study, Mhp detection was relatively low in suckling and nursery pigs as compared to finishing pigs. Bronchoalveolar lavage fluid and tracheobronchial samples have been reported as specimen types with greater sensitivity when tested with nested PCR to detect Mhp infection, as compared to other specimen types such as nasal, tonsil, and tracheobronchial swabs and lung tissues.7 In this study, Mhp qPCR detection rate was 49% in bronchoalveolar lavage fluid, 64% in bronchial swab, and 34% in tracheal swab. Likewise, nasal swabs and lung tissues had a relatively lower Mhp detection rate in experimentally infected pigs.7 In our study, Mhp qPCR detection rate was 10% in nasal swab, and 33% in lung.
The number of cases submitted and the percentage testing positive for Mhp by qPCR were highest during the fall season (September, October, and November). Although not entirely comparable, similar results have been reported in Mycoplasma pneumoniae infection in humans.15 The increased sample submissions in the fall season may be partly attributed to the seasonality of other respiratory pathogens including porcine reproductive and respiratory syndrome virus16 and influenza A virus.17 The majority of cases were from the Midwest region of the United States with the greatest number of cases from Iowa, which is not surprising due to the high density of swine in Iowa and the location of the ISU VDL.
Limitations of this study include the fact that testing positive for Mhp by qPCR does not necessarily imply clinical disease. Despite most cases being associated with field investigations, cases involving monitoring and surveillance projects were also included which could have affected conclusions. Also, this analysis was limited to cases submitted to the ISU VDL.
Implications
• Despite the relative and consistent increase of Mhp qPCR performed from 2004 to 2016, the percentage of the positive results remained relatively similar. The overall increase of Mhp qPCR performed is likely a reflection of the overall increase of diagnostic cases submitted to the ISU VDL during this period.
• While the results from samples submitted to the ISU VDL for Mhp qPCR testing had variable detection rates, it is important to keep in mind that these results are based on passive surveillance data.
• The recent increase of Mhp qPCR from samples of suckling and adult pigs may reflect more recent efforts to monitor the progress towards Mhp control and elimination projects rather than investigations of clinical disease in those age groups.
Acknowledgements
The authors appreciate the cooperation of the ISU VDL in making the database available for assessment.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Goodwin RF, Hurrell JM, Whittlestone P. Production of enzootic pneumonia in pigs with a Mycoplasma suipneumoniae grown in embryonated hens’ eggs. Br J Exp Pathol.1968;49:431-435.
2. Mare CJ, Switzer WP. New species: Mycoplasma hyopneumoniae; A causative agent of virus pig pneumonia. Vet Med Small Anim Clin. 1965;60:841-846.
3. Kobisch M, Friis NF. Swine mycoplasmoses. Rev Sci Tech. 1996;15:1569-1605.
*4. Holtkamp D. Economic impact of Mycoplasma hyopneumoniae on pig farms. Pig333 Web site. https://www.pig333.com/articles/economic-impact-of-mycoplasma-hyopneumoniae-on-pig-farms_8936/. Published 2014. Accessed September 19, 2014.
*5. Schwartz M. The cost of Mycoplasma hyopneumoniae in growing pigs. Proc Allen D. Leman Swine Conf. St. Paul, Minnesota. 2015.
6. Friis NF. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma flocculare a survey. Nord Vet Med. 1975;27:337-339.
7. Kurth KT, Hsu T, Snook ER, Thacker EL, Thacker BJ, Minion FC. Use of a Mycoplasma hyopneumoniae nested polymerase chain reaction test to determine the optimal sampling sites in swine. J Vet Diagn Invest. 2002;14:463-469.
8. Sorensen V, Ahrens P, Barfod K, Feenstra AA, Feld NC, Friis NF, Bille-Hansen V, Jensen NE, Pedersen MW. Mycoplasma hyopneumoniae infection in pigs: duration of the disease and evaluation of four diagnostic assays. Vet Microbiol. 1997;54:23-34.
9. Strait EL, Madsen ML, Minion FC, Christopher-Hennings J, Dammen M, Jones KR, Thacker EL. Real-time PCR assays to address genetic diversity among strains of Mycoplasma hyopneumoniae. J Clin Microbiol. 2008;46:2491-2498.
10. Hartigan JA, Wong MA. Algorithm AS 136: A K-means clustering algorithm. J R Stat Soc Ser C Appl Stat. 1979;28:100-108.
11. Done SH. Enzootic pneumonia (mycoplasmosis) revisited. Pig J. 1996;38:40-61.
12. Holst S, Yeske P, Pieters M. Elimination of Mycoplasma hyopneumoniae from breed-to-wean farms: A review of current protocols with emphasis on herd closure and medication. J Swine Health Prod. 2015;23(6):321-330.
13. Meyns T, Maes D, Dewulf J, Vicca J, Haesebrouck F, Kruif AD. Quantification of the spread of Mycoplasma hyopneumoniae in nursery pigs using transmission experiments. Prev Vet Med. 2004;66:265-275.
14. Ross RF. Mycoplasmal diseases. In: Straw BE, D’ Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:495-505.
15. Kim EK, Youn YS, Rhim JW, Shin MS, Kang JH, Lee KY. Epidemiological comparison of three Mycoplasma pneumoniae pneumonia epidemics in a single hospital over 10 years. Korean J Pediatr. 2015;58(5):172-177.
16. Tummaruk P, Surapat P, Sriariyakun S, Seemakram O, Olanratmanee EO, Tantilertcharoen R, Thanawongnuwech R. Porcine reproductive and respiratory syndrome virus detection in Thailand during 2005-2010 in relation to clinical problems, pig types, regions, and seasons. Trop Anim Health Prod. 2013;45:771-779.
17. Chamba Pardo FO, Alba-Casals A, Nerem J, Morrison RB, Puig P, Torremorell M. Influenza herd-level prevalence and seasonality in breed-to-wean pig farms in the midwestern United states. Front Vet Sci. 2017;4:167. doi:10.3389/fvets.2017.00167
* Non-refereed references.
