| Original research | Peer reviewed |
Cite as: Campler M, Pairis-Garcia M, Kieffer J, et al. Sow behavior and productivity in a small stable group-housing system. J Swine Health Prod. 2019;27(2):76-86.
Also available as a PDF.
SummaryObjectives: To quantify behavior and productivity of females grouped in small static groups when fed using a single-entry/exit electronic sow feeder (ESF) over two consecutive gestation periods. Materials and methods: Fifty-eight gilts with no previous experience in group gestation housing were enrolled into 3, static, successive cohorts (Cohort 1, n = 20; Cohort 2, n = 18; and Cohort 3, n = 20) at day 35 of gestation. Pigs were housed individually throughout the farrowing period, and pigs that were healthy were moved back into their respective groups for their second gestation (Cohort 1, n = 19; Cohort 2, n = 13; Cohort 3, n = 17). Pig behavior, social rank, and post-gestation productivity was quantified for each gestation period. Results: Agonistic behaviors decreased between the first and second gestation (P < .001). High-ranked sows initiated more agonistic bouts around the ESF when compared to intermediate- and low-ranked sows (P < .001). Duration of active (P = .78) and inactive (P = .76) behaviors did not differ between gestation periods, but more active behaviors were observed near the ESF when compared to other areas of the pen (P < .001). High-ranked sows visited the feeder more frequently when compared to intermediate- and low-ranked sows (P < .001). No differences in subsequent litter or female productivity measures were found based on sow ranking. Implications: Housing gestating females in small static groups with an ESF decreased aggression between the first and second parity without detrimentally affecting general pig behavior or productivity. | ResumenObjetivos: Valorar la conducta y productividad de las hembras agrupadas en grupos estáticos pequeños cuando se alimentaron utilizando un comedero electrónico para hembras (ESF por sus siglas en inglés) con entrada/salida única durante dos periodos consecutivos de gestación. Materiales y métodos: En el día 35 de gestación, se agruparon cincuenta y ocho hembras primerizas, sin experiencia previa en alojamiento en grupo durante la gestación en 3 cohortes estáticos, sucesivos, (Cohorte 1, n = 20; Cohorte 2, n = 18; y Cohorte 3, n = 20). Las cerdas fueron alojadas individualmente durante el periodo de parto, las cerdas saludables fueron regresadas a sus grupos respectivos durante la segunda gestación (Cohorte 1, n = 19; Cohorte 2, n = 13; Cohorte 3, n = 17). Para cada periodo de gestación, se cuantificó la conducta de la cerda, la clasificación social, y la productividad post gestación. Resultados: Las conductas agresivas disminuyeron entre la primera y la segunda gestación (P < .001). Las hembras de clasificación alta iniciaron más episodios combativos alrededor del ESF en comparación con las hembras de clasificación intermedia y baja (P < .001). La duración de las conductas activas (P = .78) e inactivas (P = .76) no difirieron entre los periodos de gestación, pero se observaron más conductas activas cerca del ESF al compararse con otras áreas del corral (P < .001). Las hembras de clasificación alta visitaron el alimentador más frecuentemente en comparación con las hembras de clasificación intermedia y baja (P < .001). En base a la clasificación de la hembra, no se encontraron diferencias en las medidas de la camada subsecuentes o productividad de las hembras. Implicaciones: Alojar hembras gestantes en pequeños grupos estáticos con un ESF disminuyó la agresión entre la primera y segunda paridad sin afectar negativamente la productividad o la conducta general de las cerdas. | ResuméObjectifs: Quantifier le comportement et la productivité de femelles regroupées en petits groupes statiques lorsque nourries en utilisant un système électronique d’alimentation à entrée/sortie unique (ESF) au cours de deux périodes consécutives de gestation. Matériels et méthodes: Cinquante-huit cochettes sans expérience préalable d’hébergement en groupe de gestation furent recrutées dans trois cohortes statiques successives (Cohorte 1, n = 20; Cohorte 2, n = 18; et Cohorte 3, n = 20) au 35e jours de gestation. Les porcs étaient logés individuellement durant la période de mise-bas, et les porcs qui étaient en santé ont été retournés dans leurs groupes respectifs pour la seconde gestation (Cohorte 1, n = 19; Cohorte 2, n = 13; Cohorte 3, n = 17). Le comportement des porcs, leur rang social, et la productivité post-gestation ont été quantifiés pour chaque période de gestation. Résultats: Les comportements agonistiques diminuèrent entre la première et la seconde gestation (P < .001). Les truies de rang social élevé initièrent plus d’attaques agonistiques autour de l’ESF comparativement aux truies de rangs intermédiaires et faible (P < .001). La durée des comportements actifs (P = .78) et inactifs (P = .76) ne différaient pas entre les périodes de gestation, mais des comportements plus actifs étaient observés à proximité des ESF lorsque comparé aux autres endroits dans l’enclos (P < .001). Les truies de rang élevé visitèrent la mangeoire plus fréquemment comparativement aux truies de rangs intermédiaire et faible (P < .001). Aucune différence dans les portées subséquentes ou les mesures de productivité des femelles ne fut trouvée en fonction du rang social des truies. Implications: L’hébergement de femelles gestantes en petits groupes statiques avec un ESF diminua l’agressivité entre la première et la deuxième parité sans affectant négativement le comportement général des porcs ou la productivité. |
Keywords: swine, aggression, gestation, group housing, electronic sow feeder
Search the AASV web site
for pages with similar keywords.
Received: December 22, 2017
Accepted: September 26, 2018
In the United States, legislation in 10 states currently mandates the use of group-housing systems to house pregnant sows during gestation, with Michigan and Ohio being the most recent states to pass legislation that will be implemented by 2019 and 2026 respectively.1,2 Meeting the group housing mandate will require producers to either convert existing facilities or to build new. Regardless of approach, sow gestation housing must be constructed in a way that minimizes pig aggression while assuring optimal welfare, nutritional support, and productivity. Previous research has shown the transition from gestation stalls to group housing can improve breeding female welfare by minimizing abnormal behaviors and improving physical condition.3,4 However, gestation group housing enables aggressive interactions amongst females, particularly as they establish a group hierarchy and when they compete over restricted resources. This aggression occurs at the greatest intensity within the first 48 hours post mixing.5,6 Aggression, which occurs most commonly during feeding can result in severe injuries.7,8 The intensity and frequency of aggressive behavior can be influenced by many factors including age and experience, familiarity, and the feed system. For example, it has been reported that sows fed utilizing unguarded electronic sow feeders (ESF) display more aggressive behavior around the feeder when compared to conventionally group-housed sows fed using a trickle feeding system. This difference in aggressive behavior highlights the issue with sequential versus simultaneous feeding.9 However, presenting a feed resource during the mixing of sows and throughout the initial establishment of a social hierarchy may not always be a cause for concern as it has been reported that mixing unfamiliar sows does not always increase the frequency of aggressive behaviors.10,11 Additional factors that may affect sow aggressive behaviors in group-housing systems are space allowance and mixed-parity groups, where older and larger sows tend to be dominant over smaller gilts.12,13 Whereas group size reportedly has little impact on aggression levels.14-16
Thus, increased understanding of swine aggression and pen dynamics in housing and feeding systems commonly used by the industry will provide additional insight for refinement and management improvements of current systems as well as for future implementation of new feeding and housing strategies. Therefore, the objective of the present study was to investigate the effect of a single-entry/exit ESF in small static group housing on behavior, productivity, and social rank of females during the first 48 hours post mixing over two consecutive gestation periods. The hypothesis was that the static pen and familiarity with the ESF across successive gestation periods would reduce aggression in early post-mixing of the second gestation period, resulting in fewer injuries within the group and improved gilt and sow production.
Materials and methods
The research protocol was approved by The Ohio State University Institutional Animal Care and Use Committee.
Animals and housing
The study was conducted at The Ohio State University Swine Research Facility between December 2015 and June 2016. Fifty-eight, Landrace × Yorkshire gilts (DNA Genetics, Columbus, Nebraska) with no previous group gestation housing experience were enrolled in the study at approximately day 35 of gestation. After the first gestation, all gilts were managed and housed similarly until day 35 of their second gestation period when they were moved back into group housing with their previous pen mates. Between gestation periods, 9 sows were unable to join their previous cohorts and were omitted from the study leaving 49 sows for the second gestation period. Throughout the study, the grouped sows were managed as three static cohorts (first gestation: cohort 1, n = 20; cohort 2, n = 18; cohort 3, n = 20; second gestation: cohort 1, n = 19; cohort 2, n = 13; cohort 3, n = 17) with approximately 42 days between each cohorts’ initial establishment during their first gestation period. Gilt first-mating criteria were (1) a minimum age of 300 days, (2) experiencing the second estrus period or greater, and (3) a minimum body weight of 136 kg. Prior to the initiation of group housing, all females in both gestation periods were housed and mated in standard individual gestation stalls (1.28 m2; 2.14 × 0.60 m, length × width) with partially slatted concrete flooring (slat width = 15.24 cm and gap width = 2.54 cm) and maintained in stalls until pregnancy confirmation. Females were fed parity-specific diets in gestation and lactation, and diets were formulated to meet or exceed the National Research Council nutrient guidelines.17 Electronic identification tags (Allflex, USA Inc, Dallas, Texas) for monitoring ESF usage and daily feed intake were placed in the ear of each female during the individual housing period prior to the study.
Experimental housing and design
The group gestation-housing area was composed of 3 pens (6.8 × 5.5 × 1.1 m, length × width × height) retrofitted across a section of the facility that previously contained gestation stalls. Each pen (Figure 1) consisted of two areas of solid concrete flooring (Lying area [A], 5.5 × 1.1 × 1.1 m; and ESF[C], 5.5 × 3.3 × 1.1 m; length × width × height) and a middle section of slatted concrete flooring (Water access area [B], 5.5 × 2.4 × 0.9 m). Pen sides consisted of covered hard polyethylene side walls (height 1.1 m) mounted to steel posts surrounding solid concrete areas, and a 3.5 m steel-barred gate separating pens allowed for visual and nose-to-nose contact with females from other pens (height: 0.9 m; 0.1 m distance between bars).
Figure 1: Schematic of a former gestation stall area retrofitted to a pen equipped with an electronic sow feeder (ESF) and two dual-nipple water drinkers. The pen was designed to accommodate 15 to 20 sows. Pen zones were defined as (A) lying area, (B) water nipple, or (C) electronic sow feeder.
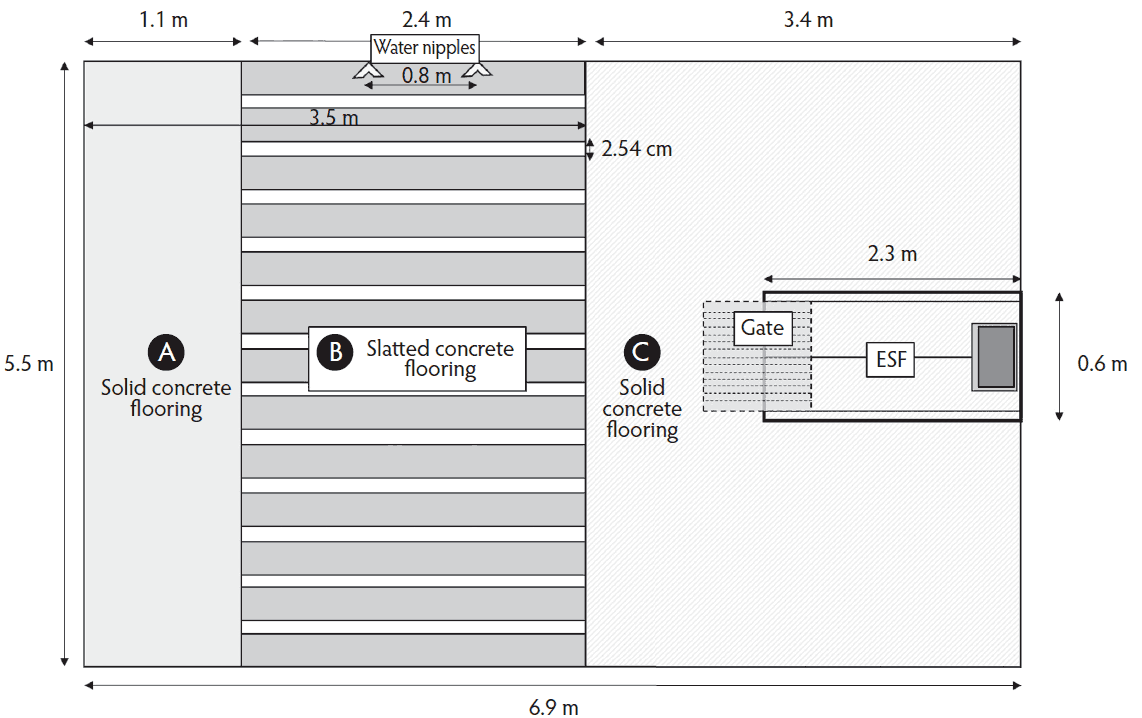
Pens were fitted with one, single-animal, single-entry/exit ESF (Gestal 3G, Jyga Technologies, Greeley, Kansas) that was installed in area C of the pen (Figure 1). Females had free access to the ESF station throughout the day; however, between midnight and 2 am, no feed was delivered. Feed disbursement occurred in 113 g per 30 second meals and feed was provided until the individual’s daily allocation was delivered. Feed allocation required 8.0 and 10.6 min/female/d for first and second gestation periods respectively. Females were provided ad libitum access to water through two, twin-nipple drinkers (Edstrom Industries, Inc, Waterford, Wisconsin) located on one side of each pen above the slatted floor (Figure 1). The pen and ESF system were designed for a maximum group size of 20 females per feeding station and each female had 1.87 m2 of space allowance.
Data collection
To investigate behavioral changes over time, individual females were evaluated during parity 1 (gilts) and parity 2 (sows). Females were identified using a non-toxic animal identification paint (Marksman, Rumenco/Nettex, Staffordshire, United Kingdom) to place a unique number on the back and both flanks. All gilt and sow behaviors were recorded continuously over the first 48 hours post mixing by 1 color Internet Protocol (IP) based video camera (Model F19805P, 30 frames/sec, Wireless IP Camera; Foscam, Houston, Texas) attached at a height of 3 m overlooking each pen. Agonistic behaviors, including fights, were obtained exclusively as frequency data while all other behaviors were collected using duration and frequency data. Video recordings were analyzed by two observers using behavioral observation software (Noldus Observer XT 12, Wageningen, the Netherlands). To ensure inter-observer reliability, both observers were trained prior to initiation of data collection by scoring three, 2-hour segments of the video recordings and achieving at least 95% accuracy. The 2-hour segments were selected to capture all behaviors specified in the ethogram to assure that all observers were comfortable and accurate viewing the videos during the data collection. The selected time periods were the first 2 hours post mixing (08:00 to 10:00), behavior around the ESF and active feeding behavior (14:00 to 16:00), and night time behavior (22:00 to 00:00).
Agonistic behavior. The frequency of all initiated and received agonistic behavior (biting, chasing, and displacement) were recorded using continuous observation. The agonistic behaviors were registered as mutually exclusive, thus two behaviors could not occur at the same time. The frequency of all initiated and received agonistic behavior for each animal was recorded throughout the observed 48-hour period. For biting, every individually distinguishable bite was recorded. A chasing bout was defined as the time from when one sow was biting the hindquarter of another sow while running or running after another sow without any active biting until the initiating sow stopped running or switched focus to another sow. For displacement, the act of physically moving another pig from a resource or lying area would be counted as one bout. The frequency of agonistic behaviors targeted towards a specific body region (flank, head, or hindquarter) and occurring within a pen zone (Lying area [A], Water nipple [B], or ESF [C]; Figure 1) was recorded. A fight was defined as an active reciprocal head to head biting interaction between two sows until one of the sows stopped the biting activity or performed a different non-biting behavior. An ethogram of aggressive behaviors is found in Table 1.
Table 1: Definitions of sow behaviors used in analyses
| Behavioral category | Behavior | Definition |
|---|---|---|
| Active behavior | Standing | Standing up with at least 3 legs touching the floor. |
| Walking | Walking or running in a forward or backwards direction. | |
| Agonistic behavior | Biting flank | Biting at flank. |
| Biting head | Biting on head or neck. | |
| Biting hindquarter | Biting on hindquarter or tail. | |
| Chasing | Actively following another sow while biting hindquarter or flank. | |
| Displacement | Removal of sow from ESF, water nipples, or lying area by actively biting the receiving sow’s head, flank, or hindquarter. | |
| Fighting | Active reciprocal head to head biting between two sows until one of the sows stops the biting activity. | |
| Inactive behavior | Lying | Lying sternally or laterally with sternum or side of the body touching the floor. |
| Sitting | Sitting on hind legs with front legs touching the floor in front of body. | |
| Maintenance behavior | Drinking | Actively drinking from the water nipple. |
| Feeder visits | Walking into the ESF with entire body or with head in the feed bin. | |
| Feeding | Actively consuming feed in the ESF with head in the feed bin. | |
| Oral manipulation of ESF gate | Mouth or snout manipulating the ESF gate. | |
| Other | Out of view | Pen mates blocking the focal sow from camera view. |
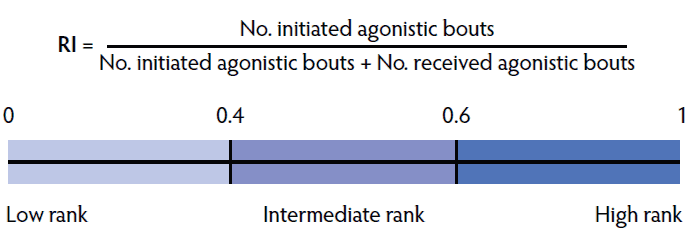
Social-rank index. A social-rank index (RI) score was calculated for each individual animal by dividing the number of initiated agonistic bouts with the summed total of initiated and received agonistic bouts per individual over 48 hours (methodology adopted from Galindo and Broom;18 Figure 2). The calculation yielded an RI score between 0 and 1 which was translated into 3 social-rank categories (high: RI ≥ 0.6; intermediate: 0.4 ≥ RI < 0.6; and low: RI < 0.4). Females in the high category initiated more aggressive bouts, whereas females in the low category received a greater number of aggressive bouts.
Figure 2: Social-rank index (RI) calculation adapted from Galindo and Broom18 and the respective social-rank categories.
General behavior. The duration of feeding, drinking, lying, sitting, standing, and walking was recorded using continuous observation for each female by zone (Lying area [A], Water nipple [B] or ESF [C]). In addition, feeder visit frequency and duration and frequency of oral manipulation of the ESF gate was also recorded for each female within this 48-hour period.
Production and animal care. Litter traits recorded were the number of born alive, light weight pigs, mummified, nursed, stillborn, total born, and total weaned per litter. Piglet weight at weaning was collected, summed, and averaged within the litter. Female body weights were collected at the time of weaning. The daily feed intake and the feed intake for the total lactation period was recorded for each female. Daily observations of animal care were conducted per the farm’s standard operating procedures and details of animal treatments, including duration and outcomes, were recorded.
Treatments and removals. Throughout the study, injuries or illness were reported and treated by farm staff in 13 of 58 (22.4%) females during the first gestation period and 8 of 49 (16.3%) females during the second gestation period. Reasons listed for treatment across both parity groups included musculoskeletal injury or lameness (n = 18; 85.7% of animals treated), “off feed” (n = 2), and thin body condition/diarrhea (n = 1). All treatments were administered per label directions according to the farm’s standard operating procedures or under the direction of the attending veterinarian. Duration of treatment administration ranged from 1 to 8 consecutive days, with an average of 3.2 days in which the female was receiving treatment. Three females were humanely euthanized via penetrating captive bolt and 1 female died during the study, but none during the 48 hours post mixing. Reasons for euthanasia included a broken leg (n = 1), extremely poor body condition in conjunction with unresolving diarrhea (n = 1), and worsening lameness despite treatment (n = 1). One female died unexpectedly and was diagnosed with a ruptured mesenteric artery upon necropsy. Overall mortality rate was 6.9% (4 of 58 females) during the duration of the study. Females culled after completing their first parity were removed due to failure to return to estrus (n = 3), failure to conceive (n = 3), poor body condition at regrouping (n = 2), and failure to train to ESF (n = 1).
Statistical analysis
Data were analyzed using SAS software (Version 9.4; SAS Institute Inc, Cary, North Carolina). Zones A and B (Lying area and Water) were merged for a more accurate comparison with zone C (ESF) based on zone area footprint within the pen (Figure 1). Lying and sitting behaviors were merged into an inactive category and standing and walking were merged into an active category, while feeding and drinking were analyzed as their own mutually exclusive behaviors. Additionally, all initiated flank, head, and hindquarter bites were merged into one biting category to be able to analyze the effect of social-rank on the pen zone where the most bites occurred. The normality of the data was assessed using the PROC univariate procedure and by evaluating residual plot distribution.
All behavior categories were considered not normally distributed and were therefore analyzed with a generalized linear mixed model (PROC GLIMMIX) using a Poisson distribution for frequency data. Fixed effects of parity (1 or 2), rank (high, intermediate, and low), body region (flank, head, or hindquarters), pen area (Lying area or ESF), and their interactions, with individual nested within pen as a random effect, were tested in the initial model. Non-significant interactions (P > .20) were removed from the final models. Contrast statements with a Bonferroni adjustment were used to identify statistical differences.
Measures of female productivity were analyzed using a generalized linear mixed model (PROC MIXED) with fixed effects of parity (1 or 2; ie, post hoc analysis after farrowing), rank (high, intermediate, and low), and their interactions, as well as using a random effect of static cohort group (1, 2, or 3) for initial models. Interaction effects were not significant (P > .20) for all measures and were removed from final models. A linear covariate for weaning age was used to adjust sow weight, feed intake, and litter weight measurements to a 21-day weaning age basis. Least squares means and standard errors were estimated and assessed using the PDIFF option in SAS.
Treatment and removals were reported as frequency and proportions within and across cohort and parity for explanation of changes in animal numbers between consecutive parities and to acknowledge measured characteristics of the population. No statistical analyses were performed.
Results
Agonistic behavior over gestation periods
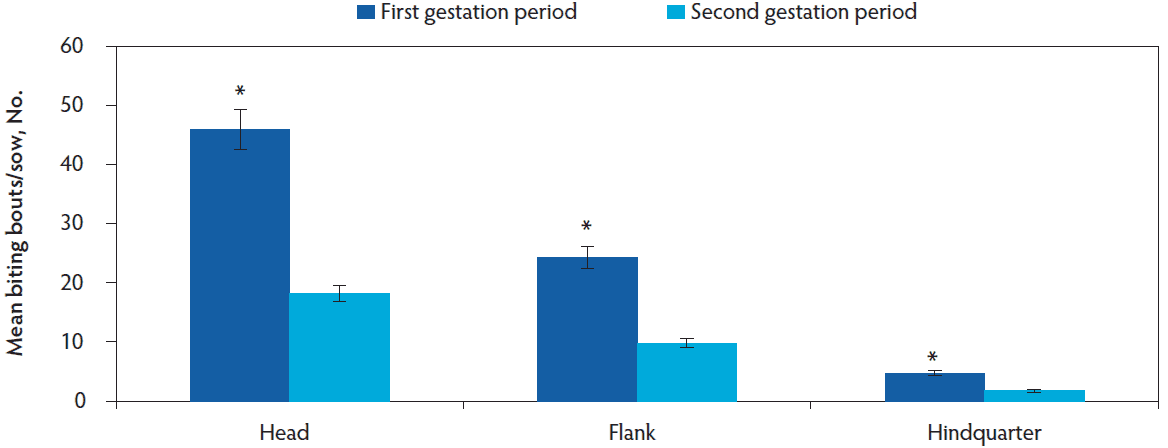
A total of 6999 agonistic bouts were recorded for all females for both gestation periods (first gestation period, n = 5215; second gestation period, n = 1784), of which 6831 (97.6%) of these agonistics behaviors were biting bouts, 112 (1.6%) were displacement bouts, and 56 (0.8%) were chasing bouts. The total number of initiated biting bouts decreased between the first and second gestation period (mean [SE]; 20.8 [2.1] vs 7.7 [0.8] bouts/female; F1,145 = 554.75, P < .001). Additionally, the number of biting bouts decreased in both the water/lying area and around the ESF between the first and second gestation period (Figure 3; F1,151 = 9.74, P = .002). The most targeted body area was the head region followed by the flank and hindquarter of the sow regardless of gestation period (Figure 4, F2,256 = 827.63, P < .001). The number of fights were more frequent during the first gestation period when compared to the second gestation period (mean [SE]; 2.1 [0.3] vs 1.1 [0.2] fights/female; F1,145 = 22.31, P < .001) and more frequent in the ESF area compared to the water/lying area regardless of gestation period (mean [SE]; 3.5 [0.3] vs 0.7 [0.2] fights/female; F1,145 = 177.90, P < .001). The number of displacements were infrequent and did not differ between gestation periods (mean [SE]; 0.38 [0.1] vs 0.23 [0.1] bouts/female; F1,145 = 0.20, P = .65), but more displacements were performed around the ESF when compared to the water/lying area within the pen (mean [SE]; 0.41 [0.1] vs 0.22 [0.1] bouts/female; F1,145 = 7.53, P = .007). Chasing bouts were infrequent but a greater number tended to occur during the first gestation period when compared to the second gestation period (mean [SE]; 0.15 [0.05] vs 0.06 [0.02] bouts/female; F1,145 = 2.88, P = .06) while no difference was observed between the ESF and the water/lying area (mean [SE]; 0.16 [0.05] vs 0.07 [0.03] bouts/female; F1,145 = 0.56, P = .46).
Figure 3: Least squares means of biting bouts per sow by pen location and gestation period (first gestation period, n = 58; second gestation period, n = 49). Data was analyzed using a generalized linear mixed model (PROC GLIMMIX). An * indicates significant differences between gestation periods within the pen area (P = .002). ESF = electronic sow feeder.
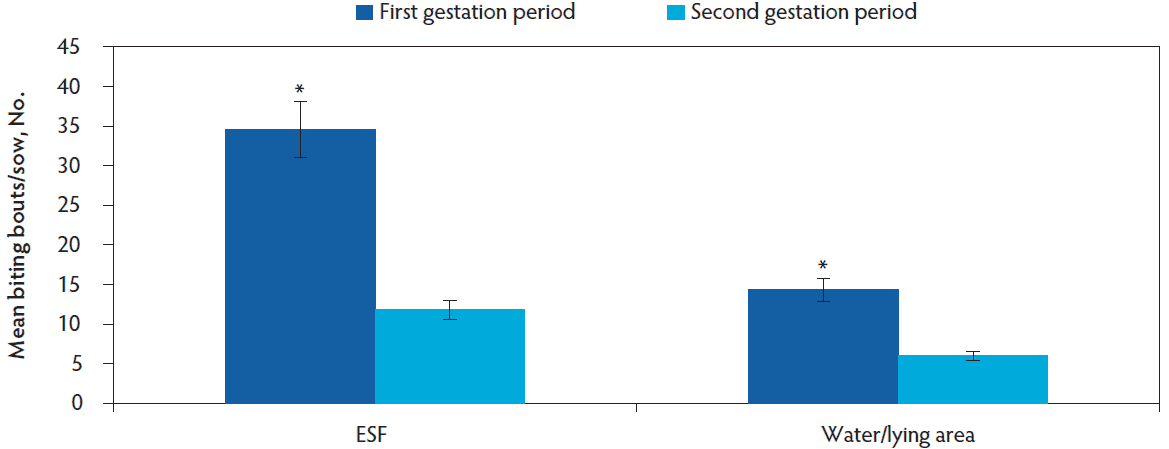
Figure 4: Least squares means of biting bouts per sow by body location and gestation period (first gestation period, n = 58; second gestation period, n = 49). Data was analyzed using a generalized linear mixed model (PROC GLIMMIX). An * indicates significant differences between gestation periods within a body location (P < .001).
Agonistic behavior and social rank
The social-rank scores shifted between the first (of the 58 females, 9 [15.5%] ranked high, 28 [48.3%] ranked intermediate, and 21 [36.2%] ranked low) and second gestation period (of the 49 females, 15 [30.6%] ranked high, 13 [26.5%] ranked intermediate, and 21 [42.8%] ranked low). A social rank × pen zone interaction was significant with high-ranked sows initiating a greater number of biting bouts around the ESF when compared to intermediate- and low-ranked females. High-ranked females initiated the most biting bouts, followed by intermediate-ranked females and lastly low-ranked females for both ESF area and water/lying area (Figure 5; F2,145 = 26.21, P < .001).
Figure 5: Least squares means of biting bouts per sow across both gestation periods by pen location and sow social rank (high, n = 23, intermediate, n = 42, low, n = 42). Data was analyzed using a generalized linear mixed model (PROC GLIMMIX). Different letters indicate significant differences (P < .001). ESF = electronic sow feeder.
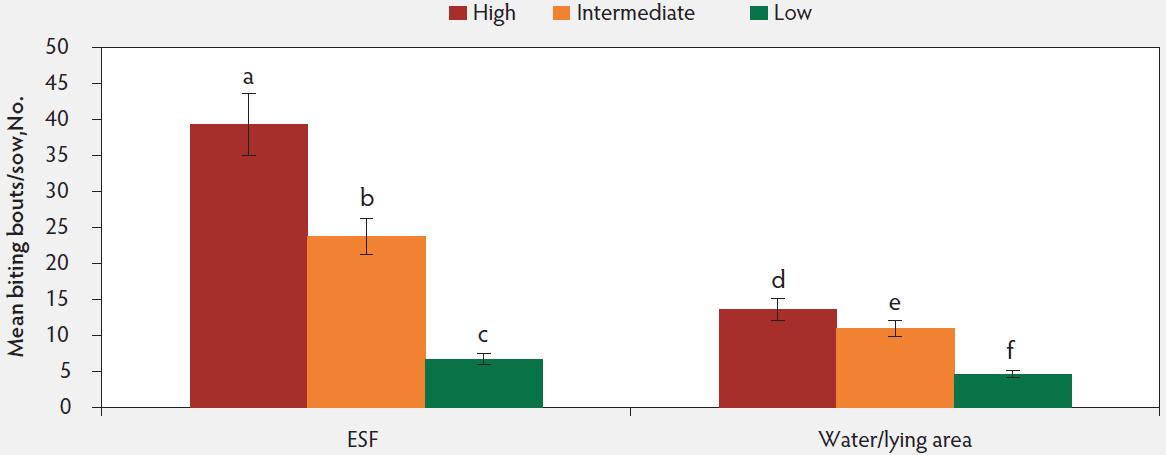
High- and intermediate-ranked females performed a greater number of fights when compared to low-ranked females during the first gestation period (mean [SE]; 2.3 [0.8], 2.1 [0.4], and 0.8 [0.2] fights/female for high-, intermediate-, and low-ranked females, respectively; F2,145 = 15.42, P < .001).
A tendency in the number of displacements between social rankings was observed with intermediate-ranked females more frequently displacing high- and low-ranked females (mean [SE]; 0.17 [0.1], 0.42 [0.1], and 0.21 [0.1] displacements/female for high-, intermediate-, and low-ranked females, respectively; F2,145 = 2.95, P = .06). Similarly, a tendency in the number of chasing events between social rankings was observed with high- and intermediate-ranked females more frequently chasing low-ranked females (mean [SE]; 0.18 [0.1], 0.20 [0.1], and 0.04 [0.03] bouts/female for high-, intermediate-, and low-ranking females, respectively; F2,145 = 2.88, P = .06).
General behavior
The time spent active did not differ between gestation periods (mean [SE]; 167.03 [8.38] vs 159.17 [8.3] min/female; F1,145 = 0.51, P = .47). Overall, most active behaviors were observed around the ESF when compared to the water/lying area (mean [SE]; 243.9 [8.2] vs 82.3 [8.2] min/female; F1,145 = 232.37, P < .001). No difference in the time spent inactive was observed between gestation periods (mean [SE]; 911.0 [66] vs 905.2 [64] min/female; F1,141 = 0.01, P = .95). Females also spent the most time inactive around the ESF when compared to the water/lying area (mean [SE]; 1258.2 [63.3] vs 557.9 [64.0] min/female; F1,141 = 62.27, P < .001). Total time spent in the feeding station (mean [SE]; 84.4 [7.4] vs 112.8 [7.1] min/female; F1,97 = 7.41, P = .008) and the number of feeder visits (6.5 [0.7] vs 8.7 [0.8] visits/female; F1,103 = 3.89, P = .05) increased during the second gestation period when compared to the first gestation period. The length of feeder visits did not increase between the first and second gestation period (mean [SE]; 13.5 [1.3] vs 15.8 [1.3] minutes per female per feeding bout, F1,103 = 1.43, P = .23). No other significant results were found.
General behavior and social rank
Female social rank and gestation period did not affect time spent performing active behaviors (mean [SE] per female; First gestation period: 378.4 [48.6], 406.2 [27.5], and 440.8 [31.8]; Second gestation period: 407.9 [39.0], 381.3 [39.0], and 431.4 [31.8] min/d for high-, intermediate-, and low-ranked females, respectively; F2,101 = 0.24, P = .78) or inactive behaviors (First gestation period: 2312.2 [62.6], 2291.5 [35.5], and 2251.6 [40.0]; Second gestation period: 2313.5 [50.2], 2365.9 [50.2], and 2304.1 [41.0] min/d for high-, intermediate-, and low-ranked females, respectively; F2,101 = 0.26, P = .76).
High-ranked females visited the feeder more frequently when compared to intermediate- and low-ranked females during the first gestation period (mean [SE]; 7.5 [1.1], 6.2 [0.7], and 6.1 [0.8] bouts per female per 48 hours for high-, intermediate-, and low-ranked females, respectively, F2,256 = 8.47, P < .001) but high- and intermediate-ranked females visited the feeder less frequently compared to low-ranked females during the second gestation period (7.5 [0.8], 7.8 [0.9], and 10.1 [0.8] bouts per female per 48 hours for high-, intermediate-, and low-ranked females, respectively; F2,256 = 8.47, P < .001). High-ranked sows spent less time feeding compared to low-ranked sows with intermediate-ranked sows not differing from other sows during the first gestation period (mean [SE]; 10.2 [1.9], 16.2 [1.2], and 12.4 [1.3] min/visit for high-, intermediate-, and low-ranked females, respectively; F2,256 = 3.15, P = .04) but no difference was seen during the second gestation period (16.2 [1.5], 15.8 [1.5], and 16.0 [1.3] min/visit for high-, intermediate-, and low-ranked females, respectively; F2,256 = 0.28, P = .81). No difference in total feeding time between social-rank categories was found (mean [SE]; 97.5 [10.4], 92.1 [8.4], and 106.6 [8.0] min/48h, for high-, intermediate-, and low-ranked females, respectively; F2,95 = 0.83, P = .43). High- and intermediate-ranked females spent more time manipulating the ESF gate when compared to low-ranked females (mean [SE]; 46.2 [6.5], 25.6 [5.0], and 13.5 [5.0] min/48h, high-, intermediate-, and low-ranked females, respectively; F2,100 = 7.69, P < .001) but no female social rank × gestation period interaction was observed (F2,100 = 2.06, P = .13).
High-ranked females spent the most time drinking followed by intermediate-ranked females, while low-ranked females spent the least amount of time drinking (mean [SE]; 17.6 [2.0], 12.0 [1.5], and 10.0 [1.5] minutes per female per 48 hours; F2,101 = 6.84, P < .01). No female social rank × gestation period interaction was observed (F2,101 = 0.97, P = .38).
Production
Performance measures for the commercial females evaluated in the present study were indicative of highly prolific, strong maternal genetic resources. No differences in litter and female productivity measures (Table 2) were observed when comparing social rank categories in the present study, a strong indication that, while females clearly demonstrated hierarchy differences early following mixing, the implanted fetuses survived and female productivity was maintained equally across aggression categories through the first and second lactations. Parity effects were present as expected, with parity 2 females producing a greater number of total piglets born (mean [SED]; 2.12 [0.69] piglets; F1,95 = 9.25, P < .001), piglets born alive (1.85 [0.71] piglets; F1,95 = 6.83, P < .001), weaned piglets per litter (1.22 [0.28] piglets; F1,95 = 19.28, P < .001), and litter weight (6.39 [6.26] kg; F1,95 = 7.90, P < .001) when compared with parity 1 females.
Table 2: Least squares means for female and litter performance measures by social rank across first and second gestation period*
| Social rank† | ||||||
|---|---|---|---|---|---|---|
| High | Intermediate | Low | SE | F Value | P Value‡ | |
| Female traits§ | ||||||
| Average daily feed intake, kg | 6.2 | 6.1 | 6.5 | 0.4 | F2,95 = 1.80 | .17 |
| Total lactation feed intake, kg | 139.4 | 139.9 | 145.8 | 8.4 | F2,95 = 1.13 | .33 |
| Weight at weaning, kg | 202.0 | 205.1 | 205.2 | 5.4 | F2,95 = 0.35 | .70 |
| Litter traits | ||||||
| Light weight pigs (< 0.68 kg), No. | 0.21 | 0.23 | 0.33 | 0.11 | F2,95 = 0.34 | .72 |
| Litter weaning weight, kg | 78.6 | 78.1 | 82.6 | 2.96 | F2,92 = 1.78 | .18 |
| Mummies, No. | 0.46 | 0.43 | 0.40 | 0.19 | F2,95 = 0.16 | .86 |
| Piglets born alive, No. | 14.23 | 14.83 | 14.59 | 0.73 | F2,95 = 0.21 | .80 |
| Piglets nursed, No. | 13.54 | 13.94 | 13.71 | 0.34 | F2,95 = 0.82 | .44 |
| Piglets weaned, No. | 12.69 | 12.62 | 12.89 | 0.38 | F2,95 = 0.39 | .85 |
| Piglet weaning weight, kg | 6.24 | 6.22 | 6.45 | 0.13 | F2,92 = 1.03 | .36 |
| Stillborn piglets, No. | 1.74 | 1.23 | 1.01 | 0.25 | F2,95 = 2.02 | .14 |
| Total piglets born, No. | 16.42 | 16.14 | 15.89 | 0.72 | F2,95 = 0.17 | .85 |
* Information provided for both gestation periods (first gestation period, n = 58; second gestation period, n = 49).
† A social-rank index score was calculated for each individual animal by dividing the number of initiated agonistic bouts with the summed total of initiated and received agonistic bouts per individual over 48 hours. The calculation yielded an index score which was categorized to high (≥ 0.6), intermediate (≥ 0.4 and < 0.6), and low (< 0.4).
‡ All female and litter traits were analyzed using a generalized linear mixed model (PROC MIXED) with fixed effects of parity (1 or 2; ie, post hoc analysis after farrowing), rank (high, intermediate, and low), and their interactions, and static cohort group (1, 2, or 3) as a random effect. Significance was treated as P < .05.
§ Measures adjusted to a 21-day weaning age.
Discussion
Behavior during the first 48 hours post mixing
Primary challenges with group-housed gestating females are the provision of a feeding system that can provide control over an individual female’s feed intake and the implementation of methods or approaches that reduce aggression caused by mixing and resource guarding. Despite reports of increased aggression around unguarded ESF stations when compared to trickle feeding or free access stalls,9 the consistent upgrades in technology and system design contributes to ESF stations now being a commonly chosen gestation feeding system.19,20 In addition, commercial entities have established that utilizing static grouping strategies can help alleviate aggression levels for the entire group-housed gestation period, depending upon total space allocation provided. In the present study, agonistic behavior levels during the first 48 hours in group gestation housing were reduced by approximately 60% and the number of fights were reduced by approximately 40% between the first gestation period, when sows were unfamiliar with their pen mates, and the second gestation period, when sows were housed back together with their previous pen mates. These results coincide with two earlier studies that reported lower levels of aggression when sows were familiar with each other in either group housing20 or when housing pigs temporarily in pairs to quantify agonistic behaviors.21 Thus, it is reasonable to ascertain that a key factor influencing the reduced aggression observed in this study was pen-mate familiarity as reported by contemporary studies.22,23 However, familiarity should not be interpreted as the sole contributing factor to the observed reduced aggression as age and experience are likely to affect aggression levels in group housed sows.11 Results from the present study are in contrast to studies that reported no difference in the level of aggression in sows during a 2- or 8-hour observation window post mixing in either small or large groups.16,24 Furthermore, a recent large-scale study reported no effect of group size on sow aggression, suggesting that a large number of sows may be housed together without increased aggression levels.15 The lack of aggression in larger groups observed by Hemsworth et al15 could potentially be due to the disruption or change of displayed dominance behavior due to a large number of competitors.25 The present study, by design, kept a targeted group size of 20 females to accommodate the ESF feeding system design capacity. In contrast, other commercially available ESF systems can accommodate access for 60 to 80 animals per group depending on ESF design and management. Considering larger group sizes require either multiple stations with single-animal entry/exit or a pass-through station design that allows females to leave the ESF on the opposite side from where the next female in line will attempt to enter, the present study findings represent data most comparable to small-scale production systems using small group sizes during gestation.
In the present study, most aggression was reported near and around the ESF when compared to the water/lying area, a finding that identifies feed as a valued resource. In addition, being a single-entry/exit ESF design, it is likely that a greater level of aggression may occur as antagonists have more opportunities to attack entering and exiting females compared to an ESF with a pass-through design. It is possible that having an additional single-entry/exit ESF or having a separate exit, or pass-through ESF, may have decreased aggression levels. Additionally, it was not feasible to implement a pass-through ESF system in the small, retrofitted, group pens used in this study due to cost and the physical footprint required.
The greater number of initiated agonistic bouts around the ESF by high-ranked females when compared to lower-ranked females may be due to resource-based guarding given the value and motivation of feed for a limit-fed gestating female. Furthermore, the single-entry, backward-exit station design of the ESF may have allowed higher-ranking females to attempt to deny entrance of other individuals to the feeder by engaging in agonistic behaviors and impede or antagonize lower-ranked females as they were backing out of the ESF system after feeding. High-ranked females chased and displaced lower-ranked females which corresponds with previous knowledge regarding social relationships in swine and their interactions around resources within a pen.26,27 Thus, the overall level of aggression cannot be solely interpreted as an effect of the hierarchy establishment within the group, but rather a combination of rank and resource guarding for feed. However, ESFs in combination with static groups may be a better option compared to mixed groups as previous pen-mate familiarity could potentially reduce overall aggression normally occurring at mixing.
There was no difference in activity or inactivity levels between gestation periods, but females spent more time overall in close proximity to the ESF when compared to the other pen zones. Inactive behaviors were greater in this study (85% of the daily time budget for the 48-hour period) compared to previously reported research in group housing (66%-73%), stall systems (63%-84%),28 or outdoor housing (78%).28-31 It is likely that the lack of enrichments in the current study had an impact on the sows’ time budgets resulting in a higher degree of inactivity as there was less opportunity to perform a wide range of active behaviors. It is also possible that exhaustion due to fighting or inability to rest due to the initial social unrest in the pen may have contributed to higher inactivity levels as sows may have overcompensated once aggression levels decreased. This suggests that during the 48-hour period, the female’s behavioral repertoire is focused on fighting or resting with the females performing little to no exploratory behavior. No effect of social rank on activity was found suggesting that high-ranked sows interacted and possibly kept intermediate- and low-ranked sows alert and on their feet to avoid confrontation. This result is in contrast with an earlier study showing low-ranked sows being more inactive compared to their high-ranked pen mates.32
As expected, the total feeding time and feeder visit length increased during the second gestation period as the females returning were older heavier animals with greater maintenance nutritional demands and previous ESF experience. Time spent feeding in the present study (2%-4% of daily time budget; 42-55 min/d) was greater when compared to earlier work with group gestation housing with other types of ESF units (eg, 31 min/d for unspecified ESF unit;29 22 min/d for unprotected ESF - Fitmix33). Differences in results from the present study are likely due to the feed drop rate (113 g per 30 seconds) or total feed delivered which may have increased the time spent in the feeder to get the entire meal delivered and consumed. However, no difference in feeding time was seen between sow social rankings. Furthermore, the enclosed design of the ESF protected females from being displaced from the feeder prematurely, thus allowing for an uninterrupted and sequentially slower feeding rate compared to unprotected feeding systems. It is possible that high-ranked sows may have been able to get access to the feeder more often simply by entering more frequently to search for leftover feed from the previous sow. Although high-ranked females visited the feeder more frequently when compared to intermediate- and low-ranked females during the first gestation period, low-ranked sows had the longest feeder visit on average. Additionally, low-ranked females had a larger number of feeder visits when compared to high- and intermediate-ranked females during the second gestation period making it hard to interpret the change in visit frequency between high- and low-ranked sows. A possible explanation is that the high-ranked females defended what would be regarded as the high-value resource in the pen during the first gestation period preventing or reducing the frequency of visits in low-ranked females. An indication that supports this explanation is that most agonistic bouts were recorded around the ESF, a finding supported by previous studies4,34,35 as well as the larger amount of time spent at the water nipples by high-ranking sows in the current study. Moreover, it is possible that vocal threats or postural threats (variables not recorded in this study) by high-ranked females could have forced low-ranked females out of the ESF without any physical contact. Physical contact was a requirement in the definition of displacement, therefore any vocal or postural threat-based displacement was not assessed.
Production
No overall differences in female performance or litter traits were observed between social rank categories, a finding that is in contrast to two earlier studies that reported a relationship between high-ranking females and heavier offspring body weight at birth and weaning.32,36 No differences were noted in total piglets born alive between gestation periods, a result supported by earlier findings that did not report any differences in total piglets born alive between dynamic and static groups during gestation33 or housing effects on live litter sizes at birth and weaning when comparing gestation stalls or small and large group pens.37 However, results from the current study are in contrast to a recent study that reported fewer piglets born alive from high-ranked females when compared to low-ranked females, with intermediate-ranked females in between.38 Verdon et al11 found that high-ranking females had increased cortisol levels (a parameter not measured in the present study) due to increased aggression from remixing. It was speculated that the increased cortisol resulted in greater oxidative stress, which in turn could have contributed to a decrease in litter size, although this remains unclear. No difference in total piglets born was seen between social-rank categories, a result supported by recent studies.11,39
Parity influences on productivity measures were as expected in commercial production with the second parity litter size increasing, litter and piglet weight increasing, and female weight increasing with maturation. The combination of the female maturing physiologically and immunologically leads to a greater number of eggs ovulated, fertilized, and fully developed into live piglets40 and improved colostrum quality and milk production, which lead to heavier, healthier piglets at weaning.41,42
Conclusions
The results from this exploratory study showed that aggression decreased between the first and second gestation period during the initial 48 hours when sows were in static group housing with a gated ESF. The outcome is likely to be linked to familiarity with previous pen mates, even with individual housing during lactation and post weaning through confirmation of pregnancy, but age and experience may also play a significant role. The use of a gated single-entry/exit ESF ensured that all animals received their daily feed allocation and performed at industry expected levels, but also resulted in aggression near the feeder due to resource guarding. In situations where housing style is dictated by regulation or where new or retrofit construction options are being considered, the single-entry/exit ESF system can be considered. Additional research to alleviate agonistic behaviors in group-housed females, particularly early post mixing, is still warranted to continue to improve individual pig welfare.
Implications
- Small, static, sow groups in gestation decreases aggression between first and second parity during the first 48 hours post mixing.
- Electronic sow feeder systems tailored for small-group gestation housing presents an alternative for sow barns that are being converted.
Acknowledgements
The authors would like to sincerely thank Corie Herbert for her help in collecting data for this study and the caretakers at The Ohio State University Swine Research Facility for helping with daily maintenance.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
*1. HR 5127, 95th Leg, Regular Session (MI 2009).
*2. Ohio Admin. Code 901:12-8-02 (May 11, 2017).
3. McGlone JJ. Updated scientific evidence on the welfare of gestating sows kept in different housing systems. Prof Anim Sci. 2013;29:189-198.
4. Zurbrigg K, Blackwell T. Injuries, lameness, and cleanliness of sows in four group-housing gestation facilities in Ontario. J Swine Health Prod. 2006;14:202-206.
5. Hoy S, Bauer J. Dominance relationships between sows dependent on the time interval between separation and reunion. Appl Anim Behav Sci. 2005;90:21-30.
*6. Marchant-Forde JN. Welfare of dry sows. In: Marchant-Forde, JN, ed. The Welfare of Pigs. Queensland, Australia: Springer Science. 2010:113-114.
7. Bench CJ, Rioja-Lang FC, Hayne SM, Gonyou HW. Group gestation housing with individual feeding—I: How feeding regime, resource allocation, and genetic factors affect sow welfare. Livest Sci. 2013;152:208-217.
8. Jang JC, Jung SW, Jin SS, Ohh SJ, Kim JE, Kim YY. The effects of gilts housed either in group with the electric sow feeding system or conventional stall. Asian-Australas J Anim Sci. 2015;28:1512-1518.
9. Chapinal N, Ruiz-de-la-Torre JL, Cerisuelo A, Gasa J, Baucells MD, Manteca X. Aggressive behavior in two different group-housing systems for pregnant sows. J Appl Anim Welf Sci. 2010;13:137-153.
10. Barnett JL, Cronin GM, McCallum TH, Newman EA. Effects of food and time of day on aggression when grouping unfamiliar adult pigs. Appl Anim Behav Sci 1994;39:339-347.
11. Verdon M, Morrison RS, Rice M, Hemsworth PH. Individual variation in sow aggressive behavior and its relationship with sow welfare. J Anim Sci. 2016;94(3):1203-1214.
12. Remience V, Wavreille J, Canart B, Meunier-Salaun MC, Prunier A, Bartiaux-Thill N, Nicks B, Vandenheede M. Effects of space allowance on the welfare of dry sows kept in dynamic groups and fed with an electronic sow feeder. Appl Anim Behav Sci. 2008;112:284-296.
13. Li YZ, Wang LH, Johnston LJ. Sorting by parity to reduce aggression toward first-parity sows in group-gestation housing systems. J Anim Sci. 2012;90:4514-4522.
14. Rodenburg BT, Koene P. The impact of group size on damaging behaviours, aggression, fear and stress in farm animals. Appl Anim Behav Sci. 2007;103:205-214.
15. Hemsworth PH, Rice M, Nash J, Giri K, Butler KL, Tilbrook AJ, Morrison RS. Effects of group size and floor space allowance on grouped sows: Aggression, stress, skin injuries, and reproductive performance. J Anim Sci. 2013;91:4953-4964.
16. Samarakone TS, Gonyou HW. Domestic pigs alter their social strategy in response to social group size. Appl Anim Behav Sci. 2009;121:8-15.
*17. National Research Council. Nutrient Requirements of Swine. 11th ed. Washington, DC: The National Academies Press. 2012.
18. Galindo F, Broom DM. The relationships between social behaviour of dairy cows and the occurrence of lameness in three herds. Res Vet Sci. 2000;69:75-79.
*19. Backus GBC, Vermeer HM, Roelofs PFMM, Vesseur PC, Adams JHAN, Binnendijk GP, Smeets JJJ, van der Peet-Schwering CMC, van der Wilt FJ. Comparison of four housing systems for non-lactating sows. Rosmalen, The Netherlands: Research Institute for Pig Husbandry. 1997. Report P1.171.
20. Arey DS. Time course for the formation and disruption of social organization in group-housed sows. Appl Anim Behav Sci. 1999;62:199-207.
21. Puppe B. Effects of familiarity and relatedness on agonistic pair relationships in newly mixed domestic pigs. Appl Anim Behav Sci. 1998;58:233-239.
22. Turner SP, Horgan GW, Edwards SA. Effect of social group size on aggressive behavior between unacquainted domestic pigs. Appl Anim Behav Sci. 2001;74:203-215.
23. Li YZ, Johnston LJ. Behavior and performance of pigs previously housed in large groups. J Anim Sci. 2009;87:1472-1478.
24. Schmolke SA, Li YZ, Gonyou HW. Effects of group size on social behavior following regrouping of growing-finishing pigs. Appl Anim Behav Sci. 2004;88:27-38.
25. Estevez I, Andersen IL, Nævdal E. Group size, density and social dynamics in farm animals. Appl Anim Behav Sci. 2007;103:185-204.
26. O’Connell NE, Beattie VE, Moss BW. Influence of social status on the welfare of sows in static and dynamic groups. Anim Welfare. 2003;12:239-249.
*27. Verdon M, Hemsworth P. The relationship between aggression, feeding times and injuries in pregnant group-housed sows. Proc ISAE. Indianapolis, Indiana. 2011:139.
28. Harris MJ, Pajor EA, Sorrells AD, Eicher SD, Richert BT, Marchant-Forde JN. Effects of stall or small group gestation housing on the production, health and behaviour of gilts. Livest Sci. 2006;102:171-179.
29. Weng RC, Edwards SA, Hsia LC. Effect of individual, group or ESF housing in pregnancy and individual or group housing in lactation on sow behavior. Asian-Australas J Anim Sci. 2009;22:1574-1580.
30. Bergeron R, Bolduc J, Ramonet Y, Meunier-Salaün MC, Robert S. Feeding motivation and stereotypies in pregnant sows fed increasing levels of fibre and/or food. Appl Anim Behav Sci. 2000;70:27-40.
31. Buckner LJ, Edwards SA, Bruce JM. Behaviour and shelter use by outdoor sows. Appl Anim Behav Sci. 1998;57:69-80.
32. Mendl M, Zanella AJ, Broom DM. Physiological and reproductive correlates of behavioural strategies in female domestic pigs. Anim Behav. 1992;44:1107-1121.
33. Chapinal N, Ruiz-de-la-Torre JL, Cerisuelo A, Baucells MD, Gasa J, Manteca X. Feeder use patterns in group-housed pregnant sows fed with an unprotected electronic sow feeder (Fitmix). J Appl Anim Welf Sci. 2008;11(4):319-336.
34. Anil L, Anil SS, Deen J, Baidoo SK, Walker RD. Effect of group size and structure on the welfare and performance of pregnant sows in pens with electronic sow feeders. Can J Vet Res. 2006;70:128-136.
35. Jensen KH, Sorensen LS, Bertelsen D, Pedersen AR, Jorgensen E, Nielsen NP, Vestergaard KS. Management factors affecting activity and aggression in dynamic group housing systems with electronic sow feeding: a field trial. Anim Sci. 2000;71:535-545.
36. Kranendonk G, Van der Mheen H, Fillerup M, Hopster H. Social rank of pregnant sows affects their body weight gain and behavior and performance of the offspring. J Anim Sci. 2007;85:420-429.
37. Johnston LJ, Li YZ. Performance and well-being of sows housed in pens retrofitted from gestation stalls. J Anim Sci. 2013;91:5937-5945.
38. Zhao Y, Flowers WL, Saraiva A, Yeum KJ, Kim SW. Effect of social ranks and gestation housing systems on oxidative stress status, reproductive performance, and immune status of sows. J Anim Sci. 2013;91:5848-5858.
39. Greenwood EC, Plush KJ, van Wettere WHEJ, Hughes PE. A novel method for the analysis of social structure allows in-depth analysis of sow rank in newly grouped sows. Appl Anim Behav Sci. 2017;189:29-35.
40. Tummaruk P, Lundeheim L, Einarsson S, Dalin A-M. Effect of birth litter size, birth parity number, growth rate, backfat thickness and age at first mating of gilts on their reproductive performance as sows. Anim Repod Sci. 2001;66:225-237.
41. Xu RJ, Wang F, Zhang SH. Postnatal adaptation of the gastrointestinal tract in neonatal pigs: a possible role of milk-borne growth factors. Livest Prod Sci. 2000;66:95-107.
42. Le Dividich J, Rooke JA, Herpin P. Nutritional and immunological importance of colostrum for the new-born pig. J Agric Sci. 2005;143:469-485.
*Non-referred references.
