| Original research | Peer reviewed |
Cite as: Jenvey CJ, Van Wettere WHEJ, Reichel MP, et al. Investigation of the impact of oral rennet supplementation on serum globulin concentration in neonatal piglets. J Swine Health Prod. 2014;22(6):282–286..
Also available as a PDF.
SummaryObjective: The objective of this study was to determine whether oral supplementation of piglets with rennet increases immunoglobulin absorption and thereby piglet serum globulin concentrations. Serum immunoglobulin concentrations in piglets derived from induced farrowing and non-induced farrowing multiparous (MP) and primiparous (PP) sows were compared. Materials and methods: A total of 20 MP and 20 PP sows were used in this trial, with half of the MP and PP sows induced to farrow using prostaglandin F2α by injection. Each piglet from induced and non-induced MP and PP sows was conveniently assigned to one of three treatment groups: no supplementation, oral supplementation with rennet, or oral supplementation with saline. Rennet and saline treatments were administered to piglets twice during their first 12 hours of life. A blood sample was collected from each piglet 48 to 72 hours post farrowing. Results: Serum globulin concentrations did not differ with rennet supplementation in piglets derived from either induced or non-induced PP or MP sows. Implication: Within the power of this study, oral rennet supplementation does not increase piglet serum globulin concentrations. | ResumenObjetivo: El objetivo de este estudio fue determinar si el suplemento oral de lechones con cuajo incrementa la absorción de inmunoglobulina y por consiguiente las concentraciones de globulina sérica del lechón. Se compararon las concentraciones de inmunoglobulina sérica en lechones nacidos de partos inducidos y partos no inducidos de hembras multíparas (MP por sus siglas en inglés) y primíparas (PP por sus siglas en inglés). Materiales y métodos: Se utilizaron un total de 20 hembras MP y 20 hembras PP en este estudio, con la mitad de las hembras MP y PP inducidas al parto utilizando prostaglandina F2α inyectada. Cada lechón de hembra MP y PP inducida o no inducida fue apropiadamente asignado a uno de tres grupos: sin suplemento, suplemento oral con cuajo, o suplemento oral con solución salina. Se administraron tratamientos con cuajo y solución salina a lechones dos veces durante sus primeras 12 horas de vida. Se recolectó una muestra de sangre de cada lechón 48 a 72 horas post parto. Resultados: Las concentraciones de globulina sérica no difirieron con el suplemento de cuajo en lechones provenientes de hembras PP o MP inducidas o no inducidas. Implicación: Dentro del poder de este estudio, el suplemento oral de cuajo no incrementa las concentraciones de globulina sérica del lechón. | ResuméObjectif: La présente étude visait à déterminer chez des porcelets si une supplémentation orale en présure augmentait l’absorption des immunoglobulines et par conséquent les concentrations de globulines sériques. Les concentrations d’immunoglobulines sériques chez des porcelets obtenus par mises-bas induites et non-induites chez des truies multipares (MP) et primipares (PP) ont été comparées. Matériels et méthodes: Un total de 20 truies MP et 20 truies PP ont fait partie de l’étude, la mise-bas chez la moitié des truies de chacun de ces deux groupes étant induite par injection de prostaglandine F2α. Chaque porcelet provenant des truies MP et PP induites et non-induites était assigné à un des trois groupes de traitement: aucun supplément, supplément oral de présure, ou supplément oral de saline. La présure et la saline furent administrées aux porcelets deux fois durant les 12 premières heures de vie. Un échantillon sanguin fut prélevé de chaque porcelet 48 à 72 heures suivant la mise-bas. Résultats: Les concentrations de globulines sériques ne différaient pas suivant une supplémentation en présure chez des porcelets provenant de truies MP ou PP et que la mise-bas soit provoquée ou non. Implication: En tenant compte des limites de la présente étude, on peut conclure qu’une supplémentation orale en présure n’augmente pas les concentrations des globulines sériques. |
Keywords: swine, piglets, rennet, serum globulin
Search the AASV web site
for pages with similar keywords.
Received: September 20, 2013
Accepted: March 25, 2014
Piglets are born hypogammaglobulinemic and therefore require ingestion of maternal immunoglobulins (Igs) via colostrum immediately after birth to provide protection against infections. Immunoglobulins are absorbed in the small intestine and pass directly into the bloodstream. However, this process is time dependent, with gut closure in piglets occurring 24 to 36 hours after birth.1
Rennet is composed of a group of enzymes that occur in the stomach of the newborn mammal. The active enzyme, chymosin (also referred to as rennin), assists in release of Igs from the colostrum and milk ingested by coagulating the casein, resulting in formation of a solid curd and immunoglobulin-rich (Ig-rich) whey. Curd formation is thought to be important for maintaining the health of newborn dairy calves.2 The absorption of Igs from colostrum by the neonate can be indirectly measured by an assay of serum gamma-glutamyl transferase (GGT),3,4 an enzyme produced by the ductile cells in the mammary gland. In a study by Gregory,5 feeding calves colostrum that had previously been incubated with rennet resulted in 20% fewer calves with suboptimal GGT activity (< 500 U per L). Additionally, the proportion of calves that still had suboptimal GGT activity was also 60% lower.5 To the authors’ knowledge, supplementation of piglets with rennet to improve piglet Ig absorption has not been investigated. The objective of this study was to determine whether oral supplementation of piglets with rennet increases Ig absorption, thereby improving piglet serum globulin concentrations. Serum globulin concentrations were compared in piglets derived from induced farrowing and non-induced farrowing multiparous (MP) and primiparous (PP) sows.
Materials and methods
All experimental procedures were conducted at the University of Adelaide’s Roseworthy Piggery, South Australia, with approval of the University of Adelaide Animal Ethics Committee.
Sow selection
The study was performed on an intensive commercial pig farm in South Australia from May to November 2012. Twenty MP and 20 PP sows from this herd were sampled (Figure 1). Half of each group (10 MP and 10 PP sows; the first 10 sows on the farrowing list in each category) were selected for induction of parturition. Induction was performed the day before the estimated due date, which was based upon an assumed gestation length of 115 days. Each sow was injected with 1 mL of prostaglandin F2α (Lutalyse; Pfizer, West Ryde, New South Wales), administered into the stroma of the vulval lips on two occasions. The first injection was given in the morning and the second 6 hours later in the afternoon.
Figure 1: Experimental design of a study to determine whether oral supplementation of piglets with rennet increases immunoglobulin absorption and thereby piglet serum globulin concentrations. Among 40 sows in the breeding herd (20 Multiparous, MP; 20 Primiparous, PP), the first 10 sows on the farrowing list in each category (10 MP and 10 PP sows) were selected for induction of parturition. Half of the sows in each group were induced to farrow by injection of prostaglandin into the vulvar lips. A maximum of nine piglets per sow were each assigned to one of three treatments: None (control), Saline (control), or Rennet (treatment). Administration of the saline or rennet was performed twice within the first 12 hours of life.
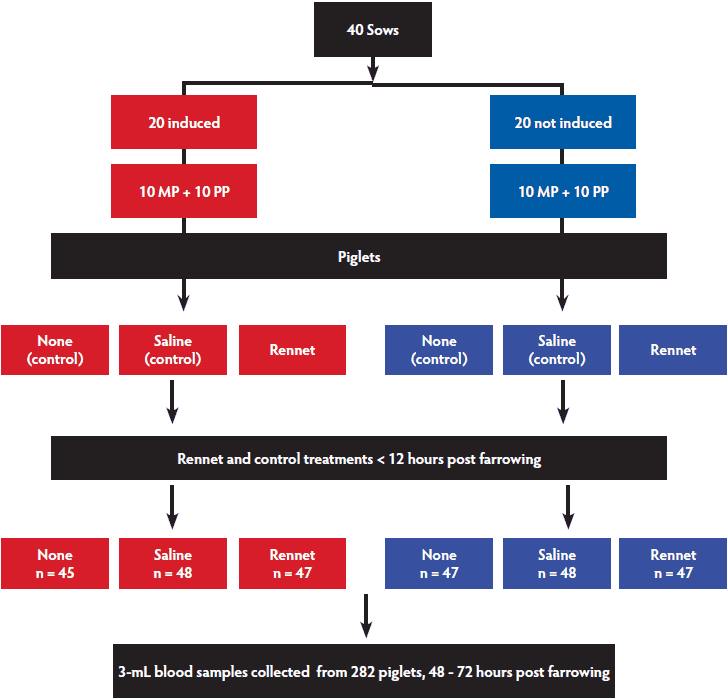
Piglet allocation to treatments
Either three or six or nine piglets from each litter were included in the trial. This number was maximised according to the size of the litter. For example, if there were five piglets in the litter, three piglets were conveniently selected and assigned to the trial. The remaining two piglets were excluded from the trial but remained with the litter. Sows were housed in individual crates and cross-fostering was allowed.
The piglets to be included in the trial within each litter were weighed within 6 hours of birth and were ranked according to their birth weights. The ranked list of piglets was sequentially divided into groups of three, starting with the heaviest piglet. Each piglet within each weight-rank group was conveniently assigned to either the rennet treatment group (Rennet) or one of two control groups. The control groups consisted of no oral supplementation (None) and oral 0.9% saline supplementation (Saline). Saline supplementation was included as a control treatment as it was used as the carrier for the vegetable rennet administered to the Rennet group. Piglets assigned to the Saline or Rennet groups were fed 4 mL of their designated oral supplement via stomach tube twice during the first 12 hours post parturition. The rennet supplement consisted of vegetable rennet (Cheeselinks, Little River, Victoria, Australia) diluted 1:100 with saline to achieve a final concentration of 2.02 international milk-clotting units per mL.
Piglet blood-sample collection, weaning, and serum testing
A blood sample was collected from each piglet by venipuncture of the anterior vena cava 48 to 72 hours post parturition. All piglets were weighed at weaning (21 ± 0.14 days; mean ± standard error) and all deaths between birth and weaning were recorded. Blood samples were centrifuged and the serum tested using mass spectrometry (Beckman Coulter AU480, Lane Cove, New South Wales) for total protein and albumin concentrations to determine globulin concentration (globulin concentration = total protein – albumin).
Statistical analysis
Descriptive statistics to determine normal distribution were performed. Mean, standard error, and 95% confidence intervals (CI) were calculated for parity, induction, and treatment groups (Rennet, Saline, or None). All 18 variables (Box 1) measured in this study were assessed for significance using multivariable linear regression analysis with an automated backwards stepwise elimination of nonsignificant factors (P ≥ .05). The interactions between the treatment groups and five other variables were also assessed (Box 1). Model diagnostics were performed to assess the assumptions of normality, linearity and homoscedasticity. The statistical package “R” was used for all analyses (R version 3.0.2; www.r-project.org/).
Box 1: Variables assessed for significance in relation to piglet serum globulin concentrations in a multivariable linear regression model*
| Gender (Male or Female) |
|---|
| Treatment group (None, Saline, Rennet) |
| Time of day when born (am or pm) |
| Birth date |
| Birth weight |
| Weaning date |
| Weaning weight |
| Weaning age |
| Growth rate |
| Fostered (Yes or No) |
| Primiparous/Multiparous |
| Induce (Yes or No) |
| Litter size |
| Interactions: |
| Treatment + Induce |
| Treatment + Parity |
| Treatment + Birth weight |
| Treatment + Litter size |
| Treatment + Fostered |
* Study described in Figure 1.
Results
A total of 312 piglets were recruited for the study. Forty-five piglets were cross-fostered. Twenty-one piglets died during the study (6.7% mortality) and data were incomplete for nine piglets: these 30 animals were removed from the statistical analysis.
Mean piglet serum globulin concentrations did not differ (P > .05) among the None (n = 92), Saline (n = 96), and Rennet (n = 94) groups (38.31 ± 0.92, 39.07 ± 0.92, and 39.20 ± 0.78 g per L, respectively).
Mean serum globulin concentrations of piglets derived from non-induced dams did not differ (P > .05) among the None (n = 47), Saline (n = 48), and Rennet (n = 47) groups (39.86 ± 1.42, 39.94 ± 1.15, and 38.43 ± 1.39 g per L, respectively). Mean serum globulin concentrations of piglets derived from dams induced to farrow did not differ (P > .05) among the None (n = 45), Saline (n = 48), and Rennet (n = 47) treatment groups (36.78 ± 1.14, 38.44 ± 1.04, and 39.75 ± 1.30 g L, respectively).
Mean serum globulin concentrations of piglets derived from PP sows did not differ (P > .05) among the None (n = 46), Saline (n = 48), and Rennet (n = 45) groups (34.74 ± 1.12, 36.06 ± 1.11, and 36.14 ± 1.40 g per L, respectively). Mean serum globulin concentrations of piglets derived from MP sows did not differ (P > 0.5) among the None (n = 46), Saline (n = 48), and Rennet (n = 49) groups (42.04 ± 1.27, 42.27 ± 0.90, and 41.94 ± 1.17 g per L, respectively).
Mean serum globulin concentrations of piglets derived from induced PP sows did not differ (P > 0.5) among the None (n = 24), Saline (n = 28), and Rennet (n = 25) groups (34.52 ± 1.48, 36.11 ± 1.21, and 38.12 ± 1.94 g per L, respectively). Mean serum globulin concentration of piglets derived from induced MP sows did not differ (P > 0.5) among the None (n = 21), Saline (n = 20), and Rennet (n = 22) groups (39.55 ± 1.62, 41.41 ± 1.60, and 41.68 ± 1.64 g per L, respectively). Mean serum globulin concentrations of piglets derived from non-induced PP sows did not differ (P > .05) among the None (n = 22), Saline (n = 20), and Rennet (n = 20) groups (35.00 ± 1.73, 36.00 ± 2.04, and 33.91 ± 1.98 g per L, respectively). Mean serum globulin concentration of piglets derived from non-induced MP sows did not differ (P > 0.5) among the None (n = 25), Saline (n = 28), and Rennet (n = 27) groups (44.15 ± 1.84, 42.93 ± 1.04 and 42.13 ± 1.67 g per L, respectively).
Multivariable linear regression analysis
The model met the assumptions for normality, linearity, and homoscedasticity. A total of 18 variables were assessed for significance in relation to piglet serum globulin concentrations using a multivariable linear regression model. Of the assessed variables, four were deemed to be significant by regression analysis (Table 1). These variables were time of day when born (P < .001), birth weight (P < .01), weaning weight (P < .001), and parity (P < .001). The multiple R2 and P value for the model were 0.20 and P < .001, respectively. There were no significant differences in serum globulin concentrations among the treatment groups (P = .07).
Table 1: Final model evaluating factors influencing piglet serum globulin concentrations*
| Variable | Estimate | Standard error | P |
|---|---|---|---|
| Intercept | 3.47 | 2.81 | < .001 |
| Time of day when born (pm)† | 3.17 | 1.01 | .002 |
| Birth weight (kg) | 4.79 | 1.88 | .01 |
| Weaning weight (kg) | -0.72 | 0.25 | .005 |
| Parity (MP)† | 6.15 | 1.03 | < .001 |
* Study described in Figure 1. Multivariable linear regression model, variables as follows: time of day when born (pm = born between 12 pm and 6 pm); birth weight (kg); weaning weight (kg) (mean age 21 ± 0.14 days); parity of sow (MP = multiparous). Multiple R2 = 0.20, adjusted R2 = 0.18.
† Value in parentheses is the significant variable of two variable options.
Discussion
Oral supplementation of piglets with rennet during their first 12 hours of life did not increase piglet serum globulin concentrations. In contrast with the current study, Gregory5 added calf rennet directly to colostrum before bottle feeding each calf. It is possible that combining rennet with the colostrum prior to feeding may have promoted better rennet activity than administering rennet directly into the stomach in the current study. Gregory5 also measured GGT activity rather than serum globulin. However, the positive association between GGT activity and IgG has previously been documented.4,6
Both piglets and calves are born hypogammaglobulinemic and therefore rely upon ingestion and absorption of maternal antibodies derived from colostrum to provide protection against infections. In both species, macromolecules (such as Igs) are readily absorbed in the small intestine during the neonatal period. Transmission of macromolecules across the gut epithelium declines post partum and ends within 24 to 36 hours of birth in both calves and piglets.1 This process, termed gut closure, is accelerated by ingestion of colostrum.1
It is possible that the physiological differences in digestive processes between calves and piglets may explain why the hypothesis was rejected in this study. Following ingestion of colostrum, chymosin, a proteolytic enzyme present in the stomach of newborn calves, causes a curd to form within 10 minutes of the colostrum reaching the abomasum.7 A study by Pang and Ernstrom8 demonstrated milk-clotting activity in bovine fetal cells removed from calves at 6 months of development. Curd formation slows down the digestive processes and allows the whey produced to be passed into the small intestine where absorption of immunoglobulins occurs.2 A study by Foltmann et al9 demonstrated that chymosin is present in the stomachs of newborn piglets. The study also found that the relative milk-clotting activity of the extract taken from newborn piglet stomachs was greater than the activity demonstrated by calf chymosin.9 However, curd formation may be reduced if the pH of the stomach is elevated.10 The normal abomasal pH of calves was 1.6 ± 0.2111 two hours before their first feed and 2.77 ± 0.08 twenty-four hours after birth.7 Abomasal pH increases with continued feeding, but decreases to normal ranges within 711 to 127 hours. This increase in abomasal pH following feeding is thought to be due to two factors: the ability of the calf to produce hydrochloric acid in the abomasum, which is stimulated by feeding; and production of low-pH whey during curd formation. Acid secretion has also been demonstrated in piglets. Lecce and Morgan1 showed that 1-day-old piglets had the ability to acidify their stomachs to a pH of 2, while Cranwell and Titchen,12 using a fundic pouch, were able to demonstrate acid secretion as early as 17 hours after birth and observed milk clots in the stomachs of the piglets in the study during the pouch operation. Stomach pH of piglets in these studies ranged from 2.1 to 3.91 and 1.25 to 1.90.12 Although chymosin concentration and pH were not measured in the current study, it is apparent from previous research that newborn piglets have the necessary physiological processes to allow for adequate curd formation after colostrum ingestion, not unlike the processes found in the calf. It is possible that the concentration of chymosin present within the newborn piglet stomach is already sufficient, thus supplementation with rennet will not promote further curd formation and whey production.
Within the power of this study, there was no observed relationship between treatment group and piglet serum globulin concentration. However, our results did show a positive relationship between piglet serum globulin concentrations and parity, time of day when born, and birth weight. Our study also found a negative relationship between piglet serum globulin concentrations and weaning weight. A study by Hendrix et al13 aimed to determine the effect certain dam factors had on the serum gamma globulin concentrations of piglets. They found a positive correlation between piglet serum gamma globulin concentration and parity, and with birth weight.13 However, studies by Carney-Hinkle et al14 and Nguyen et al15 could not find a relationship between piglet serum globulin concentration and parity.
Implications
• Within the power of this study, oral rennet supplementation of piglets does not increase serum globulin concentration beyond that in piglets not supplemented.
• Sow parity, time of day when born, and birth weight are positively related to piglet serum globulin concentration.
• Weaning weight is negatively related to piglet serum globulin concentration.
Acknowledgements
The funding for this experiment was provided by the CRC for High Integrity Australian Pork and is gratefully acknowledged. The authors would like to thank staff at Roseworthy Piggery for their technical assistance.
Conflict of interest
None reported.
References
1. Lecce J, Morgan D. Effect of dietary regimen on cessation of intestinal absorption of large molecules (closure) in the neonatal pig and lamb. J Nutr. 1962;78:263–268.
2. Longenbach J, Heinrichs A. A review of the importance and physiological role of curd formation in the abomasum of young calves. Anim Feed Sci Technol. 1998;73:85–97.
3. Parish SM, Tyler JW, Besser TE, Gay CC, Krytenberg D. Prediction of serum IgG1 concentration in Holstein calves using serum gamma glutamyltransferase activity. J Vet Intern Med. 1997;11:344–347.
4. Leilani K, Wilson LK, Tyler JW, Besser TE, Parish SM, Gant R. Prediction of serum IgG1 concentration in beef calves based on age and serum gamma glutamyltransferase activity. J Vet Intern Med. 1999;13:123–125.
5. Gregory NG. Effect of enhancing curd formation during the first colostrum feed on absorption of γ glutamy1 transferase by newborn calves. Aust Vet J. 2003;81:549–552.
6. Perino L, Sutherland R, Woollen N. Serum gamma-glutamyltransferase activity and protein concentration at birth and after suckling in calves with adequate and inadequate passive transfer of immunoglobulin G. Am J Vet Res. 1993;54:56–59.
7. Constable P, Ahmed A, Misk N. Effect of suckling cow’s milk or milk replacer on abomasal luminal pH in dairy calves. J Vet Intern Med. 2005;19:97–102.
8. Pang SH, Ernstrom CA. Milk clotting activity in bovine fetal abomasa. J Dairy Sci. 1986;69: 3005–3007.
9. Foltmann B, LØnblad P, Axelsen NH. Demonstration of chymosin (EC 3.4.23.4) in the stomach of newborn pig. Biochem J. 1978;169:425–427.
10. Rand AG, Ernstrom CA. Effect of pH and sodium chloride on activation of prorennin. J Dairy Sci. 1964;47:1181–1187.
*11. Birgele E, Ilgaza A, Keidane D, Mugurevics A. The functional state of the stomach in calves in the first month of postnatal life. Proc ISAH Cong Anim Hygiene. Warsaw, Poland. 2005; 219–224.
12. Cranwell P, Titchen D. Gastric acid secretion in newly born piglets. Res Vet Sci. 1974;16:105–107.
13. Hendrix W, Kelley K, Gaskins C, Hinrichs D. Porcine neonatal survival and serum gamma globulins. J Anim Sci. 1978;47:1281–1286.
14. Carney-Hinkle EE, Tran H, Bundy JW, Moreno R, Miller PS, Burkey TE. Effect of dam parity on litter performance, transfer of passive immunity, and progeny microbial ecology. J Anim Sci. 2013;91:2885–2893.
15. Nguyen K, Cassar G, Friendship RM, Dewey C, Farzan A, Kirkwood RN, Hodgins D. An investigation of the impacts of induced parturition, birth weight, birth order, litter size, and sow parity on piglet serum concentrations of immunoglobulin G. J Swine Health Prod. 2013;21:139–143.
* Non-refereed reference.