Case report |
Peer reviewed |
Ringworm in lactating sows
Tiña en cerdos de lactancia
La teigne dans les truies en la mise bas
Jeremy S. Pittman, DVM; John D. Roberts, DVM, PhD
JSP: Murphy-Brown, LLC, Waverly Division, Waverly, Virginia. JDR: College of Veterinary Medicine, North Carolina State University, Raleigh, North Carolina. Deceased. Corresponding author: Dr Jeremy S. Pittman, 6722 Lake Road, Prince George, VA 23875; Tel: 804-834-1220; Fax: 804-834-3207; E-mail: jeremypittman@carrollsva.com.
Cite as: Pittman JS, Roberts JD. Ringworm in lactating sows. J Swine Health Prod. 2005;13(2):86-90.
Also available as a PDF.
SummaryA 100-sow farrow-to-feeder farm with an outdoor gestation lot had a 4-year history of ring-like skin lesions in lactating sows. On average, 90% of sows in each 5-week batch developed lesions within a week of entering the farrowing house. Lesions beginning as 2- to 3-cm circles of dark, crusty skin coalesced into larger areas on the back, sides, and ventral abdomen. No etiologic agent was identified in hair shafts and skin biopsies in initial samples. A second sampling revealed diffuse fungal elements in exfoliated epidermis. The tentative diagnosis was ringworm. Control strategies were limited by cost and labor constraints defined by the producer. Goals agreed upon included reducing the number of organisms in the farrowing house and the number that entered the farrowing house on the sows, and limiting recontamination of the environment by aggressively treating lesions. Sows were thoroughly washed before entering the farrowing house. Washing and disinfecting protocols used in the farrowing house and topical treatment of lesions were improved. Prevalence of lesions in the next farrowing group was 30%, and in subsequent groups remained < 10%. The average number of lesions per sow decreased from > 20 to < 5, and lesions were much smaller. | ResumenUna granja de 100 hembras, de gestación a lechón de 30 kg, con gestación exterior, tenía una historia de 4 años de lesiones con forma de anillo en la piel de las hembras en lactancia. En promedio, el 90% de las hembras de cada banda de 5 semanas desarrollaban lesiones en menos de una semana después de haber entrado a la maternidad. Las lesiones que empezaban como círculos de 2 a 3 cm de piel oscura y dura se unían en grandes áreas en la espalda, los costados y el abdomen ventral. No se identificaron agentes etiológicos en las muestras iniciales de pelo ni en las biopsias de piel. Una segunda toma de muestras reveló elementos fúngicos difusos en la epidermis exfoliada. El diagnóstico tentativo fue tiña. Las estrategias de control se limitaron por restricciones de costo y mano de obra establecidas por el productor. Los objetivos acordados incluyeron reducir el número de organismos en la maternidad y el número que entraba a la maternidad en las hembras (sic), así como limitar la recontaminación del medio ambiente tratando las lesiones agresivamente. Las hembras fueron lavadas cuidadosamente antes de entrar a la maternidad. Se mejoraron los protocolos de lavado y desinfección usados en la maternidad, así como el tratamiento utilizado en las lesiones. La frecuencia de las lesiones en el siguiente grupo de maternidad fue de 30% y en los grupos subsiguientes permaneció < al 10%. El número promedio de lesiones por hembra disminuyó de > de 20 a < de 5, y las lesiones fueron mucho menores. | ResuméUne ferme avec 100 truies de gestation à porcelets de 30 kg avec une gestation extérieur avait une histoire de 4 années de lésions de la peau comme anneaux dans les truies en la mise bas. En moyenne, 90% de truies dans chaque bande de 5 semaines ont développé des lésions dans une semaine d'entrer dans la mise bas. Lésions qui commencent comme cercles de 2 à 3 centimètres de peau noir, croustillante s'est unie dans plus grandes régions sur le dos, côtés, et abdomen ventral. Aucun agent étiologique n'a été identifié dans les échantillons initiaux des cheveux et les biopsies de la peau. Un deuxième échantillonnage révélé des éléments fongiques diffuses dans l'épiderme exfolié. Le diagnostic d'essai était la teigne. Les stratégies du contrôle ont été limitées par contraintes du coût et de la main-d'oeuvre définies par le producteur. Les buts convenus inclus la réduction du nombre d'organismes dans la mise bas et le nombre qui est entré à la mise bas sur les truies (sic), et limiter la recontamination de l'environnement en traitant des lésions agressivement. Les truies ont été lavées entièrement avant d'entrer dans la mise bas. Les protocoles de nettoyage et désinfection utilisé dans la mise bas et traitement d'actualité de lésions a été amélioré. La prévalence de lésions dans le prochain groupe de la mise bas était 30%, et dans les groupes subséquents sont restés < 10%. Le nombre moyen de lésions par truie a diminuée de > 20 à < 5, et les lésions étaient plus petites. |
Keywords: swine, ringworm,
dermatophytosis, skin disease
Search the AASV web site
for pages with similar keywords.
Received: April
19, 2004
Accepted: August
4, 2004
Dermatophytosis in swine, com- monly known as ringworm, is a skin disease caused by numerous species of fungal agents, most commonly Microsporum nanum, Microsporum canis, and Trichophyton verrucosum.1 Ringworm is rare in swine, but there are reports of large numbers of adult swine being affected.2,3 Dermatophytes inhabit the hair shafts and keratinized layers of the epidermis and rarely enter into the underlying dermis.4 Lesions begin as small circular areas of reddened or darkened skin (1 to 2 cm) that then exfoliate (crusting). Lesions expand outward with crusting following the leading margin. Multiple lesions may coalesce and continue to advance in an outward pattern. Differentials that should be considered for this type of skin lesion are exudative epidermitis (Staphylococcus hyicus, or greasy pig disease), Staphylococcus aureus dermatitis, erysipelas, photosensitization, pityriasis rosea, parakeratosis (zinc deficiency), and sarcoptic mange.1
Dermatophytosis occurs where conditions permit the organism to survive, proliferate, and have intimate contact with host animals. Scheiber2 reported chronic ringworm in sows in association with dirt lots and wooden hutches. Other conditions that predispose animals to ringworm are poor sanitary conditions, warm humid environments, and disruptions in the outer layers of the skin.1 Ringworm is not detrimental to performance, is usually nonpruritic, and is self-limiting. However, the disease may be demoralizing to producers and may result in incurrence of penalties assigned by the packer.2 Ringworm is zoonotic and care should be taken when handling or treating affected animals, although zoonotic infection from M nanum is mild and rare.5
Case description
A 100-sow farrow-to-feeder swine farm in North Carolina had a 4-year history of reoccurring skin lesions in lactating sows. The farm batch-farrows 20 sows on a 5-week rotation in a one-room, 20-crate farrowing house. Sows are naturally serviced in a wood and concrete barn and are moved onto dirt gestation lots after 30 days. Near term, sows are transported on a trailer and loaded into the farrowing house. Pigs are weaned at 3 to 4 weeks of age and loaded by litter into a 20-pen room of a two-room, on-site nursery. Feeder pigs are sold to a contract grower at 10 to 12 weeks of age. Low-weight feeder pigs are kept on-site in a separate dirt lot and raised for a local barbeque market.
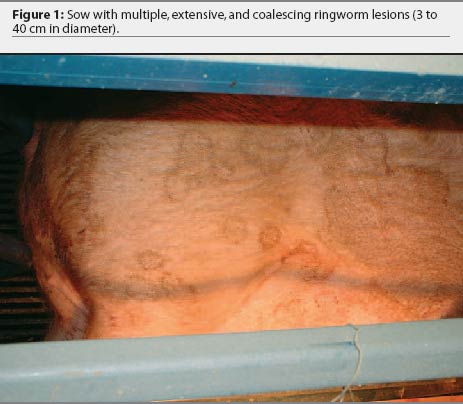
Over the 4-year period, the producer consistently noted skin lesions on sows, developing 1 to 2 weeks after sows were moved into the farrowing house. In each cohort, 16 to 20 of 20 lactating sows (80% to 100%) were affected with lesions that varied in number, size, and severity. Lesions originated behind the ears and on the neck and flank regions as small circles of dark, crusty skin (2 to 3 cm in diameter) and spread out from the center (Figure 1). The lesions coalesced into larger areas ranging in size from 8 cm to > 50 cm and covered the entire flank, neck, back, and ventral abdomen of the sow (Figures 2 and 3). The lesions were self-limiting with no alopecia or puritis, and resolved by weaning. Suckling pigs, nursery pigs, barbeque hogs, and boars never developed the lesions seen in the sows. The producer had attempted to treat the lesions by spraying them with dilute iodine. This treatment seemed only to slow, not prevent, progression of the lesions, and was terminated more than a year prior to our visit. On visits to the farm, close examination of gestation and breeding animals revealed no ring-like lesions.


Diagnosis
Six of 18 affected sows were chosen to sample. Hair shafts were collected from all six sows, and two groups of three samples were pooled and immediately placed on two Derm-Duet Dermatophyte Test (DMT) Medium cassettes (Bacti-Lab, Mountain View, California). In each of four of the sows, a skin biopsy measuring 2 cm x 2 cm was taken from the leading edge of an isolated lesion. Sites of biopsies were cleaned with a mild soap and water solution, and 2 mL of lidocaine was injected subcutaneously in a line along the dorsal aspect of the biopsy site. Two biopsy samples were placed on ice and two in 10% formalin. The inoculated DMTs and skin biopsies were submitted to the Iowa State University Veterinary Diagnostic Laboratory (ISU-VDL; Ames, Iowa) for fungal culture and identification, bacterial culture, histopathology, and special fungal staining.
Histopathology of the pooled skin biopsies revealed mild perivascular dermatitis with primarily mononuclear cells, few neutrophils, and few eosinophils, with multifocal folliculitis consistent with infectious dermatitis. The superficial epithelium was thickened. No fungal elements were detected. Results of fungal culture and periodic acid-Schiff fungal staining were negative. Bacterial culture of the skin biopsies revealed a mixed population of Staphylococcus aureus and Staphylococcus hyicus on both of two samples submitted, consistent with a commensal bacterial population. These bacteria may have contributed to the infectious dermatitis as opportunistic pathogens.
Two weeks after the first sample collection, a second sample of hair shafts, collected from four of the same group of farrowing sows, were pooled and cultured on a Derm-Duet DMT cassette at the North Carolina State University Veterinary Teaching Hospital microbiology laboratory. After 3 days of incubation, areas of the DMT culture that were suspect for a dermatophyte (indicated by a red color change in the media) were associated with exfoliated epidermis attached to the hair shafts, rather than with the hair shafts themselves. It was hypothesized that failure to culture and identify fungal agents in the samples submitted to the ISU-VDL might have been due to failure to collect and submit the proper sample (exfoliated epidermis) for visualization, culture, and identification of the causative organism.
Microscopic examination of five subsets of pooled exfoliated epidermis from the second sample collection, using a 10% KOH preparation, revealed diffuse fungal hyphae in each of the five subsets. A tentative diagnosis of ringworm was based on history, clinical presentation, suspect dermatophyte culture, and the presence of fungal elements in exfoliated epidermis. Etiologic diagnosis to distinguish the species of dermatophyte was not performed, since the treatment strategies are identical for all species and immediate initiation of control strategies were timely and desired.
Control strategies
The decision was made to attempt to resolve the lesions, assuming a diagnosis of dermatophytosis. The opportunity to implement all control strategies was immediately available as sows were being weaned.
Several control strategies were considered; however, due to a limited ability to implement changes (labor and budget), the most applicable and cost-effective management changes, which would result in the highest compliance, were selected. It was understood by the producer that failure of the initial control strategies would require implementation of some of the more labor-intensive or costly protocols. Control measures were aimed at reducing the number of organisms present on sows entering the farrowing house, decreasing the pathogen load possibly already present in the farrowing house, and minimizing recontamination of the environment by aggressively treating lesions as they developed.
Sows entering the farrowing house were usually washed as a group with a low-pressure garden hose prior to unloading from the trailer into the farrowing house; however, removal of organic material was limited. Actively washing each sow prior to entry into the farrowing house was discussed with the producer, but was not an option, as both the producer and the stockman were unwilling to increase labor in this area. Both agreed to continue washing the sows on the trailer as a group, using a high-pressure nozzle and a more diligent attempt to remove organic material. The trailer was washed with water (without disinfectant), dried, and left unused for 4 weeks after each rotation of sows out of the farrowing room. This trailer was used exclusively to transport sows to and from farrowing. Feeder pigs and barbeque hogs were transported by contract drivers.
Since the lesions appeared to be associated with the farrowing house, environmental sanitation focused on cleaning the farrowing house, with emphasis on farrowing crates. The room was soaked with water for 2 hours using a garden sprinkler, then organic material was removed with a high-pressure hose. Farrowing crates were power-washed at approximately 2000 psi with hot water and a bactericidal, fungicidal, and virucidal disinfectant (DC&R; Hess and Clark, Ashland, Ohio) at the labeled dilution of 1:128, to remove the existing biofilm. The crates were allowed to soak for 30 minutes and were then washed a second time with a 1:10 aqueous solution of povidone iodine (0.75% povidone iodine; AgriLabs, St Joseph, Missouri) using a high-pressure hose, allowed to dry, rinsed once more with a high-pressure hose, and dried a final time before sows were loaded into the farrowing house. The batch-farrowing system created a 3- to 4-day downtime that allowed for this detailed and extensive cleaning to be completed. This protocol was repeated once more between the next farrowing groups. Subsequent cleanings consisted of presoaking, washing to remove organic material, disinfecting with DC&R, rinsing, and drying.
The producer had been attempting to treat by spraying individual lesions with a range of diluted iodine solutions (1:10 to 1:1); however, this never appeared to improve the condition. Due to their watery consistency, iodine solutions have a tendency to run off the skin. Treatment was changed to a 1:1 solution of povidone iodine (0.75% povidone iodine; AgriLabs) and mineral oil that would be viscous enough to increase contact time with the affected areas. In addition, since the organism was found in the exfoliated epidermis, the lesions were generously scrubbed with a course bristle brush to remove as much of the exfoliated skin as possible, prior to the administration of the solution. It was suggested that the lesions be treated once daily until they resolved. The brush was disinfected in a 1:128 DC&R solution and rinsed between sows and after each use.
Response to treatment
After implementation of the treatment strategies, the number of sows affected, the numbers of lesions per sow, and the extent of the lesions decreased. Statistical analysis was performed on the proportion of sows affected using difference of proportions (z score) in two populations using the following formula:
![]()
where pn is the proportion of positive animals in specified group, ^p is the proportion in the total population, and nn is the total number of animals in a group, with the level of significance set at P = .05. There was a significant decrease in the prevalence of sows with skin lesions, from 90% in the first group sampled to 30% in the next farrowing group (P < .001). Prevalence remained at 0% to 10% in at least five subsequent farrowing groups. The average number of lesions per affected sow decreased from > 20 to < 5. The average size of the lesions decreased from a range of 8 to 50 cm to < 5 cm. When treatment began on individual sows, lesions resolved within 3 to 7 days. The producer and stockman saw an immediate response to the control strategies and were very satisfied with the results, which helped to encourage them that their extra efforts were being rewarded.
Discussion
Although not diagnostic, two facts help to speculate as to the possible etiologic agent in this case. The lack of fungal elements or positive culture of the hair shafts decreases the likelihood of Trichophyton species or M canis, which are commonly found in the hair shafts and follicles.4 Fungal elements of M nanum, the most common dermatophyte of swine,1 are limited to the exfoliated epidermis;6 therefore, the presumed etiologic agent in this case was M nanum. If an etiologic diagnosis is desired, culture and microscopic identification of the fungal macroconidia are required. Veterinary diagnostic laboratories suggest the following samples for culture, isolation, and identification of dermatophytes: skin scrapings, hair (including the base), and crusts (all submitted in a dry paper envelope), and skin biopsies (fresh and fixed in 10% formalin) from the leading edge of a lesion.7,8
It is likely that in this herd, the level of contamination in the sows' environment will always predispose them to develop ringworm, as the organisms can persist in the dirt lots. If the condition returns, other control measures, initially considered but not implemented, may be revisited. Disinfection of the breeding barn and dirt lot hutches (Figure 4) to further reduce the numbers of organisms in the sows' environment would be another point of control, as indicated by Scheiber.2 Disinfection protocols for these structures might be similar to that used in the farrowing house; however, practicality and environmental considerations may alter the manner in which they are cleaned and the chemicals used.

The possibility of washing individual sows prior to entering the farrowing house had been discussed earlier. It was considered that the sows could be washed with a "sow soap" or possibly an equine or feline antifungal shampoo. The labor required and the absence of a wash area were the major reasons for this step not being implemented. If the condition were to return to its original level of severity, washing of individual sows and disinfection of the breeding barn and hutches would be the first strategies recommended.
Fumigation or spraying of the farrowing house with enilconazole (Clinafarm; Schering-Plough, Union, New Jersey) was an option to reduce the fungal load in the farrowing house. Enilconazole, an antifungal available in a spray or fogger and labeled for use in poultry facilities for control of Aspergillus flavus, has been used for environmental control of feline ringworm caused by M canis in catteries.9 The use of this product in swine houses is off-label and should be considered only when there are no animals in the barn. Personnel should follow the labeled application directions and wear personal protective equipment when using the product.
Pharmaceutical treatment of ringworm in pigs has been documented with oral griseofulvin (Fulvicin; Schering-Plough, Union, New Jersey) at doses of 10 mg per kg,1 20 mg per kg,5 and 1g per 100 kg1,5 for 10 to 40 days and was considered for use in this herd. However, three limitations were noted. First, this product is labeled for cats, dogs, and horses only, and its use in this case would have been an off-label use in a lactating animal. Second, the product is expensive, with calculated costs averaging $1.50 per sow per day ($US), and was cost prohibitive for the producer. Lastly, at the time of investigation, there was a 1-year backorder on the product and the supplier was no longer accepting orders.
It is possible in this case that sows were being exposed to the organisms in the dirt lots, but did not develop lesions until they were placed indoors where conditions (eg, lack of sunlight, changes in humidity) permitted lesions to develop. Changes in the sows' physiologic status (lactation) or stress (parturition) may also have predisposed to development of lesions; however, this is purely speculation. Other conditions that might predispose these sows to developing ringworm could be further evaluated. For example, nutrient deficiencies and immunosuppression can predispose animals to diseases of the skin, such as hypovitaminosis A4 and B,1 zinc deficiency, 1 or possibly mycotoxicosis.
The response to treatment, combined with the history, clinical signs, and microscopic visualization of fungal elements in exfoliated epidermis, supports the tentative diagnosis of ringworm in these lactating sows. This case serves as an example to practitioners to ensure that they identify and collect the proper samples when faced with an uncommon or unfamiliar disease. A brief review of differentials and ideal samples for generalized disease conditions, prior to investigation, may help increase the likelihood of a correct diagnosis on the first attempt, while reducing the cost and time associated with re-sampling when initial submissions are nondiagnostic.
Implications
- Diagnosis of ringworm in swine is difficult and dependent on submission of the proper samples.
- Exfoliating epidermis must be collected for diagnosis of M nanum, and hair shafts for M canis and T verrucosum.
- Elimination of ringworm may be difficult due to persistence of the organism in the environment and lack of cost-effective control measures.
- Prevalence of ringworm in sows may be reduced by simple and cost-effective management changes in sow hygiene, environmental sanitation, and aggressive treatment of skin lesions.
References
1. Cameron RDA. Diseases of the skin. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:941-958.
2. Scheiber AR. Endemic ringworm and Staphylococcus hyicus infections: A case report. J Swine Health Prod. 1995;3:165-167.
3. Dodd DC, Newlin RW, Niksch GR. Infection of swine with Microsporum nanum. JAVMA. 1965;146:486-489.
4. Yager JA, Scott DM. The skin and appendages. In: Jubb KVF, Kennedy PC, Palmer N, eds. Pathology of Domestic Animals. 3rd ed. 1985;1:483-486.
5. Rochette F, Engelen M, Vanden Bossche H. Antifungal agents of use in animal health - practical applications. J Vet Pharmacol Therap. 2003;26:31-53.
6. Doster AR. Skin diseases of swine. J Swine Health Prod. 1995;3:256-261.
7. Iowa State University Veterinary Diagnostic Laboratory. Users Guide. Ames, Iowa; 2004:41.
8. South Dakota Animal Disease and Research Laboratory. Users Guide. Brookings, South Dakota. 2002:28.
9. Moriello KA. Monitoring treatment and preventing reinfection in cats with dermatophytosis. Vet Med. 2003;98:886-891
