| Case report | Peer reviewed |
Cite as: Gilbreath ET, Gorham SL, Anderson DL, et al. Pulmonary Paragonimus infection and other pathologic findings in feral swine (Sus scrofa) from Macon County, Alabama. J Swine Health Prod. 2019;27(3):125-132.
Also available as a PDF.
SummaryTuskegee University College of Veterinary Medicine (TUCVM) was integrated into a feral swine surveillance program to aid in monitoring feral swine in Macon County, Alabama. The program was initiated by the Wildlife Services division of the Animal Plant and Health Inspection Services of the United States Department of Agriculture. Feral swine were captured, humanely euthanized, and blood was collected for various serological analyses. The carcasses were then submitted to the TUCVM diagnostic laboratory for postmortem examination and tissues were collected for additional disease surveillance. This report highlights pathologic findings identified in 15 feral hogs captured from Macon County, Alabama between March 14, 2012 and April 16, 2013, and serves as a record of some of the diseases the feral swine in this area harbor. Some of the pertinent pathologic findings identified include pulmonary metastrongyliasis, pulmonary paragonimiasis and severe ectoparasitism. | ResumenEl Colegio de Medicina Veterinaria de la Universidad Tuskegee (TUCVM por sus siglas en inglés) se integró a un programa de vigilancia de cerdos salvajes para ayudar en el monitoreo de cerdos salvajes del condado de Macon, Alabama. El programa fue iniciado por la división de Servicios de Fauna Silvestre de los Servicios de Inspección de Animales, Plantas y Salud del Departamento de Agricultura de los Estados Unidos. Los cerdos salvajes fueron capturados, sacrificados humanamente, y se recolectó sangre para varios análisis serológicos. Las canales fueron entregadas al laboratorio de diagnóstico de TUCVM para la examinación post mortem y se recolectaron tejidos para vigilancia adicional de enfermedades. Este reporte resalta los hallazgos patológicos identificados en 15 cerdos capturados en el condado de Macon, Alabama entre marzo 14, 2012 y abril 16, 2013, y sirve como un registro de algunas de las enfermedades que los cerdos salvajes albergan en esta área. Algunos de los hallazgos patológicos pertinentes identificados incluyen metastrongiliasis pulmonar, paragonimiasis pulmonar y ectoparasitismo severo. | ResuméLa Tuskegee University College of Veterinary Medicine (TUCVM) a été intégrée dans un programme de surveillance des porcs sauvages afin d’aider à surveiller les porcs sauvages dans le comté de Macon, Alabama. Le programme fut initié par la division des Services de la faune du Animal Plant and Health Inspection Services du département de l’agriculture des États-Unis. Des porcs sauvages ont été capturés, euthanasiés de façon humanitaire, et du sang prélevé pour différentes analyses sérologiques. Les carcasses furent ensuite soumises au laboratoire de diagnostic de TUCVM pour un examen post-mortem et des tissus ont été prélevés pour la surveillance de maladies additionnelles. Le présent rapport souligne les trouvailles pathologiques identifiées chez 15 porcs sauvages capturés dans le comté de Macon, Alabama entre le 14 mars 2012 et le 16 avril 2013, et sert de registre de quelques-unes des maladies rencontrées chez les porcs sauvages dans cette région. Quelques-unes des trouvailles pathologiques pertinentes identifiées incluent la metastrongylose pulmonaire, la pargonimiase pulmonaire et un ectoparasitisme sévère. |
Keywords: swine, Alabama, feral swine, Metastrongylus, Paragonimus
Search the AASV web site
for pages with similar keywords.
Received: October 25, 2018
Accepted: January 23, 2019
Feral swine (Sus scrofa) are highly prolific and lack natural predators. Therefore, once established, they can readily overpopulate an area. Their numbers have progressively increased to high numbers in the United States, currently estimated at over 6 million,1 with highest populations in Texas, California, Florida, and Hawaii.2 They are also becoming more common in other regions of the nation, including Alabama.3 Their presence and increasing prevalence warrants concern of their potential impact on humans and other animals that share their habitat. Feral swine readily interact with and breed with domestic swine (Sus scrofa domesticus) and the ability for disease transmission between wild and domestic swine is high.4,5 Transmissible diseases known to be harbored and display seroprevalence by feral swine in the United States include pseudorabies virus6,7 and zoonotic diseases such as Brucella,5 Toxoplasma gondii,8 Trichinella spiralis,8 and influenza A virus.9,10 Furthermore, consumption of improperly cooked products (ie, skeletal muscle and intestine) of paratenic hosts of Paragonimus, such as wild boar, could result in human and animal infection as has been previously reported.11,12 Feral swine also have the potential to propagate foreign animal diseases, such as foot-and-mouth disease,13 hog cholera,14 and African swine fever.15 In addition to the potential for disease transmission to humans and various domestic species, their natural foraging behavior can result in massive crop destruction. They are also known to prey on small mammals, including goat kids and neonatal lambs,16 resulting in their classification as agricultural nuisances.
In light of the growing prevalence of feral swine in Alabama and the United States1,17 and their ability to cause adverse effects on humans, domestic animals, and other wildlife species, monitoring these animals for pathologic conditions is paramount for public health, public education, and epidemiologic surveillance. This report highlights significant pathology identified in 15 feral swine captured in Macon County, Alabama between March 14, 2012 and April 16, 2013.
Case description
The Manually Initiated Nuisance Elimination trapping system (Jager Pro Hog Control, Fortson, GA) was used to capture feral swine. The capture devices were installed on the Russell Plantation which comprises 1687 acres of forestry land owned by Tuskegee University in Macon County, Alabama. Once pigs were captured in the trap, they were humanely killed by gunshot. All pigs appeared to be in good health based on observation of adequate activity, sufficient body condition score, and absence of external lesions or adverse clinical signs. Sterile swabs were used to obtain nasal samples and blood was collected via cardiac puncture using a 60 mL syringe with a 16-gauge, 10.16-mm needle. While in right lateral recumbency, the blood collection site was between the fourth and fifth rib behind the left elbow. Enough blood was collected from each animal to fill three 8.5 mL BD vacutainer blood tubes (Becton, Dickinson and Co, Franklin Lakes, NJ). Serum samples were obtained and frozen until analysis by the US Department of Agriculture. Serum was analyzed for antibodies to pseudorabies virus (n = 15) via glycoprotein B enzyme-linked immunosorbent assay (ELISA), classical swine fever virus (n = 15), porcine hemagglutinating encephalomyelitis virus (n = 8), influenza A virus (n = 4), and Trichinella (n = 4) via ELISA, Toxoplasma via ELISA (n = 4) and microagglutination assay (n = 7), and Brucella suis (n = 15) via fluorescence polarization assay. Nasal swabs were analyzed for influenza A virus (n = 7) via real-time reverse transcription polymerase chain reaction. Tests were performed at various laboratories (Table 1). Carcasses were submitted to the Tuskegee University College of Veterinary Medicine for postmortem evaluation.
Table 1: Laboratory locations where various tests were performed on samples from 15 feral pigs captured in Macon County, Alabama
| Test performed | Laboratory |
|---|---|
| Pseudorabies virus – glycoprotein B ELISA | University of Georgia Tifton Veterinary Diagnostic Laboratory, Tifton, GA and Wisconsin Veterinary Diagnostic Laboratory, Madison, WI |
| Classical swine fever virus – ELISA | Foreign Animal Disease Diagnostic Laboratory, Greenport, NY |
| Hemagglutinating encephalomyelitis virus - ELISA | National Institutes of Health, Bethesda, MD |
| Influenza A virus – ELISA | USDA APHIS National Wildlife Disease Program, Fort Collins, CO |
| Trichinella - ELISA | USDA APHIS National Wildlife Disease Program, Fort Collins, CO |
| Toxoplasma - ELISA | USDA APHIS National Wildlife Disease Program, Fort Collins, CO |
| Toxoplasma - microagglutination | USDA Agricultural Research Service, Beltsville, MD |
| Brucella - fluorescence polarization assay | Kansas State Federal Brucellosis Laboratory, Topeka, KS |
| Influenza A virus - rRT-PCR (nasal swab) | Thompson Bishop Sparks State Diagnostic Laboratory, Auburn, AL |
Case findings
Three of 7 serum samples submitted for Toxoplasma microagglutination assay were positive. The 4 samples submitted for Toxoplasma ELISA were negative. One of 15 samples submitted for pseudorabies virus was a suspect positive. Results of all other tests previously listed were negative.
Postmortem examinations were performed on feral swine that ranged from juvenile to adult animals weighing 9 to 57 kg. There were 10 females and 5 males. Of these, 1 female was pregnant with 8 fetuses with crown-to-rump lengths of 25 cm, which is most consistent with a gestational age of approximately 99 days based on an established prediction equation for gestational age.18 Feral swine have a gestational period of approximately 115 days and usually have 1 to 2 litters per year, with an average of 4 to 8 piglets per litter.4
All 15 pigs were infected with numerous ectoparasites; 46 ectoparasites were examined. Of the 36 ticks, 33 (91.7%) were identified as Amblyomma americanum and 3 (8.3%) were identified as Dermacentor variablis. The ticks were characterized by a cephalothorax and 4 pairs of legs.19 Ten Haematopinus suis lice, (21.7% of the total ectoparasites collected) were identified. Lice had a distinct head, thorax, abdomen, and 3 pairs of legs (Figure 1).20 Speciation of ectoparasites was done by a veterinary parasitologist via observation through a dissecting microscope.
Figure 1: Representation of arthropod ectoparasites on the feral pig carcasses. The larger ectoparasite on the left is a female Amblyomma americanum tick, the middle ectoparasite is a male A americanum tick, and the ectoparasite on the right is a Haematopinus suis louse.

Intrabronchial and intrabronchiolar nematodes were macroscopically observed in 7 of 15 pigs (46.7%). In pigs with pulmonary nematodes, lung color was diffusely mottled, variably firm on palpation, and were associated with tracheobronchial lymph node hyperplasia. The pulmonary nematodes were white, thin, cylindrical, and 4 to 6 cm in length (Metastrongylus species; Figure 2). Histologically, bronchi and bronchioles contained cross-sections of intraluminal nematodes that measured 500 to 700 µm. They contained a body cavity, thin cuticle, coelomyarian musculature, intestinal tract lined by few multinucleated cells, and ovaries and uterus filled with oocytes and developing larva (Figure 3). Bronchi and bronchioles contained moderate amounts of intraluminal edema, fibrin, and mucus admixed with predominately eosinophils and fewer macrophages, lymphocytes, plasma cells, and neutrophils. Occasional free nematode eggs were present in bronchi. Bronchial and bronchiolar epithelium was hyperplastic with goblet cell metaplasia. There was marked peribronchial and peribronchiolar smooth muscle hypertrophy and bronchial associated lymphoid tissue hyperplasia. There were multifocal to coalescing areas of alveolar capillary congestion with associated intra-alveolar edema. To confirm species identity of the pulmonary nematodes, genomic DNA was extracted from 0.1 g of formalin-fixed paraffin embedded (FFPE) lung tissue.
Figure 2: Moderate numbers of white cylindrical Metastrongylus parasites (indicated by arrow) lie on the mucosal surface of 3 bronchi in the lung of a feral pig.
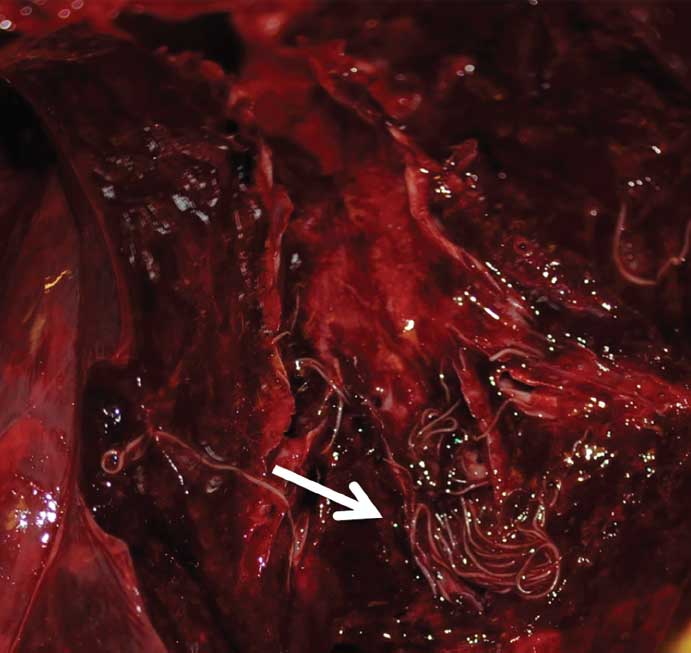
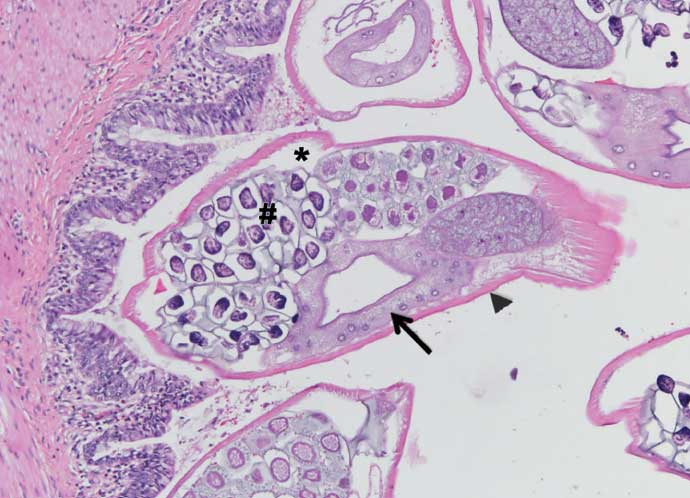
Figure 3: Cross sections of nematode parasites in a pulmonary bronchiole of a feral pig. There is a body cavity (asterisk), digestive tract (arrow), cuticle (arrowhead), and an ovary filled with eggs (hashtag). H&E stain; Magnification × 100.
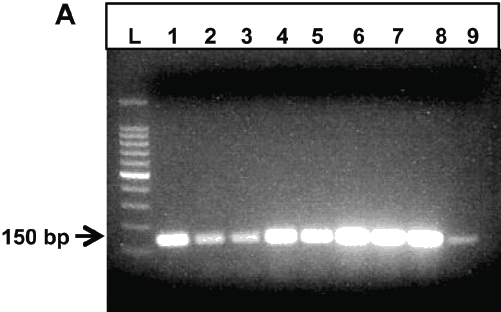
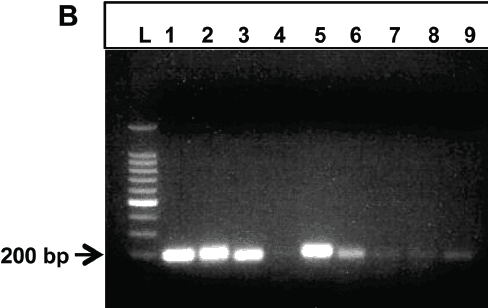
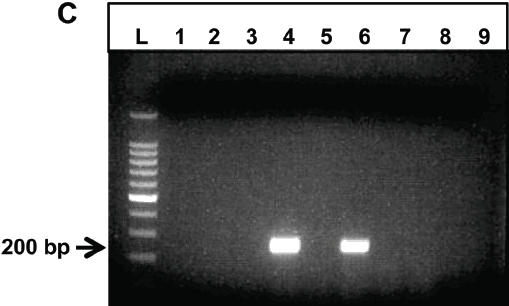
Briefly, pulverized FFPE samples were suspended in 600 μL of TSK buffer (567 μL of TE buffer [10 mM Tris and 1 mM EDTA, pH = 7.5], 30 μL of 10% sodium dodecyl sulfate, and 3 μL of 20 mg/mL proteinase K) and incubated at 50°C overnight. Then, 114 μL of 5 M NaCl and 91 μL of 1 M NaCl and 10% (vol/vol) hexadecyltrimethylammonium bromide mixture were added. After a 15-minute incubation at 65°C, DNA was extracted twice with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1, vol/vol) and then once with an equal volume of chloroform-isoamyl alcohol (24:1, vol/vol). After precipitation with 900 μL of isopropanol, the DNA was washed with 70% (vol/vol) ethanol and resuspended in TE buffer. The lung samples came from 9 different pigs. Conventional polymerase chain reaction (PCR) was performed on 20 ng of DNA using primer sets specific for detection of Metastrongylus salmi, Metastrongylus pudendotectus, Metastrongylus elongatus (apri), Paragonimus westermani, and Paragonimus kellicotti (Table 2). The cycling conditions included an initial denaturation for 1 minute at 95°C, 35 cycles of denaturation at 95°C for 30 seconds, annealing at 55°C for 30 seconds, elongation at 72°C for 30 seconds, and a final extension step at 72°C for 5 minutes. The amplified gene fragments were sequenced and found to match the gene sequences reported in the GenBank corresponding to the genes’ accession numbers. The PCR amplification targeted the 28s ribosomal RNA gene for M salmi and M pudendotectus, and the 18s ribosomal RNA gene for M elongatus (GenBank accession numbers AJ305404, AF210046 and AJ920363, respectively). As loading control, all samples were positive for S scrofa 12s ribosomal RNA gene (Figure 4A; GenBank accession number EF027294). Among the 9 samples tested by PCR, 8 were positive for M salmi (Figure 4B), while all 9 samples were negative for both M pudendotectus and M elongatus.
Table 2: Primer pairs used for detection of pulmonary parasites by polymerase chain reaction
| Genus and species tested | Primer pairs |
|---|---|
| Metastrongylus salmi | Forward 5’-TTCAGGGTTGTTAACGAT-3’ and Reverse 5’-TTGCTTGAACGGGTAA-3’ |
| Metastrongylus pudendotectus | Forward 5’-CAGTGACCGGGTCGGTT-3’ and Reverse 5’-TCCGTACCAGTTCCA-3’ |
| Metastrongylus elongatus | Forward 5’-TGCATGTCGAGTTCAACTTC-3’ and Reverse 5’-ATGCTGCGTTATTCAGAGTC-3’ |
| Paragonimus westermani | Forward 5’-AGGCAATGTGGTGTTCAGGT-3’ and Reverse 5’-ATCGGACTCGTGCAAGTA-3’ |
| Paragonimus kellicotti | Forward 5’-ATATTGCGGCCACGGGTTA-3’ and Reverse 5’-ACGTGGCACATACATAGATCA-3’ |
| Sus scrofa | Forward 5’-AAACTGGGATTAGATACCCCA-3’ and Reverse 5’-AGAACAGGCTCCTCTAGGT-3’ |
Figure 4: Conventional polymerase chain reaction of formalin-fixed paraffin-embedded lung tissue from feral pigs for parasite identification. (A) All samples were positive for Sus scrofa 12s ribosomal RNA. (B) Rows 1-3 and 5-9 were positive for Metastrongylus salmi. Row 4 was negative for M salmi. All samples were negative for Metastrongylus pudendotectus and Metastrongylus elongatus. (C) Among the 9 samples tested from the lung tissue of the wild pig with the pulmonary trematode, 2 samples (rows 4 and 6) were positive for Paragonimus westermani.
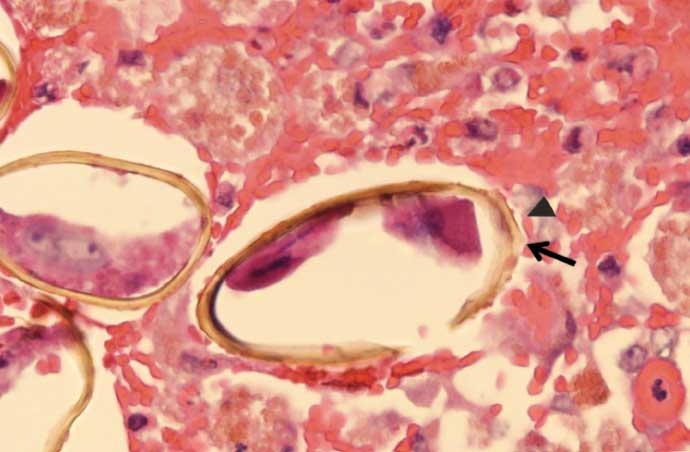
An intrapulmonary trematode was observed in a pig that did not have grossly observable pulmonary nematodes. The trematode was 1.5 cm long, brown, flat, and tapered at both ends, consistent with Paragonimus species. Microscopically, the trematode cross section was 8 × 3 mm2, contained multiple cuticular ridges and spines, had a body filled with parenchyma, and contained peripherally located vitelleria (Figure 5A). Ceca, testes with mature sperm (Figure 5B), and a uterus with yellow-shelled eggs (Figure 5C) were present. Histologically, the lung of the pig infected with pulmonary trematodes contained multinodular aggregates of innumerable oocytes surrounded by thick bands of fibrosis (Figure 6). The oocytes were ovoid, 50 to 70 × 80 to 100 µm2 with a distinct 3 µm thick, yellow-pigmented refractile cell wall, typical of trematode eggs. A single operculum with opercular ridges was occasionally evident (Figure 7). The observed opercular ridges are a characteristic feature of Paragonimus species oocytes.21 Oocytes were surrounded by moderate to numerous lymphocytes, plasma cells, and macrophages, which often contained abundant brown granular pigment (hemosiderin) and few to moderate scattered eosinophils. In the surrounding lung tissue, within bronchi, bronchioles, and alveoli, there were small to moderate numbers of eosinophils, lymphocytes, plasma cells, and histiocytes, which were sometimes infiltrating peribronchiolar regions. The interstitium was mildly expanded by macrophages. Bronchioles and alveoli occasionally contained small amounts of hemorrhage, fibrin, and edema. There was a focally extensive abscess with a central area of necrosis, hemorrhage, and numerous oocytes admixed with numerous degenerate and few viable eosinophils, lymphocytes, plasma cells, macrophages, and neutrophils. The central necrosis was surrounded by lymphocytes, plasma cells, and macrophages and rimmed by fibrosis which was further lined by hemorrhage, fibrin, and edema. The PCR was performed on DNA extracted from the 9 samples which included the FFPE trematode-infected lung tissue using primer pair for P westermani 28s ribosomal RNA gene (GenBank accession number HM172630) and P kellicotti 28s ribosomal RNA gene (GenBank accession number HQ900670). Among the 9 samples tested, 2 were positive for P westermani (Figure 4C) and P kellicotti was not detected. These results suggest that the trematode P westermani was present. Because this species of Paragonimus is not endemic in North America, additional ancillary diagnostics should be performed in future studies to definitively confirm the presence of P westermani in our samples. The PCR results do, nonetheless, confirm the macroscopic and microscopic diagnosis of Paragonimus.
Figure 5: Sections of a trematode obtained from the lung of a feral pig. (A) The trematode has a thin cuticle with cuticular ridges and spines (arrow), prominent subcuticular vitellaria (circle), multiple cross-sections of a digestive tract (asterisk), and absence of a coelomic space. H&E stain; magnification × 40. (B) Testis filled with mature sperm (hashtag). H&E stain; magnification × 600. (C) Uterus containing yellow, thick-shelled eggs. H&E stain; magnification × 400.
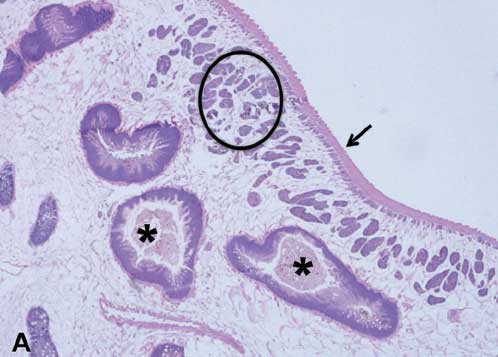
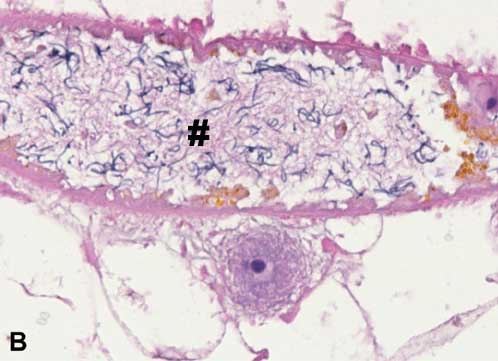
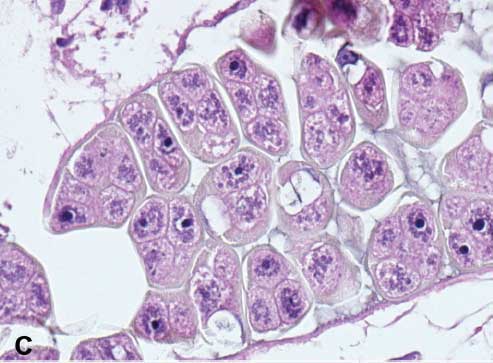
Figure 6: In the lung of a feral pig, aggregates of trematode oocytes have effaced normal lung architecture and are separated by bands of fibrous tissue and associated with inflammatory cells, hemorrhage, and hemosiderin-laden macrophages. Oocytes are round to oval with thick refractile walls. H&E stain; magnification × 40.
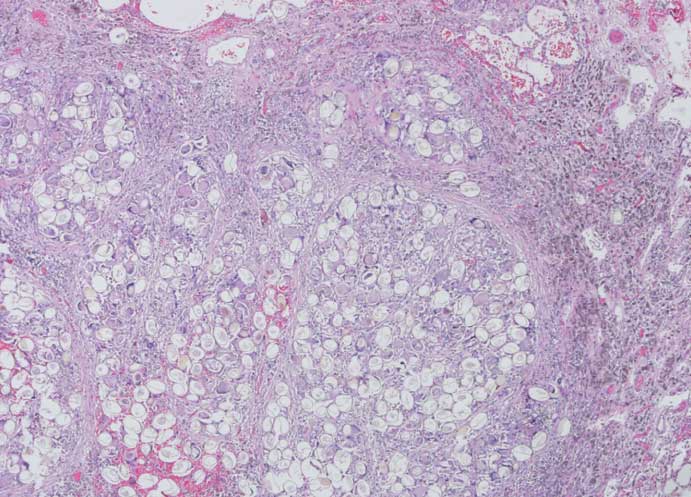
Figure 7: A trematode oocyte with a single operculum (arrowhead) and opercular ridges (arrow) in the lung of a wild pig. H&E stain; magnification × 600.
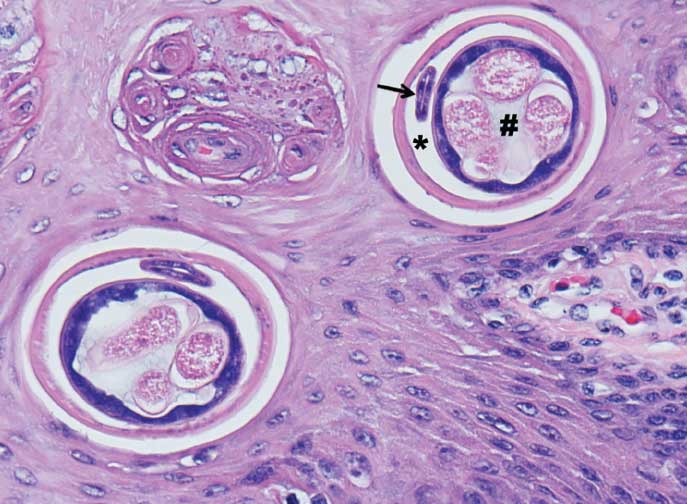
One pig (6.7%) had a tortuous nematode within the mucosa of the dorsal tongue. The nematode in the tongue was histologically observed in cross-section within the lingual epithelium. It was 60 to 70 µm in diameter and had a 5 to 7 µm cuticle, platymyarian musculature, a body cavity, and contained a small intestine and a uterus with unembryonated, thin-shelled eggs (Figure 8). The most common nematode in the tongue of the wild pig is Eucoleus (Capillaria) garfiai,22 and is the likely species in this case. This was considered an incidental finding of minimal pathologic significance.
Figure 8: Cross sections of a nematode in the lingual mucosa of a feral pig. The nematode contains a body cavity (asterisk), digestive tract (arrow), and uterus with unembryonated, thin-shelled eggs (hashtag). H&E stain; magnification × 400.
In 1 pig (6.7%), there was a firm, red, 2 × 2 × 2 cm3, nodular area of consolidation that extended into the underlying pulmonary parenchyma. Microscopically this was an area of lymphofollicular hyperplasia. Immunohistochemistry confirmed the presence of a mixed population of B and T lymphocytes, ruling out pulmonary lymphoma.
Additional incidental findings included 3 pigs with enlarged inguinal lymph nodes, 1 pig with englarged mandibular lymph nodes, and 1 pig with enlarged mesenteric lymph nodes. Other significant macroscopic and microscopic findings were not observed.
Discussion
Feral swine in Macon County, Alabama were infested with several external parasites: the H suis louse, D variablis tick (American dog tick), and A americanum tick (Lone star tick). Haematopinus suis can transmit swine pox virus23 and Mycoplasma suis,24 D variablis can transmit Rocky Mountain Spotted Fever (Rickettsia rickettsia)25 and tularemia (Francisella tularensis),26 and A americanum can transmit tularemia, Ehrlichia chaffensis, and Ehrlichia eweingii (the cause of human ehrlichiosis).27 Feral swine in Macon County, Alabama were infected with Metastrongylus organisms, which is the pig lung worm that can be transmitted between feral and domestic pigs. A novel finding in this case was the presence of the lung fluke, a Paragonimus organism. This trematode was macroscopically and microscopically observed in 1 pig; however, PCR detected DNA for P westermani in this pig as well as in 1 additional pig. This suggests that even though the trematode was not observed macroscopically or microscopically, the infection was still present. Paragonimus organisms are zoonotic agents that can be transmitted to humans and other animal species. Transmission commonly occurs by way of consuming infected crayfish, which are intermediate hosts, but can also occur through consumption of undercooked meat from paratenic hosts, such as feral swine.11,12,21 Antibodies to Toxoplasma organisms were detected in 3 pigs. This indicates that the animals were exposed to this organism and generated an immune response. Toxoplasma is a zoonotic protozoan parasite that can be transmitted to humans and other homeothermic species via ingestion of oocysts from feline (definitive host) feces, ingestion of bradyzoites in tissues of intermediate hosts (ie, wild pigs), and transplacentally.28 Transplacental transmission can cause fetal infection and abortion29; therefore, pregnant women should be especially cautious when handling feral swine tissues.
It is important to be aware of diseases prevalent in feral swine that may come in contact with domestic animals and humans. Proper precautionary measures should be taken when coming in contact with feral swine, thereby minimizing the risk of disease transmission.
Implications
The sample of feral swine in Macon County, Alabama were:
- infested with ectoparasites that can transmit a variety of diseases to domestic swine, other animal species, and humans.
- infected with endoparasites that are transmissible to domestic swine, other animal species, and humans.
- serologically positive for Toxoplasma which is transmissible to domestic swine, other animal species, and humans.
Acknowledgements
Appreciation is extended to the Auburn College of Veterinary Medicine Histopathology Laboratory for performing immunohistochemistry for this study. This study was supported by US Department of Agriculture’s Animal and Plant Health Inspection Service – Wildlife Services Cooperative Agreement (16-7100-0357-CA) and the National Institutes of Health-Tuskegee University Research Centers in Minority Institutions Core Facility (Grant G12MD007585).
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
*1. History of Feral Swine in the Americas. United States Department of Agriculture Animal and Plant Health Inspection Service. https://www.aphis.usda.gov/aphis/resources/pests-diseases/feral-swine/sa-fs-history. Updated March 23, 2018. Accessed October 4, 2018.
*2. Centers for Disease Control and Prevention. Brucella suis infection associated with feral swine hunting—three states, 2007-2008. MMWR. 2009;58(22):618-621.
*3. United States Department of Agriculture Forest Service. A guide to hunting feral swine--Bankhead National Forest. https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5389744.pdf. Accessed October 1, 2018.
*4. Kammermeyer K, Bowers J, Cooper B, Forster D, Grahl K, Holbrook T, Martin C, McDonald S, Nicholson N, VanBrackle M, Waters G. Feral Hogs in Georgia: Disease, Damage and Control. Atlanta, GA: Georgia Department of Natural Resources; 2011:1-11.
5. Olsen SC. Brucellosis in the United States: role and significance of wildlife reservoirs. Vaccine. 2010;28(Suppl 5):F73-76.
6. Cramer SD, Campbell GA, Njaa BL, Morgan SE, Smith SK II, McLin WR IV, Brodersen BW, Wise AG, Scherba G, Langohr IM, Maes RK. Pseudorabies virus infection in Oklahoma hunting dogs. J Vet Diagn Invest. 2011;23(5):915-923.
7. Corn JL, Stallknecht DE, Mechlin NM, Luttrell MP, Fischer JR. Persistence of pseudorabies virus in feral swine populations. J Wildl Dis. 2004;40(2):307-310.
8. Sandfoss M, DePerno C, Patton S, Flowers J, Kennedy-Stoskopf S. Prevalence of antibody to Toxoplasma gondii and Trichinella spp. in feral pigs (Sus scrofa) of eastern North Carolina. J Wildl Dis. 2011;47(2):338-343.
9. Corn JL, Cumbee JC, Barfoot R, Erickson GA. Pathogen exposure in feral swine populations geographically associated with high densities of transitional swine premises and commercial swine production. J Wildl Dis. 2009;45(3):713-721.
10. Hall JS, Minnis RB, Campbell TA, Barras S, Deyoung RW, Pabilonia K, Avery ML, Sullivan H, Clark L, McLean RG. Influenza exposure in United States feral swine populations. J Wildl Dis. 2008;44(2):362-368.
11. Nagayasu E, Yoshida A, Hombu A, Horii Y, Maruyama H. Paragonimiasis in Japan: a twelve-year retrospective case review (2001-2012). Intern Med. 2015;54(2):179-186.
12. Nakano N, Kirino Y, Uchida K, Nakamura-Uchiyama F, Nawa Y, Horii Y. Large-group infection of boar-hunting dogs with Paragonimus westermani in Miyazaki Prefecture, Japan, with special reference to a case of sudden death due to bilateral pneumothorax. J Vet Med Sci. 2009;71(5):657-660.
13. Mohamed F, Swafford S, Petrowski H, Bracht A, Schmit B, Fabian A, Pacheco JM, Hartwig E, Berninger M, Carrillo C, Mayr G, Moran K, Kavanaugh D, Leibrecht H, White W, Metwally S. Foot-and-mouth disease in feral swine: susceptibility and transmission. Transbound Emerg Dis. 2011;58(4):358-371.
14. Penrith ML, Vosloo W, Mather C. Classical swine fever (hog cholera): review of aspects relevant to control. Transbound Emerg Dis. 2011;58(3):187-196.
15. Costard S, Wieland B, de Glanville W, Jori F, Rowlands R, Vosloo W, Roger F, Pfeiffer DU, Dixon LK. African swine fever: how can global spread be prevented? Philos Trans R Soc Lond B Biol Sci. 2009;364(1530):2683-2696.
16. Plant JW, Marchant R, Mitchell TD, Giles JR. Neonatal lamb losses due to feral pig predation. Aust Vet J. 1978;54(9):426-429.
*17. Nelson DK, Causey MK. Feral Hogs in Alabama. Montgomery, AL: Alabama’s TREASURED Forests; 2001(Spring 2001):20-21.
18. Ullrey DE, Sprague JI, Becker DE, Miller ER. Growth of the swine fetus. J Anim Sci. 1965;24:711-717.
19. Bowman DD. Arthropods. In: Bowman DD, ed. Georgis’ Parasitology for Veterinarians. 7th ed. Philadelphia, PA: W.B. Saunders Company; 1999:46.
20. Bowman DD. Arthropods. In: Bowman DD, ed. Georgis’ Parasitology for Veterinarians.7th ed. Philadelphia, PA: W.B. Saunders Company; 1999:29-31.
21. Procop GW. North American paragonimiasis (Caused by Paragonimus kellicotti) in the context of global paragonimiasis. Clin Microbiol Rev. 2009;22(3):415-446.
22. Bowman DD. Diagnostic Parasitology. In: Bowman DD, ed. Georgis’ Parasitology for Veterinarians. 7th ed. Philadelphia, PA: W.B. Saunders Company; 1999:350-351.
23. Gibbs P. Pox Diseases. In: Kahn CM, ed. The Merck Veterinary Manual. 10th ed. Whitehouse Station, NJ: Merck & Co, Inc. 2010:793-794.
24. Allison RW. Blood Parasites. In: Kahn CM, ed. The Merck Veterinary Manual. 10th ed. Whitehouse Station, NJ: Merck & Co, Inc. 2010:26-27.
25. McQuiston JH. Rickettsial Diseases. In: Kahn CM, ed. The Merck Veterinary Manual. 10th ed. Whitehouse Station, NJ: Merck & Co, Inc. 2010:731-733.
26. Rohrback BW. Tularemia. In: Kahn CM, ed. The Merck Veterinary Manual. 10th ed. Whitehouse Station, NJ: Merck & Co, Inc. 2010:625-626.
27. Levin ML. Ticks. In: Kahn CM, ed. The Merck Veterinary Manual. 10th ed. Whitehouse Station, NJ: Merck & Co, Inc. 2010:845-847.
28. Brown CC, Baker DC, Barker IK. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 2. 5th ed. Philadelphia, PA: Saunders Elsevier; 2007:270-272.
29. Chaudhry SA, Gad N, Koren G. Toxoplasmosis and pregnancy. Can Fam Physician. 2014;60(4):334-336.
* Non-refereed references.
