| Brief communication | Peer reviewed |
Cite as: Han SJ, Oh Y, Lee CY, et al. Efficacy of dietary supplementation of bacteriophages in treatment of concurrent infections with enterotoxigenic Escherichia coli K88 and K99 in postweaning pigs. J Swine Health Prod. 2016;24(5):259–263.
Also available as a PDF.
SummaryPostweaning pigs challenged with enterotoxigenic Escherichia coli (ETEC) K88 and K99 and fed a diet supplemented with ETEC K88- and K99-specific bacteriophages exhibited greater weight gain, lower fecal consistency score, and less fecal shedding and intestinal adhesion of ETEC K88 than did pigs fed the unsupplemented diet. | ResumenLos cerdos post-destete probados con Escherichia coli enterotoxigénica (ETEC) K88 and K99 y alimentados con una dieta suplementada con bacteriófagos específicos de ETEC K88 y K99 mostraron mayor ganancia de peso, puntaje menor de consistencia fecal, y menos eliminación fecal y adhesión intestinal del ETEC K88 que los cerdos alimentados con una dieta no suplementada. | ResuméDes porcs en période post-sevrage ont été infectés avec des souches entérotoxinogènes d’Escherichia coli (ETEC) K88 et K99 et nourris avec une diète supplémentée avec des bactériophages spécifiques contre des ETEC K88 et K99. Ceux-ci ont montré un gain de poids supérieur, un score plus faible de la consistance fécale, et moins d’excrétion fécale et d’adhésion intestinale des ETEC K88 que les porcs nourris avec une nourriture non supplémentée. |
Keywords: swine, bacteriophage, postweaning diet, enterotoxigenic Escherichia coli, feces, E. coli
Search the AASV web site
for pages with similar keywords.
Received: September 15, 2015
Accepted: February 2, 2016
Postweaning diarrhea or colibacillosis is a costly disease causing substantial mortality, as well as growth retardation, in swine production.1-3 Colibacillosis is typically associated with avid intestinal adhesion and fecal shedding of enterotoxigenic Escherichia coli (ETEC). The ETEC causing diarrhea in postweaning pigs carries the F4 (K88) or F18 fimbrial antigen in most cases.4-6 The F5 (K99) antigen has also been found in postweaning diarrhea in Central China7 as well as in South Korea (Jeong-Hee Han, unpublished data, 2014), although piglets are less susceptible to ETEC K99 than to K88 with increasing age.8
Common therapies used for prevention and treatment of colibacillosis are antibiotics9,10 and pharmacological concentrations of zinc oxide (ZnO) ranging from 2000 to 4000 mg per kg diet in many countries,2-4,9-11 including the United States.11 Use of antibiotics as feed additives has been banned in the European Union since 2006, and subsequently in Korea since mid-2011,12 because of increasing concerns about the emergence of antibiotic-resistant pathogens.13 Moreover, the concentration of ZnO in feed is now limited to 150 mg per kg by regulation in the European Union,14 which may lead non-European countries to adopt a similar regulation. It is thus necessary to find alternatives to in-feed antibiotics as well “pharmacological” ZnO.
Bacteriophages or phages have recently received re-emerging attention as alternatives to antibiotics because of several merits as feed additives, including their high stability within the feed and digestive tract as well as their high specificity of transfection.15-17 However, only limited information is available as to the effects of phage therapy in the pig, although dietary phages have been shown to be effective for alleviating the severity of diarrhea in postweaning pigs challenged with a hemolytic K88-positive ETEC strain as well as in unchallenged piglets.18 Thus, more studies are needed before dietary phages can be established as prophylactic or therapeutic agents against porcine colibacillosis. The present study was therefore initiated to evaluate the efficacy of dietary phages on treatment of colibacillosis induced by a concurrent oral challenge with ETEC K88 and K99 in postweaning pigs.
Materials and methods
The experimental protocol for the present study was approved by the Institutional Animal Care and Use Committee of Kangwon National University.
The phages used in the present study were prepared by iNtRON Biotechnology, Inc (Sungnam, Korea), as follows. Briefly, the ETEC K88-specific and K99-specific phages were isolated on agar plates of K88 and K99 bacterial cultures, respectively, from the feces of 30- to 70-day-old grower pigs on a commercial swine farm The isolated phages, which were identified as Myoviridae and Siphoviridae families, respectively, were plaque-purified, diluted in a 0.2 M Tris buffer (pH 7.5) containing 0.1 M NaCl, 1 mM MgSO4, and 0.01% gelatin, and freeze-dried. The ETEC K88-specific and K99-specific phages added to a common pig diet are known to retain their titers for 12 months (Dr Sang-Hyeon Kang, iNtRON Biotechnology, Inc; oral communication, December, 2015).
Thirty candidate piglets, which had been born to Duroc-sired Landrace × Yorkshire dams on a commercial farm, were castrated on day 2 after birth. Pigs were injected intramuscularly with 4 mg ceftiofur sodium per kg body weight once a day for 3 consecutive days during the suckling period, beginning on day -7 of the experiment, to attempt to remove commensal ETEC if present. Of the 30 candidates, 18 piglets that did not excrete either ETEC K88 or K99, as determined by real-time polymerase chain reaction (PCR) on genomic DNA extracted from feces,9 were selected at weaning (28 days of age). The animals were transported to a university animal experimental station and allotted arbitrarily to three pens (groups) of six animals each on the day of selection and transportation, corresponding to day 0 of the experiment. Two groups of animals were challenged orally with 3.0 × 108 colony-forming units (cfus) of each of ETEC K88 and K99 in a total volume of 6 mL of phosphate buffered saline (pH 7.4). Animals in the remaining group were administered the same volume of vehicle. The ETEC K88 used for the challenge was identified as F4 fimbriae-positive, heat-labile, enterotoxin-positive, hemolysin serogroup O8; ETEC K99 was identified as F5 fimbriae-positive, heat-stable, enterotoxin-positive, hemolysin serogroup O8. The unchallenged group and one challenged group were provided with a basal nursery diet (Control and Chal-Basal groups, respectively) that had been used in previous studies.9,19,20 The remaining challenged group received the same diet supplemented with 1.0 × 109 plaque-forming units of each of ETEC K88- and K99-specific phages per kg (Chal-Phage group). The animals were on the feeding trial for 7 days, beginning on day 0.
Fecal consistency was scored daily beginning on day 1 according to a four-ladder whole-number scale5 as described previously: 0 = normal feces; 1 = soft feces; 2 = mild diarrhea; 3 = severe diarrhea.19 Rectal temperature was measured on days 0, 3, and 7 using an electronic thermometer (ThermoScan; Braun GmbH, Kronberg, Germany). Fecal samples were collected on days 1, 3, and 7, also as described previously.9,19 All animals were euthanized at the end of the 7-day feeding trial. After measuring digesta pH of the stomach, jejunum, and ascending colon using litmus paper (Thomas Scientific, Swedesboro, New Jersey), mucosal tissues from the small intestinal segments and the mesenteric lymph node were collected as described.18,20 The numbers of ETEC K88 and K99 shed in feces and bound to the tissue were determined by real-time PCR targeting the genes coding for fimbriae K88 and K99, respectively, also as described.9
All data were analyzed using the general linear model procedure (SAS 9.2; SAS Institute Inc, Cary, North Carolina), except for fecal shedding and intestinal adhesion of ETEC K88 and K99. The model included treatment only when there was a single observation per animal. In an analysis of repeated measurements, in which the model included treatment, day, and their interaction, the effects of treatment and day, including its interaction with treatment, were tested using animal and day × animal nested within treatment as error terms, respectively. For fecal shedding and intestinal adhesion of ETEC K88 and K99, data from unchallenged animals were excluded from statistical analysis after confirmation of the absence of either pathogen in the feces and intestinal tissues. Frequency of the appearance of pathogen-positive feces and pathogen-positive intestinal tissue were analyzed using the chi-squared test. Means were separated by t test; P < .05 was considered statistically significant and P < .10 was considered a tendency.
Results
The rectal temperature of the Control group did not change during the 7-day experimental period (Table 1). Mean rectal temperature was lower in the Chal-Phage group than in the Chal-Basal group, but did not differ between the Chal-Phage and Control groups. Fecal consistency score increased transiently after day 1 in the challenged groups; mean score was greatest in the Chal-Basal group, followed sequentially by the Chal-Phage and Control groups. Average daily gain, which was less in the challenged groups than in the Control group, was greater in the Chal-Phage group than in the Chal-Basal group. The digesta pH value measured at necropsy was lower in the Control group than in the Chal-Basal group for the stomach, jejunum, and colon and also in the Chal-Phage group versus the Chal-Basal for the colon, with a tendency to be lower in the Chal-Phage group than in the Chal-Basal for the jejunum (P = .07). The ETEC K88 (Figure 1, Panel A) and K99 (Figure 1, Panel B) were detected in feces of the challenged groups, but not the Control group. The mean number of cfus of ETEC K88 transformed to base 10 logarithm (log) per gram feces was greater in the Chal-Basal group than in the Chal-Phage group, but the log number of cfus of ETEC K99 did not differ between the two groups.
Table 1: Effects of dietary supplementation of enterotoxigenic Escherichia coli (ETEC) K88- specific and K99-specific bacteriophages on clinical signs, growth performance, and digesta pH in postweaning pigs challenged with ETEC K88 and K99*
| Control | Challenged | SEM | P | ||
|---|---|---|---|---|---|
| Basal (n = 6) | Basal (n = 6) | +Phage (n = 6) | |||
| Rectal temperature (°C) | |||||
| Day 0 | 38.72 | 38.68x | 38.72x | 0.097† | NA |
| Day 3 | 38.72a | 39.87b,y | 39.05c,y | ||
| Day 7 | 38.68 | 38.90x | 38.83xy | ||
| Overall‡ | 38.71a | 39.15b | 38.87a | 0.057 | < .01 |
| Fecal consistency score§ | |||||
| Day 1 | 0.17 | 0.33x | 0.17x | 0.237† | NA |
| Day 2 | 0.17a | 1.33b,y | 0.50a,xy | ||
| Day 3 | 0.17a | 2.17b,z | 1.33c,z | ||
| Day 4 | 0.33a | 2.00b,z | 1.00c,yz | ||
| Day 5 | 0.17a | 1.33b,y | 0.67a,xy | ||
| Day 6 | 0.17a | 1.17b,y | 0.50a,xy | ||
| Day 7 | 0.17a | 1.00b,y | 0.50ab,xy | ||
| Overall¶ | 0.19a | 1.33b | 0.67c | 0.111 | < .01 |
| Growth performance | |||||
| Initial weight (kg) | 11.4 | 10.1 | 10.6 | 0.46 | .18 |
| Final weight (kg) | 16.4a | 11.5b | 13.2c | 0.52 | < .01 |
| ADG (kg) | 0.361a | 0.098b | 0.186c | 0.021 | < .01 |
| Digesta pH | |||||
| Stomach | 2.75a | 3.65b | 3.28ab | 0.188 | .02 |
| Jejunum | 6.67a | 7.17b | 6.87ab | 0.111 | .02 |
| Colon | 6.70a | 7.23b | 6.80a | 0.100 | < .01 |
* A total of eighteen 28-day-old postweaning pigs received an oral administration of 3.0 × 108 colony-forming units (cfu) of ETEC K88 and of ETEC K89 in 3 mL phosphate buffered saline (PBS) each or 6 mL PBS (Control) on day 0 of the experiment. The animals were fed a nursery diet containing no phage (Basal) or 1.0 × 109 plaque-forming units (pfu) of ETEC K88-specific bacteriophages and the same number of pfus of ETEC K99-specific bacteriophages per kg diet (+Phage) for 7 days and were subjected to necropsy, including measurement of digesta pH. Data are means of six animals. Overall average daily feed intakes were 0.406, 0.392, and 0.397 kg per animal for the Control-Basal, Challenged-Basal, and Challenged-Phage groups, respectively.
† Applies to all day × treatment combinations.
‡ Both for the day and day × treatment P < .01 (ANOVA).
§ 0 = normal feces; 1 = soft feces; 2 = mild diarrhea; 3 = severe diarrhea.
¶ For day and day × treatment, P < .01 and P = .09, respectively (ANOVA).
a,b,c Means within a row with no common superscript differ (P < .05; t test).
x,y,z Means within a column with no common superscript differ (P < .05; t test).
SEM = standard error of the mean; ADG = average daily gain; NA = not applicable.
Figure 1: Fecal shedding of Escherichia coli (ETEC) K88 (Panel A) and K99 (Panel B) of post-weaned pigs after oral administration of the ETEC K88 and K99 pathogens: effects of in-feed phages. Treatments described in Table 1. Colony forming units (cfus) were transformed to base 10 logarithms. Data are means (with standard error of the means) of six animals. The P values for treatment, day, and treatment × day were < .01, < .01, and .26, respectively, for ETEC K88, and .53, .76, and .89, respectively, for ETEC K99 (ANOVA). Chal = challenged.
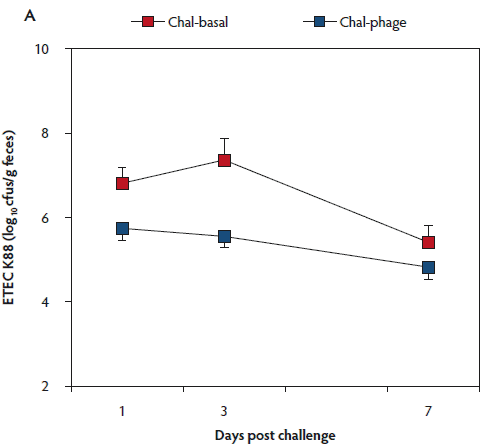
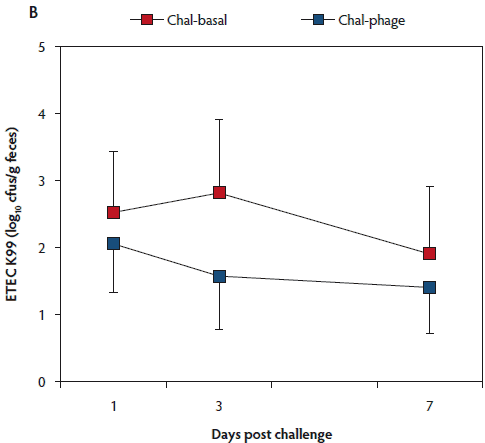
The log number of cfus of ETEC K88 bound to the tissue was less in the Chal-Phage group than in the Chal-Basal group for the ileum and cecum, but did not differ between the two groups for the duodenum, jejunum, colon, or mesenteric lymph node (Table 2). Adhesion of ETEC K99, however, did not differ between the two groups in any region of the digestive tract.
Table 2: Effects of dietary supplementation of enterotoxigenic Escherichia coli (ETEC) K88-specific and K99-specific bacteriophages on intestinal adhesion of ETEC in postweaning pigs challenged with ETEC K88 and K99*
| Basal (n = 6) | +Phage (n = 6) | P | |
|---|---|---|---|
| ETEC K88, log10 cfus/g tissue (no. of ETEC-positive pigs) | |||
| Duodenum | 1.57 ± 0.996 (2) | 0.74 ± 0.738 (1) | .52 |
| Jejunum | 3.03 ± 1.37 (3) | 1.42 ± 0.902 (2) | .35 |
| Ileum | 7.24 ± 0.460 (6) | 5.57 ± 0.263 (6) | .01 |
| Cecum | 6.32 ± 0.504 (6) | 3.92 ± 0.800 (6) | .03 |
| Colon | 3.31 ± 1.510 (3) | 1.84 ± 1.175 (2) | .46 |
| Mesenteric lymph node | 3.84 ± 1.233 (4) | 2.19 ± 0.979 (3) | .32 |
| ETEC K99, log10 cfus/g tissue (no. of ETEC-positive pigs) | |||
| Duodenum | 0 (0) | 0 (0) | NA |
| Jejunum | 2.43 ± 1.108 (3) | 1.42 ± 0.905 (2) | .50 |
| Ileum | 2.59 ± 1.184 (3) | 1.52 ± 0.965 (2) | .50 |
| Cecum | 1.91 ± 1.238 (2) | 0.83 ± 0.83 (1) | .49 |
| Colon | 1.79 ± 1.129 (2) | 0.93 ± 0.932 (1) | .57 |
| Mesenteric lymph node | 2.80 ± 1.277 (3) | 2.18 ± 0.984 (3) | .71 |
* Treatments described in Table 1. The numbers of cfus of ETEC K88 and K99 were determined by real-time polymerase chain reaction targeting the respective fimbrial genes using genomic DNA extracted from the intestinal tissue as template and were transformed to base 10 logarithms. Data are means ± standard errors of the means of six animals, with the log10 cfu value for the ETEC-negative animal calculated as 0. The numeral in parenthesis represents the number of the corresponding ETEC-positive animals out of six. The effect of the dietary treatment on the frequency of the corresponding ETEC-positive samples was not significant (chi-squared test) in any of the fecal and tissue samples. Data from Control animals without either ETEC K88 or K99 in fecal or intestinal tissue samples were excluded from this table.
Cfus = colony-forming units; NA = not applicable.
Discussion
Clinical measurements in the present study indicated that the postweaning pigs concurrently challenged with ETEC K88 and K99 developed the intended colibacillosis as manifested by the higher body temperature and fecal consistency score, as well as lower weight gain in the Chal-Basal and Chal-Phage groups versus the Control group, and severity of clinical signs was less in the Chal-Phage group than in the Chal-Basal group. These results, as a whole, were similar to the effects of the ETEC K88 challenge and dietary supplementation of antibiotics or 2500 mg ZnO per kg diet, respectively, in weanling pigs in earlier studies.9,19
The greater digesta pH value of the stomach in the Chal-Basal group, compared to the Control group, was consistent with the earlier result of ETEC K88 challenge in weanling pigs,19 but not with that of the study of Wellock et al,21 where digesta pH did not change in pigs challenged with ETEC O149 239/03. It thus remains to be determined why different ETEC strains exerted varying effects on gastric acidity. It has been reported that proliferation of beneficial microflora was favored at a lower pH,22 whereas at higher pH, ETEC colonization was enhanced.23 It is also well known that ETEC enterotoxins cause electrolyte losses and diarrhea.2,3,24 Thus, the higher pH of the jejunal digesta, as well as the better fecal consistency score in the Chal-Basal group versus the Control group, which was consistent with the results of Kwon et al19 and Wellock et al,21 is likely to have been the result of the ETEC infection in the Chal-Basal group. Conversely, the lower digesta pH in the colon, as well as the tendency to lower pH in the jejunum for the Chal-Phage group versus the Chal-Basal group, is presumed to have resulted from alleviated ETEC infection as a consequence of the phage therapy.
Fecal shedding and intestinal adhesion of ETEC, which were lower for the K99 strain than for the K88 strain, were lower in the Chal-Phage group versus the Chal-Basal group for ETEC K88, but not for K99. This may reflect the lower infectivity of the K99 antigen compared with that of K88 in postweaning pigs,25 although a possibility of confounding effects of ETEC K88 and K99 as well as the two phage strains could not be ruled out under the present experimental conditions.
Implications
• Phage therapy appears to be effective for treatment of ETEC K88 infection, but not that of K99 infection, in postweaning pigs.
• More studies on the effects of the ETEC K88-specific phage in piglets infected with ETEC K88 alone are needed.
Acknowledgements
This work was supported by the College of Veterinary Medicine and Institute of Veterinary Science of Kangwon National University. The authors thank Dr Sang-Hyeon Kang (iNtRON Biotechnology, Inc, Seoul) for providing the ETEC K88-specific and K99-specific bacteriophages used in the present study. Dr Seung-Jae Han and Dr Yeonsu Oh contributed equally to this work.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Weary DM, Jasper JJ, Hotzel MJ. Understanding weaning stress. Appl Anim Behav Sci. 2008;110:24–41.
2. Kim JC, Hansen CF, Mullan BP, Pluske JR. Nutrition and pathology of weaner pigs: nutritional strategies to support barrier function in the gastrointestinal tract. Anim Feed Sci Technol. 2012;173:3–16.
3. Heo JM, Opapeju FO, Pluske JR, Kim JC, Hampson DJ, Nyachoti CM. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol Anim Nutr. 2013;97:207–237.
4. Huang S, McFall M, Cegielski AC, Kirkwood RN. Effect of dietary zinc supplementation on Escherichia coli septicemia in weaned pigs. Swine Health Prod. 1999;7:109–111.
5. Marquardt RR, Jin LZ, Kim J-W, Fang L, Frohlich AA, Baidoo SK. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and and early-weaned piglets. FEMS Immunol Med Microbiol. 1999;23:283–288.
6. Fairbrother JM, Nadeau E, Gyles CL. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Rev. 2005;6:17–39.
7. Wang J, Jiang SW, Chen XH, Liu ZL, Peng J. Prevalence of fimbrial antigen (K88 variants, K99 and 987P) of enterotoxigenic Escherichia coli from neonatal and post-weaning piglets with diarrhea in central China. Asian-Aust J Anim Sci. 2006;19:1342–1346.
8. Runnels PL, Moon HW, Schneider RA. Development of resistance with host age to adhesion of K99+ Escherichia coli to isolated intestinal epithelial cells. Infect Immun. 1980;28:298–300.
9. Kim SJ, Kwon CH, Park BC, Lee CY, Han JH. Effects of a lipid-encapsulated zinc oxide dietary supplement, on growth parameters and intestinal morphology in weanling pigs artificially infected with enterotoxigenic Escherichia coli. J Anim Sci Technol. 2015;57:4. doi:10.1186/s40781-014-0038-9.
10. Hill GM, Mahan DC, Carter SD, Cromwell GL, Ewan RC, Harrold RL, Lewis AJ, Miller PS, Shurson GC, Veum TL. Effect of pharmacological concentrations of zinc oxide with or without the inclusion of an antibacterial agent on nursery pig performance. J Anim Sci. 2001;79:934–941.
11. Piva A, Grilli E, Fabbri L, Pizzamiglio V, Gatta PP, Galvano F, Bognanno M, Fiorentini L, Wolinski J, Zabielski R, Patterson JA. Intestinal metabolism of weaned piglets fed a typical United States or European diet with or without supplementation of tributyrin and lactitol. J Anim Sci. 2008;86:2952–2961.
12. Classification and Specification of Hazardous Feeds [in Korean]. Notification No. 2010-142. Ministry of Food, Agriculture, Forestry and Fisheries, Republic of Korea; 2010.
13. Aarestrup FM. Association between the consumption of antimicrobial agents in animal husbandry and the occurrence of resistant bacteria among food animals. Int J Antimicrobial Agents. 1999;12:279–285.
14. Amending the conditions for authorization of a number of additives in feedingstuffs belonging to the group of trace elements. Commission Regulation (EC) No 1334/2003. Official J Eur Union. 2003;L187:11–15.
15. Johnson RP, Gyles CL, Huff WE, Ojha S, Huff GR, Rath NC, Donoghue AM. Bacteriophages for prophylaxis and therapy in cattle, poultry and pigs. Anim Health Res Rev. 2008;9:201–215.
16. Endersen L, O’Mahony J, Hill C, Ross RP, McAuliffe O, Coffey A. Phage therapy in the food industry. Annu Rev Food Sci Technol. 2014;5:327–349.
17. Jamalludeen N, Johnson RP, Shewen PE, Gyles CL. Evaluation of bacteriophages for prevention and treatment of diarrhea due to experimental enterotoxigenic Escherichia coli O149 infection of pigs. Vet Microbiol. 2009;136:135–141.
18. Yoo A, Cha SB, Shin MK, Park HT, Seo HS, Kim JW, Yoo HS. Field evaluation of enterotoxi- genic Escherichia coli-specific bacteriophage (ФCJ19) as a feed additive. Korean J Vet Res. 2013;53:83–88.
19. Kwon CH, Lee CY, Han SY, Kim SJ, Park BC, Jang I, Han JH. Effects of dietary supplementation of lipid-encapsulated zinc oxide on colibacillosis, growth and intestinal morphology in weaned piglets challenged with enterotoxigenic Escherichia coli. Anim Sci J. 2014;85:805–813.
20. Jang I, Kwon CH, Ha DM, Jung DY, Kang SY, Park MJ, Han JH, Park BC, Lee CY. Effects of a lipid-encapsulated zinc oxide supplement on growth performance and intestinal morphology and digestive enzyme activities in weanling pigs. J Anim Sci Technol. 2014;56:29. doi:10.1186/2055-0391-56-29.
21. Wellock IJ, Fortomaris PD, Houdijk JGM, Kyriazakis I. Effects of dietary protein supply, weaning age and experimental enterotoxigenic Escherichia coli infection on newly weaned pigs: health. Animal. 2008;2:834–842.
22. Fuller R. The importance of lactobacilli in maintaining normal microbial balance in the crop. Br Poultry Sci. 1977;18:85–94.
23. Nagy B, Fekete PZ. Enterotoxigenic E. coli (ETEC) in farm animals. Vet Res. 1999;30:259–284.
24. Dubreuil JD. The whole shebang: the gastrointestinal tract, Escherichia coli enterotoxins and secretion. Curr Issues Mol Biol. 2012;14:71–82.
25. Cox E, Houvenaghel A. Comparison of the in vitro adhesion of K88, K99, F41 and P987 positive Escherichia coli to intestinal villi of 4- to 5-week-old pigs. Vet Microbiol. 1993;34:7–19.