Pittman JS, Shepherd G, Thacker BJ, et al. Trichuris suis in finishing pigs: Case report and review. J Swine Health Prod. 2010;18(6):306–313.
| Case Report | Peer reviewed |
Cite as: Pittman JS, Shepherd G, Thacker BJ, et al. Trichuris suis in finishing pigs: Case report and review. J Swine Health Prod. 2010;18(6):306–313..
Also available as a PDF.
SummaryTrichuris suis, the swine whipworm, can cause severe diarrhea, anorexia, and performance losses owing to reduced average daily gains and decreased feed efficiency. Severity of disease and impact on performance are related to infectious dose or concurrent infections. Trichuris suis is present in modern swine operations, but is an uncommon and perhaps neglected diagnosis. This paper describes an incidental finding of T suis in a finishing facility, with negligible impact on production, but demonstrates the continued presence of the parasite in modern swine production. This paper also provides a current review of T suis, trichuriasis, and control strategies. | ResumenEl Trichuris suis, el gusano látigo del cerdo, puede causar diarrea severa, anorexia, y pérdidas de producción debido a una reducción en la ganancia diaria y la eficiencia alimenticia. La severidad de la enfermedad y el impacto en el desempeño están relacionados a la dosis infecciosa y a las infecciones concurrentes. El T suis está presente en las operaciones modernas de producción porcina, pero su diagnóstico es poco frecuente y relegado. Este artículo describe un hallazgo incidental de T suis en una engorda, con poco efecto en la producción, pero que demuestra la presencia del parásito en la producción porcina moderna. Este artículo también aporta una revisión actual del T suis, la tricuriasis, y sus estrategias de control. | ResuméTrichuris suis, le ver en fouet du porc, peut causer une diarrhée sévère, de l’anorexie, et une réduction des performances due à une diminution du gain journalier moyen et une réduction de l’efficacité alimentaire. La sévérité de la maladie et l’impact sur les performances sont reliés à la dose infectieuse ou à des infections concomitantes. Trichuris suis est présent dans les élevages porcins modernes, mais l’infection est diagnostiquée peu fréquemment et probablement négligée. Le présent article décrit la trouvaille fortuite de T suis dans une unité de finition, avec un impact négligeable sur la production, mais démontre la présence continue du parasite dans les élevages porcins modernes. L’article fait également une revue récente des données sur T suis, la trichuriase, et des stratégies de maîtrise de l’infection. |
Keywords: swine, Trichuris suis, swine whipworm, Ascaris suum, large roundworm
Search the AASV web site
for pages with similar keywords.
Received: February 3, 2010
Accepted: June 25, 2010
Trichuris suis, the swine whipworm, is present in modern swine production, but its prevalence has declined over time, likely due to the transition to raising swine in confinement and away from pasture or soil lots where T suis is more prevalent. However, recent trends in outdoor production have demonstrated its presence.1-8 A national survey in the United States in 1988 showed that T suis was the second most prevalent intestinal parasite of swine (after Ascaris suum), with 45% of all farms testing positive, a majority of which represented finishing pigs.9 A report in 1998 of intestinal-parasite prevalence in the Nordic countries found T suis sporadically, and mostly in breeding herds.10 Reports from China, Kenya, and Western and South Australia showed a continued prevalence of T suis in modern swine production.11-14
Parasite description and life cycle
Trichuris suis is primarily a parasite of swine, although infections can be established in humans.15-17 The life cycle of T suis is direct and does not require any intermediate host. The eggs are oval (60 × 25 μm) and yellow-brown with bipolar plugs (operculate) (Figure 1). Eggs are passed in feces from infected animals, but are single-celled and are not initially infectious. Infective L1 stage larvae develop within the shell in 3 weeks to 2 months, depending on environmental temperature. The infective L1 stage within the egg is highly resistant and can remain in this form for several years in favorable conditions. Once the infective L1 egg is ingested, the bipolar plugs are digested and the L1 larvae hatch in the small intestine and cecum. The L1 larvae penetrate the mucosa via the crypts of Lieberkühn in the distal ileum, cecum, and colon. It is important to note that unlike A suum larvae, T suis larvae do not migrate extra-intestinally, and thus there are no respiratory tract or liver lesions. During the next 5 weeks, the larvae undergo four molts (L2, L3, L4) to the adult stage (L5) within the mucosal layers. The adult’s thicker posterior one-third then emerges through the mucosal surface into the lumen while the thin anterior two-thirds remains embedded in the mucosal layers. The variations in thickness of the anterior and posterior segments give the parasite the characteristic “whip-like” appearance. Adult females and males are 6 to 8 cm long and 3 to 4 cm long, respectively. Adults can be recovered anywhere from the distal ileum to the rectum, but most are located in the cecum and proximal colon.18 The prepatent period is 6 to 8 weeks and life span is 4 to 5 months.19-24
Figure 1: Fecal floatation demonstrating the large number of Trichuris suis eggs (n = 27) isolated from 1 g of feces in a single low-power field (× 28 magnification). Insert: Oval-shaped eggs of T suis demonstrating thick walls and bipolar plugs (× 280 magnification). 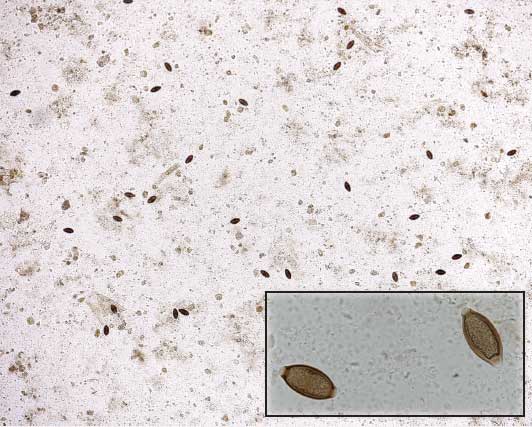 |
Trichuris suis egg production is sporadic, making diagnosis by fecal floatation difficult. Under experimental conditions, peak eggs per gram of feces (EPG) occurs around 7 weeks post infection, but this is followed by a rapid decrease in egg production over the next 4 to 5 weeks, possibly due to acquired immunity of the host or expulsion of adults.18,25,26 This is an important feature for fecal diagnostics, as a false-negative result might occur due to sporadic shedding and low egg production (< 1 EPG).25 Fecundity of an individual T suis female has not been documented, and focus has been on overall egg production post infection from individual pigs. However, Pedersen and Saeed26 estimated peak average egg production by a single female T suis to be approximately 6500 to 7800 eggs per day, which could result in approximately 6 to 8 EPG per adult female during peak egg production.
The minimal infectious dose of T suis has not been documented. However, under experimental conditions, infection has been established with inoculation of five eggs per kg of body weight administered eight times during a 4-week period (2000 total eggs per animal),7,26 during a 5-day period when pigs were fed soil containing infective T suis eggs at a rate of 200 eggs per day (1000 total eggs per pig),27 or with 400 eggs per animal.28 Other studies have demonstrated infection with single inoculations of 5000 to 50,000 eggs per animal.18,29 In addition, in experimental infections, there is a wide range (9% to 61%) of the proportion of infective eggs that develop into adults.25,29
Acquired immunity to T suis has been demonstrated26 and could explain the reduced number of adult worms in the host and a population over time, as demonstrated by reduction in fecal egg counts and lower worm burden. The immunity reduces secondary adult worm formation and induces an overall reduction in fecal egg counts. Trichuris suis excretory secretory products stimulate a T-helper 2 response in vitro through increased production of IL-6 and IL-10 cytokines from intestinal pig epithelial cells.30 These products also damage intestinal epithelial cells.31 Worm expulsion has been demonstrated experimentally by Kringel and Roepstorff18 at 9 weeks post inoculation, with most adults expelled by 11 weeks post inoculation. Subsequent work by the same authors demonstrated serum antibodies specific to T suis excretory secretory products, with increasing IgA, IgM, IgG1, and IgG2 levels through 9 weeks post inoculation.32 At 11 weeks post inoculation, levels of IgM, IgG1, and IgG2 started to decline, concurrent with expulsion of adult T suis. Serum levels of IgA remained elevated at 11 weeks post inoculation, and while this antibody is associated with mucosal immunity, relationships between IgA levels and mucosal immunity to T suis have not been evaluated.
One of the T suis excretory-secretory products, a 20-kDa glycoprotein, has been used to develop a Trichuris-specific antigen test, using Western blot and enzyme-linked immunosorbent assay (ELISA) methods.33 The ELISA test was able to detect seropositive swine by day 21 or 28 post inoculation, depending on infectious dose. Early detection suggests that this particular excretory-secretory product is excreted during the larval stages of T suis and thus might be used to detect early infections of T suis, prior to emergence of adult worms into the lumen and fecal excretion of eggs. The ELISA was also able to detect Trichuris-specific antibody in dogs chronically infected with Trichuris vulpis. At the time of publication, the test was not commercially available.33
Pathology
Infections with T suis can cause diarrhea, anorexia, anemia, poor growth, dehydration, and emaciation, but severity is usually related to the infective dose or concurrent bacterial enteritis.22,29,34 Mucohemorrhagic catarrhal enteritis (dysentery), anemia, and death have also been reported in infections in younger pigs.35-38 Severe infestations of T suis may cause acute morbidity and mortality in gilts.6
Key differentials for trichuriasis should include swine dysentery (Brachyspira hyodysenteriae), porcine proliferative enteropathy (Lawsonia intracellularis), salmonellosis, hemorrhagic bowel syndrome, intestinal parasites including A suum, and other causes of colitis (eg, Brachyspira pilisicoli).
In experimental infections, loose to watery diarrhea usually begins approximately 14 to 21 days post infection, with blood and mucous a few days prior to death or euthanasia at 43 to 60 days post infection.25,29,34,36 Development of diarrhea and presence of blood and mucous appear to be correlated with development of the larvae within the mucosal layers of the cecum and colon and subsequent emergence of adults into lumen.34 Anorexia develops 16 to 26 days post infection,25,29 with a significant weight loss in infected animals, as compared to non-inoculated controls, occurring during the third to fourth weeks post infection.25,36 Clinical anemia and severe hematological anemia begin approximately 3325 and 42 days post infection,34 respectively. Anemia and hypoalbuminemia34 develop secondary to physical damage caused by T suis migration and resultant inflammation of the deep mucosal layers, leading to destruction of capillaries and vasodilatation.
Necropsy of clinical cases of trichuriasis may be necessary to confirm a diagnosis, since clinical signs may develop prior to patency, thus preventing diagnosis by fecal examination alone. On gross necropsy, the intestine may be filled with semisolid to watery to bloody mucoid feces, depending on severity of infection and concurrent bacterial infections. The anterior portion of adult worms may be visible penetrating the cecal and colonic mucosa (Figure 2). Inflammatory nodules may be seen surrounding the adults where they penetrate the mucosa. In earlier infections, the nodules may indicate pre-erupted larvae beneath the mucosa. There is generalized moderate to severe typhlitis and colitis, again depending on severity of infection. In severe infections, the walls of the intestine may be thickened and a necrotic membrane may be present on the surface of the mucosa.29
Figure 2: Cecum of Trichuris suis-infected pig. Adult worms can be seen imbedded in the mucosa. 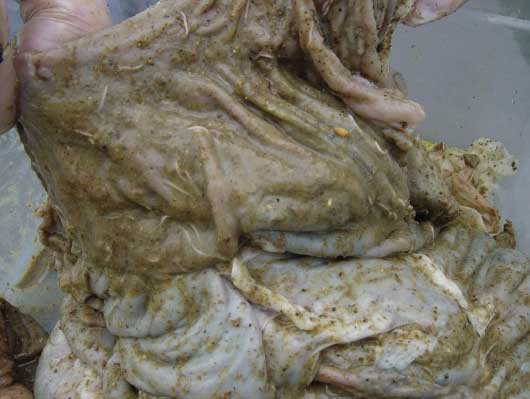 |
Histologically, cross-sections of T suis larvae or adults can be seen in various layers of the mucosa (Figure 3). Inflammation of the surrounding mucosa, specifically the lamina propria, is evident.29 The crypts of Lieberkühn, where T suis resides, are often filled with necrotic debris and mucus.29 In addition, areas of hemorrhage can be seen in the surrounding tissue.
Figure 3: Histopathological section of cecum demonstrating adult Trichuris suis (T) and mononuclear infiltrates (M) (H&E stain; magnification × 200) 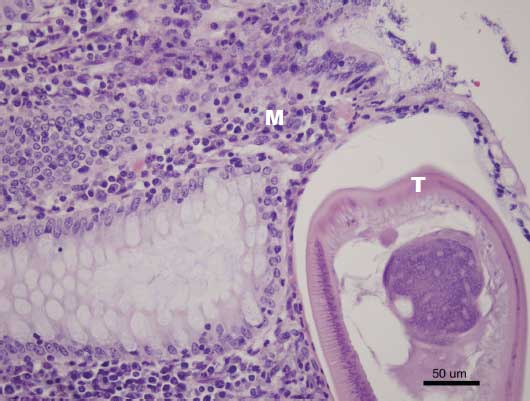 |
Hale and Stewart39 demonstrated that in pigs inoculated with moderate to high numbers of T suis eggs (1100 and 1650 eggs per kg body weight [BW], respectively) average daily gain (ADG) was lower than in pigs not infected, while in pigs inoculated with low numbers of T suis eggs (550 eggs per kg BW), ADG did not differ significantly from that of non-inoculated controls. Clinical disease, characterized by severe diarrhea, was more prevalent in the higher infective dose groups, and the poorest performing pigs were those with bloody diarrhea.
Diet also affects the pathogenesis and severity of T suis infections in swine. Protein and iron deficiencies increase the severity of infection and reduce host immunity to T suis.40,41 Inclusion in the diet of highly fermentable carbohydrates results in poorer growth and earlier expulsion of worms, while total number of T suis is lower in pigs fed diets containing inulin than in pigs fed diets that do not contain inulin.42,43
Case description
Housing
The case farm was a two-barn finishing site. Barn One was a curtain-sided, naturally ventilated finishing barn with a 950-head capacity and partially slatted flooring (50% solid). The barn contained 28 pens (5.5 × 11 m) housing approximately 33 pigs per pen, plus two 5.5 × 5.5-m pens. Barn Two was a naturally ventilated finishing barn with a 300-head capacity and totally slatted flooring. This barn contained 20 pens (5.5 × 5.5 m) housing approximately 15 pigs per pen. All pens in both barns were separated by metal gating, allowing contact between animals. Previously, the site had been a one-site farrow-to-finish operation. Pigs were last finished outside in dirt lots with wooden paddocks in 1994, after which they were raised in confinement (in Barns One and Two) on this site for approximately 2 years. There were no pigs on-site from 1996 until late 1999, when the farm was populated with feeder pigs from a production company. When this case occurred, the farm had recently received 32-kg, 10-week-old feeder pigs for finishing from an off-site nursery within the production system, with no history of T suis infection. Each barn was filled and emptied in an all-in, all-out manner, with washing and sanitation between groups; however, both barns on the site were not necessarily completely empty before receiving the next batch of feeder pigs.
Clinical description
No clinical disease was present in the population during the finishing period. The initial diagnosis of T suis infection was made subsequent to evaluation of the site as part of a larger study in which all finishing sites within a production system were being surveyed for intestinal parasites.44 The owner frequently recognized A suum adults in the barns and routinely treated with piperazine (Wazine-34; Fleming Laboratories, Inc, Charlotte, North Carolina) metered into the drinking water. The infected group had been treated with piperazine at a rate of 110 mg per kg the day after placement (Day 1). Expelled A suum adults were seen in this group of growing pigs on several occasions by the owner, by a production-company service person, and by several of the authors.
Diagnostic methods and results
As part of the large parasite survey, the authors collected a five-sample fecal composite from fresh fecal piles on the floor of each pen in each barn. Samples were collected three times, on Days 63, 85, and 106. All samples were dispatched overnight on ice to Myers Parasitology Services, Magnolia, Kentucky, for fecal flotation and EPG quantification. The fecal flotation method employed has been previously described by the authors.45
In Barn One, twenty-eight of 30 samples collected on Day 63 were positive for A suum eggs, with an average EPG of 116.2 (range 4 to 443). One pen was positive for T suis at 1 EPG. Samples collected from three pens on Day 85 were positive for T suis eggs, with 5, 3, and 7 EPG, respectively. Samples collected from three pens on Day 106 were positive for T suis eggs, with 2, 2, and 425 T suis EPG, respectively. In Barn Two, the same eight of 20 pens were positive for A suum eggs at each sampling point, but T suis eggs were never found in any samples.
It was recognized that two of three composite samples from one pen in Barn One were positive for T suis and that the Day 106 sample had a higher egg count (425 EPG) than the other positive samples. This pen was located in the back corner of the barn, and was a smaller pen reserved for poor-performing pigs. To further investigate the high T suis egg count in this pen and potentially harvest adult T suis for future experimental studies, the authors returned to the farm on Day 112. The pen contained eight pigs that were noticeably smaller than other pigs in the barn. Each of the eight pigs was identified with a numbered ear tag. Half to one kilogram of fresh fecal material was collected directly from the rectum of each of the eight pigs. Samples were processed the same day by the authors as previously described by Pittman et al45 and evaluated for the presence of T suis and A suum eggs. Seven of the eight pigs were positive for T suis eggs and all eight were positive for A suum eggs. Pig 1 had a high T suis EPG count of 575 (Figure 1), while counts in the others ranged from 1 to 10 EPG. Pig 1 also had 98 EPG of A suum.
The authors returned to the farm on Day 113. Pig 1, the smallest pig in the barn, weighed approximately 55 kg. Start weight on the individual pig was not known, but assuming the average start weight of the group (32.0 kg), average daily gain was estimated at 0.20 kg per day. It was noted that the pig defecated in large amounts and frequently, but that manure was of normal consistency and color. Pig 1 was euthanized and necropsy revealed large numbers of T suis adults in the cecum and colon (Figure 2). No other significant lesions were noted.
The cecum and colon of Pig 1 were submitted to Myers Parasitology Services for further processing. More than 100 T suis adults were collected from the cecum. A section of the cecum was collected in 10% formalin for reference at the time of necropsy. A small section of this sample and some fecal material were later submitted to the Iowa State University Veterinary Diagnostic Laboratory (ISU-VDL), Ames, Iowa, for histological evaluation and culture. Histopathology revealed moderate catarrhal typhlitis with mild to moderate mononuclear infiltrates in the lamina propria. A cross-section of a nematode (T suis) was present in the tissue section (Figure 3). Culture of the fecal sample revealed no significant growth; however, it must be noted that the sample had been refrigerated for 3 weeks prior to submission. Histological evidence for concurrent enteric bacterial pathogens, such as Brachyspira species, Salmonella serovars, or Lawsonia intracellularis, was not observed. No additional testing was performed on these samples.
Sampling of the source sow farm during this period, as part of a larger study,44 did not reveal evidence of T suis infection. The source nursery was not tested; however, other groups of finishing pigs at other finishing sites and originating from the same nursery were negative for T suis when tested,44 suggesting infection occurred after the feeder pigs arrived at the case farm.
It was suspected that the finishing site was contaminated, given the history of pigs on dirt lots, a common risk factor for trichuriasis.2,4,8 To better understand the epidemiology of the T suis infection, five 1-gallon bags of soil were collected from the premises: one near the entrance to the affected barn, one near an old nursery facility, one from the dirt lot, and two from the vacated paddocks. Multiple samples were taken from the surface layer of soil, no deeper than 2.54 cm, and represented several areas within approximately a 1.5-m diameter circle. Composite soil samples were submitted to Myers Parasitology Services and tested for T suis eggs using the same method as described for fecals. Each sample was tested twice using 1 and 3 g of soil. One paddock sample (Paddock 1) was positive for one T suis egg per 1 g of soil. Paddocks 1 and 2 were positive for one T suis egg per 3 g of soil. Soil samples from the barn entrance, nursery, and soil lot were negative for T suis eggs.
Control and treatment
No treatment was initiated in this group of pigs because of the lack of clinical impact on the animals and an inability to allow adequate withdrawal time for effective anthelmintics, as the pigs were near market weight. Subsequent groups could not be treated for T suis due to an inability to justify and implement effective feed-grade anthelmintics to a single farm within a large production system, and a lack of effective anthelmintics currently available for medication through the water system. Groups were treated for A suum with piperazine at arrival and 6 weeks post placement to minimize the impact of that parasite on growth performance.
Sanitation of the barns and site
Emphasis was placed on sanitizing the barns between groups of pigs, especially detailed washing of areas where organic material could reside from group to group, in order to reduce the level of environmental contamination by the resistant eggs of T suis and A suum. Sanitation of this site between groups consisted of an initial water-only wash to remove most of the organic material, followed by a 10- to 15-minute soak with a detergent (Barnstorm; Preserve International, Zepher Cove, Nevada) at a rate of 1:64. Fresh and cold water pressure washing (2000 psi) of floors, walls, feeders, and penning was performed after the detergent soak. The barn was allowed to completely dry, then a quaternary ammonium and glutaraldehyde combination disinfectant (Synergize; Preserve International) was applied, per label, to all surfaces at a rate of 1:256. The barn was once more allowed to dry completely before the next group of pigs was placed. This protocol alone was not sufficient to prevent infection in subsequent groups of pigs, as sampling of these groups revealed T suis and A suum eggs. While there is no apparent documentation of disinfectant efficacy against T suis in the literature, Trichuris trichiura (human whipworm) is sensitive to 2% glutaraldehyde or 1% sodium hypochlorite.46
Finishing performance and outcome
Individual pig performance was not available, so only group performance data was used for analysis. The lot was started with 839 pigs at an average body weight of 32.0 kg. Throughout the finishing phase, 11 pigs died (1.31%) and two pigs (0.24%) were sold to a cull market. Average sale weight of the market pigs was 130.58 kg and average weight of the cull pigs was 102.5 kg. Average daily gain and feed conversion (FC) of the group were 0.85 kg per day and 2.69, respectively. No pigs were condemned, and 98% of all pigs were sold as prime or above grade, meaning a carcass dress weight > 75.5 kg.
It stands to reason that, given the history of the farm and evidence of contamination of dirt lots, T suis was present on this site prior to 1994 and is likely the source of T suis infection in this case. Eight barns of finishing pigs from the same sow and nursery source, but grown at different sites, had the following production value ranges for comparison: mortality: (2.74% to 3.71%), culls (4.31% to 9.20%), average market weight (121.7 to 125.3 kg), average cull weight (62.3 to 75.1 kg), ADG (0.78 to 0.81 kg per day), FC (2.30 to 2.54).
Discussion
Eggs of T suis have remained in contaminated pastures for between 7 and 11 years.47 Survivability studies with T suis eggs have shown large reductions in percent of eggs remaining in contaminated pasture over short periods, related to season and exposure to the elements.47-49 In one of these studies, for eggs placed experimentally in pasture, recovery rate (ability to mechanically re-collect eggs from the pasture) decreased over a short period when eggs were placed in the spring to early summer months, while recovery rate was higher for eggs placed later in the year. A series of studies have shown that eggs of T suis disappear more slowly than eggs of A suum, and disappearance of both types occurs faster in summer months than in winter months and when placed on top of grass pastures as compared to being buried in the top layers (0 to 30 cm) of soil.47-49 Several factors may be responsible for these observations. Trichuris suis has a slower rate of development in ovo than does A suum, during which time the eggs are more susceptible to dehydration and high temperatures (> 50°C), which thus kill many of the eggs during the summer periods. It was hypothesized that soil covering was a protective factor, preventing eggs from dehydration and protecting them from higher temperatures, direct sunlight, and removal by rain or earthworms. On the basis of these observations, it is believed that plowing or tilling contaminated pastures may result in increased exposure of infective eggs or may increase survivability of eggs over seasons, depending on the timeline of plowing and placement of pigs.
In the survivability studies,47-49 it was shown that pigs exposed to pasture where eggs had been placed in late spring or early summer could develop infection before the end of that summer period, while pigs exposed to eggs placed in the late summer, fall, or winter did not develop trichuriasis until the next summer or even the second summer period. This effect of environmental conditions on the development of T suis eggs may allow for strategic pasture rotation of pigs to prevent clinical trichuriasis or reduce further environmental contamination.
Trichuris suis eggs survive in sludge during aerobic and anaerobic digestion, so contamination of pastures and fields via application of pig manure, slurry, or lagoon effluent is possible.1,50 However, significant and rapid destruction of T suis and A suum eggs (unembryonated and embryonated) has been demonstrated when slurry was treated using a composting method, whereby the manure was maintained at 55°C.51 Cold temperatures appear to be protective for T suis eggs, and 25% of eggs still remained infective after 80 days in -18°C storage.36 Therefore, overwintering or assuming frost or frozen pastures are no longer contaminated would be ill advised as a control strategy for T suis. Flaming of heat-resistant facilities and equipment between groups may help reduce environmental contamination if temperatures above 55°C can be achieved.
Another strategy for pasture rotation to reduce environmental contamination or potential clinical disease would be to house sows or older finishing pigs on known contaminated pastures. Egg excretion post infection is lower in sows than in growing pigs, and lower in growing pigs than in weaner pigs.52 In addition, gestating sows should be removed from contaminated areas prior to farrowing and during lactation to reduce the risk of transmission to and clinical disease in the suckling pigs. These strategies, along with properly timed treatment with anthelmintics, if not prohibited, can reduce contamination and the subsequent health or production impact of T suis and other helminths. Also, clinical trichuriasis in pastured pigs requires an extremely large infectious dose, which is not commonly seen when pastures are sampled, and would likely require a very high number of eggs to be applied to a concentrated area in a short period, with subsequent placement of animals, or constant contamination of the environment with subsequent en masse development of the eggs. Therefore, management or treatment strategies should focus on overall reduction of environmental contamination and not eradication.
In the case reported, T suis eggs were found only within the dirt lots, suggesting a level of environmental protection provided by the shelters. Eggs tend to stay in the upper 0 to 300 mm of soil.47,48 Samples in this case were taken from the top 0 to 25 mm of soil. The low number of eggs found in the soil samples is consistent with the levels seen in previous experimental and personal observations (GH Myers, personal communication, 2009).27,53
It is worth noting that a review of the literature revealed a sampling scheme by Roepstorff and Nansen54 for detection of parasite eggs in pastures. The protocol describes a “two W-route” scheme whereby the entire pasture is sampled along a series of four diagonal lines tracing a “W,” subsampling soil at 15 equally spaced points along the route. This is repeated with an inverted “W” pattern, so that a total of 30 subsamples are taken from the pasture, totaling approximately 200 to 300 grams of soil. Care should be taken to avoid sampling vegetation and feces directly. This method would prove beneficial to anyone exploring epidemiology of cases similar to this one, or in surveying the parasite burden of pastures where swine are being housed or where housing is anticipated.
It is not fully understood how the pigs in this case became infected with T suis. The farm staff commonly walked in the dirt lots with the same footwear that was used in the finishing barns, which could lead to mechanical transmission of T suis eggs. However, the low numbers of T suis eggs in the soil and the high numbers of T suis adults in pigs suggest another aspect of the epidemiology. It would seem that infection of this level would require a higher infectious dose or continuous exposure to a contaminated environment. However, continuous low-level inoculations with T suis result in acquired immunity and reduced worm burden as time progressed, which might explain the negligible impact on this population of pigs.26 It seems more likely that the direct source of exposure to the group of pigs was the barn itself, which had become contaminated over time from either the contaminated dirt lots or from previously exposed pigs. The highly resistant nature of T suis eggs and the lack of any T suis-specific treatment or management protocols would allow constant re-contamination and re-infection of the barns on this site.
Control of trichuriasis on this site must incorporate reducing contamination from the soil lots via a footwear change and washing of equipment exposed to these lots prior to entering the finishing facilities. Historical control has focused on separation of swine from outdoor areas, feral hogs, or from contaminated sources, such as housing and soil.4,8,24,55 Chemical elimination of T suis eggs from the soil is likely not feasible.56
An anthelmintic program using piperazine (as in this case) is not effective against T suis. Piperazine only paralyzes the adult stage of ascarids, does not kill A suum larvae, and does not kill any stages of T suis. The only effective anthelmintics approved for use against T suis in swine in the United States at the time of publication are fenbendazole (Safe-Guard; Intervet Schering-Plough Animal Health, DeSoto, Kansas) and dichlorvos (Atguard; Boehringer Ingelheim Vetmedica, Inc, St Joseph, Missouri). Fenbendazole has efficacy against adults and larval stages L2-L4, while dichlorvos has efficacy against adults and L4 larvae.57-59 Levamisole hydrochloride, ivermectin, and doramectin have variable efficacy against adult T suis; however, neither is labeled for this use in swine in the United States.22,58
Treatment protocols for groups of pigs positive for T suis, A suum, or both, should incorporate an anthelmintic and timing scheme that would address the life cycle and larval stages of both helminths. Therefore, on the basis of current labeling, only products containing fenbendazole or dichlorvos should be used. Animals should be treated before the minimum prepatent period (7 to 8 weeks for both T suis and A suum) is expected post exposure, to minimize re-contamination of the environment with new eggs. If animals are arriving infected (or infection is suspected), then treatment should be administered at the source farm before transport, if possible, or immediately upon arrival. Repeated treatments may be needed periodically if re-infection from the environment is anticipated. Further reviews of anthelmintics and the stages of parasites they are effective against have been published.57-59
Surprisingly, there was no clinical disease or impact on growth performance within the population. The one severely infested pig did show poor growth rate, but lacked other signs of clinical disease. Clinical disease is correlated with severity of infestation or presence of secondary bacterial pathogens,26,35,36,38 neither of which was present in this case.
Trichuris suis has been shown experimentally to act synergistically with C jejuni, B hyodysenteriae, and other enteric pathogens to cause severe colitis and mucohemorragic colitis in swine.60-62 It is hypothesized that this synergy is due to the pathogenesis of T suis first-stage larval destruction of colonic gland enterocytes or the ability of T suis to suppress or manipulate mucosal immunity.30,61,63 In contrast, concurrent infection of T suis with Salmonella serovar Typhimurium did not increase severity of salmonellosis in pigs;64 however, the authors suggest that this was due to a low infectious dose of the helminth. Co-infection of the ascarid and trichurid species are not uncommon,65 likely due to similar epidemiology, geographic distributions, resistance of eggs in environment, life-cycles, and infectious risk factors.
There was no clinical or diagnostic evidence in this case to suggest secondary bacterial enteritis. The single more severely affected animal was located in the poor-performance pen due to its poor growth performance, which is assumed to have been associated with T suis infection. It is not known why this pig alone was so severely affected and had high numbers of adult T suis, when other pigs in the barn had evidence of trichuriasis (positive fecal egg counts) but appeared healthy.
This case describes an incidental finding of Trichuris suis infection on a finishing site, and particularly one severely infested pig. It highlights the point that T suis is still present in modern swine production and serves as an opportunity to review T suis and current management strategies. Even with effective anthelmintics and a properly timed program, egg-contaminated environments, especially those contaminated with T suis and A suum, pose a constant risk of re-infection and potential for environmental recontamination. However, potential risk of severe disease or performance on future groups and a long-term health program must be considered. In severe cases, treatment with an antibiotic may be warranted to control secondary infections (eg, Lawsonia intracellularis, Brachyspira species).
Implications
- Evidence suggests that T suis may survive on a farm for at least 15 years and re-infect naive pigs.
- Infection with T suis or A suum may impact only the growth rate of specific animals within an infected population, which is likely related to the severity of infestation or concurrent disease. The impact of worm burden on growth performance of populations of pigs may be overestimated.
- Treatment and control of swine parasites requires a complete understanding of the life cycle, pathology, and flow of pigs to implement effective management, sanitation, and anthelmintic strategies.
Acknowledgments
The authors would like to thank Drs Kent Schwartz and Darin Madson, Iowa State University Veterinary Diagnostic Laboratory, and Dr H. John Barnes, North Carolina State University College of Veterinary Medicine, for providing assistance with the included histopathology and fecal floatation images. The authors would also like to thank the owner of the farm for his cooperation with this diagnostic investigation.
References
1. Elliot DC, Robinson AJ. Trichuris infection in pigs: a treatment trial in the field. NZ Vet J. 1972;20:98–101.
2. Roepstorff A, Murrell KD. Transmission dynamics of helminth parasites of pigs on continuous pasture: Ascaris suum and Trichuris suis. Int J Parasitol. 1997;27:563–572.
*3. Leiting R. Parasites: an overlooked problem. National Hog Farmer. Dec 15, 2004. Available at: http://nationalhogfarmer.com/mag/farming_parasites_overlooked_problem/. Accessed 28 June 2010.
4. Eijck IAJM, Borgsteede FHM. A survey of gastrointestinal pig parasites on free-range, organic and conventional pig farms in The Netherlands. Vet Res Commun. 2005;29:407–414.
5. Mejer H, Roepstorff A. Oesophagostomum dentatum and Trichuris suis infections in pigs born and raised on contaminated paddocks. Parasitology. 2006;133:295–304.
*6. Maldonado J, Mallol E, Soriano J, Martinez E, Riera P. Mortality in gilts caused by a massive Trichuris suis infestation. Proc 20th IPVS. Durban, South Africa. 2008;1:184.
7. Nejsum P, Thamsborg SM, Petersen HH, Kringel H, Fredholm M, Roepstorff A. Population dynamics of Trichuris suis in trickle-infected pigs. Parasitology. 2009;136:691–697.
8. Yaeger MJ, Karriker LA, Layman L, Halbur PG, Huber GH, Van Hulzen K. Survey of disease pressures in twenty-six niche herds in the midwestern United States. J Swine Health Prod. 2009;17:256–263.
*9. Kennedy TJ, Bruer DJ, Marchiondo AA, Williams JA. Prevalence of swine parasites in major hog producing areas of the United States. Agri-Practice. 1988;9:25–32.
10. Roepstorff A, Nilsson O, Oksanen A, Gjerde B, Richter SH, Örtenberg E, Christensson D, Martinsson KB, Bartlett PC, Nansen P, Eriksen L, Helle O, Nikander S, Larsen K. Intestinal parasites in swine in the Nordic countries: prevalence and geographical distribution. Vet Parasitol. 1998;76:305–319.
11. Mercy AR, de Chaneet G, Emms Y. Survey of intestinal parasites in Western Australian pig herds. Aust Vet J. 1989;66:4–6.
12. O’Callaghan MG, Langston PG. Internal parasites from pigs in South Australia. Aust Vet J. 1990;67:416.
13. Weng YB, Hu YJ, Li Y, Li BS, Lin RQ, Xie DH, Gasser RB, Zhu XQ. Survey of intestinal parasites in pigs from intensive farms in Guangdong Province, People’s Republic of China. Vet Parasitol. 2005;127:333–336.
14. Nganga CJ, Karanja DN, Mutune MN. The prevalence of gastrointestinal helminth infections in pigs in Kenya. Trop Anim Health Prod. 2008;40:331–334.
*15. Beer RJS. Experimental infection of man with pig whipworm [letter]. Br Med J. 1971;3:44.
16. Beer RJ. The relationship between Trichuris trichiura (Linnaeus 1758) of man and Trichuris suis (Schrank 1788) of the pig. Res Vet Sci. 1976;20:47–54.
17. Kradin RL, Badizadegan K, Auluck P, Korzenik J, Lauwers GY. Iatrogenic Trichuris suis infection in a patient with Crohn disease. Arch Pathol Lab Med. 2006;130:718–720.
18. Kringel H, Roepstorff A. Trichuris suis population dynamics following a primary experimental infection. Vet Parasitol. 2006;139:132–139.
19. Beer RJS. Morphological descriptions of the egg and larval stages of Trichuris suis Schrank, 1788. Parasitology. 1973;67:263–278.
20. Beer RJS. Studies on the biology of the life-cycle of Trichuris suis Schrank, 1788. Parasitology. 1973:67:253–262.
21. Murrell KD. Epidemiology, pathogenesis, and control of major swine helminth parasites. Vet Clin N Am Food Anim Pract. 1986;2:439–453.
22. Stewart TB, Hoyt PG. Internal parasites. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, eds. Diseases of Swine. 9th ed. Ames, Iowa; Blackwell Publishing; 2006:901–914.
23. Taylor MA, Coop RL, Wall RL. Parasites of pigs. In: Taylor MA, Coop RL, Wall RL. Veterinary Parasitology. 3rd ed. Ames, Iowa: Blackwell Publishing; 2007:316–355.
24. Bowman DD. Helminths. In: Bowman DD, ed. Georgis’ Parasitology for Veterinarians. 9th ed. St. Louis, Missouri: Saunders Elsevier; 2009:115–239.
25. Powers KG, Todd AC, McNutt SH. Experimental infections of swine with Trichuris suis. Am J Vet Res. 1960;21:262–268.
26. Pedersen S, Saeed I. Acquired immunity to Trichuris suis infection in pigs. Parasitology. 2001;123:95–101.
*27. Gauthier J, Thacker B, Murphy A, Nonaka N, Donoghue A. Experimental infection of pigs with Trichuris suis using contaminated soil. Proc 12th IPVS. The Hague, The Netherlands. 1992;365.
28. Pedersen S, Saeed I. Experimental infection of pigs with three dose levels of Trichuris suis. Parasite. 2000;7:275–281.
29. Beer RJS, Lean IJ. Clinical trichuriasis produced experimentally in growing pigs. Part I: Pathology of infection. Vet Rec. 1973;93:189–195.
30. Parthasarathy G, Mansfield LS. Trichuris suis excretory secretory products (ESP) elicit interleukin-6 (IL-6) and IL-10 secretion from intestinal epithelial cells (IPEC-1). Vet Parasitol. 2005;131:317–324.
31. Abner SR, Hill DE, Turner JR, Black ED, Bartlett P, Urban JF, Mansfield LS. Response of intestinal epithelial cells to Trichuris suis excretory-secretory products and the influence on Campylobacter jejuni invasion under in vitro conditions. J Parasitol. 2002;88:738–745.
32. Kringel H, Roepstorff A. Trichuris suis excretory/secretory antigen-specific antibodies in serum from single-inoculated pigs. Parasite Immunol. 2007;29:327–330.
33. Hill DE, Romanowski RD, Urban JF. A Trichuris specific diagnostic antigen from culture fluids of Trichuris suis adult worms. Vet Parasitol. 1997;68:91–102.
34. Batte EG, McLamb RD, Muse KE, Tally SD, Vestal TJ. Pathophysiology of swine trichuriasis. Am J Vet Res. 1977;38:1075–1079.
35. Schoneweis DA, Rapp WR. Trichuris suis infection in young pigs. Vet Med Sm Anim Clin. 1970;65:63–66.
36. Batte EG, Moncol DJ. Whipworms and dysentery in feeder pigs. JAVMA. 1972;161:1226–1228.
37. Hass DK, Collins JA. Swine whipworm: a clinical case. Vet Med Sm Anim Clin. 1973;68:1371–1375.
38. Thienpont D, Vanparijs O, Hermans L. Treatment of Trichuris suis infections in pigs with flubendazole. Vet Rec. 1982;110:517–520.
39. Hale OM, Stewart TB. Influence of an experimental infection of Trichuris suis on performance of pigs. J Anim Sci. 1979;49:1000–1005.
40. Pedersen S, Saeed I, Friis H, Michaelsen KF. Effect of iron deficiency on Trichuris suis and Ascaris suum infections in pigs. Parasitology. 2001;122:589–598.
41. Pedersen S, Saeed I, Michaelsen KF, Friis H, Murrell KD. Impact of protein energy malnutrition on Trichuris suis infection in pigs concomitantly infected with Ascaris suum. Parasitology. 2002;124:561–568.
42. Thomsen LE, Petkevicius S, Bach Knudsen KE, Roepstorff A. The influence of dietary carbohydrates on experimental infection with Trichuris suis in pigs. Parasitology. 2005;131:857–865.
43. Krag L, Thomsen LE, Iburg T. Pathology of Trichuris suis infection in pigs fed an inulin- and non-inulin-containing diet. J Vet Med A Physiol Pathol Clin Med. 2006;53:405–409.
*44. Shepherd G, Pittman JS, Thacker BJ, Myers GH, Francisco CJ. Prevalence of internal parasites in an integrated production system. Proc AASV. Omaha, Nebraska. 2010;341–342.
45. Pittman JS, Shepherd G, Thacker BJ, Myers GH. Modified technique for collecting and processing fecal material for diagnosing intestinal parasites in swine. J Swine Health Prod. 2010;18:249-252.
46. Spickler AR. Trichuriasis. Technical Factsheet. 2005. Center for Food Security and Public Health, Iowa State University, Ames, Iowa. Available at: http://www.cfsph.iastate.edu/DiseaseInfo/factsheets.php. Accessed 28 June 2010.
47. Burden DJ, Hammet NC, Brookes PA. Field observations on the longevity of Trichuris suis ova. Vet Rec. 1987;121:43.
48. Burden DJ, Hammet NC. The development and survival of Trichuris suis ova on pasture plots in the south of England. Res Vet Sci. 1979;26:66–70.
49. Larsen MN, Roepstorff A. Seasonal variation in development and survival of Ascaris suum and Trichuris suis eggs on pastures. Parasitology. 1999;119:209–220.
50. Black MI, Scarpino PV, O’Donnell CJ, Meyer KB, Jones JV, Kaneshiro ES. Survival rates of parasite eggs in sludge during aerobic and anaerobic digestion. Appl Envir Micobiol. 1982;44:1138–1143.
51. Burden DJ, Ginnivan MJ. The destruction of pig helminth ova and larvae in a slurry treatment process. Vet Rec. 1978;103:373–375.
52. Pedersen S, Saeed I. Host age influence on the intensity of experimental Trichuris suis infection in pigs. Parasite. 2002;9:75–79.
53. Thomsen LE, Mejer H, Wendt S, Roepstorff A, Hindsbo O. The influence of stocking rate on transmission of helminth parasites in pigs on permanent pasture during two consecutive summers. Vet Parasitol. 2001;99:129–146.
54. Roepstorff A, Nansen P. Epidemiology, diagnosis and control of helminth parasites of swine. FAO Animal Health Manual. 1998;3:111–129.
55. Gipson PS, Veatch JK, Matlack RS, Jones DP. Health status of a recently discovered population of feral swine in Kansas. J Wildl Dis. 1999;35:624–627.
56. Burden DJ, Whitehead A, Green EA, McFadzean JA, Beer RJS. The treatment of soil infested with the human whipworm Trichuris trichiura. J Hygiene. 1976;77:377–382.
*57. Guide to strategic parasite control for swine using Safe-Guard (fenbendazole). SG-SW-Swine Monograph. Intervet Schering-Plough Animal Health, Desoto, Kansas. 2007.
58. Lynn RC. Antiparasitic drugs. In: Bowman DD, ed. Georgis’ Parasitology for Veterinarians. 9th ed. St Louis, Missouri: Saunders Elsevier; 2009:254–294.
59. Jacela JY, DeRouchey JM, Tokach MD, Goodband RD, Nelssen JL, Renter DG, Dritz SS. Feed additives for swine: Fact sheets – carcass modifiers, carbohydrate-degrading enzymes and proteases, and anthelmintics. J Swine Health Prod. 2009;17:325–332.
60. Beer RJ, Rutter JM. Spirochaetal invasion of the colonic mucosa in a syndrome resembling swine dysentery following experimental Trichuris suis infection in weaned pigs. Res Vet Sci. 1972;13:593–595.
61. Rutter JM, Beer RJS. Synergism between Trichuris suis and the microbial flora of the large intestine causing dysentery in pigs. Infect Immun. 1975;11:395–404.
62. Mansfield LS, Urban JF Jr. The pathogenesis of necrotic proliferative colitis in swine is linked to whipworm induced suppression of mucosal immunity to resident bacteria. Vet Immunol Immunopath. 1996;50:1–17.
63. Mansfield LS, Gauthier DT, Abner SR, Jones KM, Wilder SR, Urban JF. Enhancement of disease and pathology by synergy of Trichuris suis and Campylobacter jejuni in the colon of immunologically naïve swine. Am J Trop Med Hyg. 2003;68:70–80.
64. Steenhard NR, Roepstorff A, Baggesen DL, Boes J, Jensen TK, Aasted B, Ornbjerg N. Studies on the interaction between Salmonella enterica ser. Typhimurium and intestinal helminths in pigs. Vet Parisitol. 2006;139:158–167.
65. Gilles HM. Soil-transmitted helminths. In: Cook GC, Zumla A, eds. Manson’s Tropical Diseases. 21st ed. London, England: WB Saunders; 2003:1527–1560.
* Non-refereed references.