| Commentary | Peer reviewed |
Cite as: O’Connor AM, Totton SC, Shane D. A systematic review and network meta-analysis of injectable antibiotic treatment options for naturally occurring swine respiratory disease. J Swine Health Prod. 2019;27(3):133-149.
Supplementary materials 1: Protocol (SM1) is available as a separate PDF.
Supplementary materials 2: Tables and Figures (SM2) is available as a separate PDF. < https://doi.org/10.54846/jshap/1104 a href=”v27n3p133.pdf”>
Also available as a PDF.
SummaryBased on an a priori protocol, a review of injectable antibiotic regimens to treat swine respiratory disease (SRD) in weaned swine was conducted to assess the first-treatment failure at 5 to 14 days post-treatment. Information sources included Cambridge Agricultural and Biological Index, MEDLINE, Food and Drug Administration New Animal Drug Approval summaries, Swine Information Library abstracts, and bibliographies of relevant studies and reviews. Two reviewers screened the records, extracted data, and assessed bias risk. From 1266 records screened, 25 relevant records described 41 relevant studies. Thirty-four relevant studies were included in a meta-analysis. The top 3 model-estimated SRD treatments based on mean rank were enrofloxacin (7.5 mg/kg once or 2.5-5 mg/kg once daily for 3-5 days; n = 5; rank = 2; 95% CI, 1-4), gamithromycin (6 mg/kg once, n = 2; rank = 5; 95% CI, 1-14), and marbofloxacin (8 mg/kg once, n = 1; rank = 6; 95% CI, 1-16). When treating SRD, this information should be combined with antibiotic treatment selection criteria including sensitivity testing results, the farm’s pathogen susceptibility monitoring data, local antibiotic prescribing policies, product label recommendations for use and warnings, cost, convenience, importance of the antibiotic to human health, and prudent antibiotic use guidelines. | ResumenEn base de un protocolo a priori, se realizó una revisión de los regímenes de antibióticos inyectables para tratar la enfermedad respiratoria porcina (SRD, por sus siglas en inglés) en cerdos destetados para evaluar el fracaso del primer tratamiento entre los 5 y los 14 días posteriores al tratamiento. Las fuentes de información incluyeron el Índice Agrícola y Biológico de Cambridge, MEDLINE, resúmenes de la Aprobación de Nuevos Medicamentos para Animales de la Administración de Alimentos y Medicamentos, resúmenes de la Biblioteca de Información Porcina y bibliografías de estudios y revisiones relevantes. Dos revisores seleccionaron los registros, extrajeron los datos y evaluaron el riesgo de parcialidad. De los 1266 registros seleccionados, 25 registros relevantes describieron 41 estudios relevantes. Se incluyeron 34 estudios relevantes en un meta-análisis. Los 3 principales modelos de tratamiento para SRD basados en la categoría promedio fueron enrofloxacina (7.5 mg/kg una vez o 2.5-5 mg/kg una vez al día durante 3-5 días; n = 5; categoría = 2; IC 95%, 1-4), gamitromicina (6 mg/kg una vez, n = 2; categoría = 5; IC 95%, 1-14) y marbofloxacina (8 mg/kg una vez, n = 1; categoría = 6; IC 95%, 1-16). Cuando se trata la SRD, esta información debe combinarse con los criterios de selección del tratamiento con antibióticos, incluidos los resultados de las pruebas de sensibilidad, los datos de monitoreo de susceptibilidad a patógenos de la granja, las políticas locales de prescripción de antibióticos, las recomendaciones de uso y las advertencias en la etiqueta del producto, el costo, conveniencia, la importancia del antibiótico con relación a la salud humana, y pautas prudentes sobre uso de antibióticos. | ResuméSur la base d’un protocole a priori, une analyse des schémas thérapeutiques d’antibiothérapie par injection pour traiter les maladies respiratoires porcines (MRP) chez les porcs sevrés a été réalisée pour évaluer l’échec du premier traitement 5 à 14 jours après le traitement. Les sources d’information comprenaient le Cambridge Agricultural and Biological Index, MEDLINE, les résumés du Food and Drug Administration sur les approbations des nouveaux médicaments pour les animaux, les résumés de la Swine Information Library, et les bibliographies des études et revues pertinentes. Deux examinateurs ont étudié les dossiers, extrait les données et évalué le risque de biais. Sur 1266 dossiers examinés, 25 dossiers pertinents décrivaient 41 études pertinentes. Trente-quatre études pertinentes ont été incluses dans une méta-analyse. Les trois principaux traitements des MRP estimés selon le modèle sur la base du rang moyen étaient l’enrofloxacine (7.5 mg/kg une fois ou 2.5 à 5 mg/kg une fois par jour pendant 3-5 jours; n = 5; rang = 2; IC à 95%, 1-4), la gamithromycine (6 mg/kg une fois, n = 2; rang = 5; IC 95%, 1-14) et la marbofloxacine (8 mg/kg une fois, n = 1; rang = 6; IC 95%, 1-16). Lors du traitement des MRP, ces informations doivent être associées à des critères de sélection de traitement aux antibiotiques, notamment les résultats des tests de sensibilité, les données de surveillance de la sensibilité des agents pathogènes de la ferme, les règles locales de prescription d’antibiotiques, les recommandations d’utilisation et avertissements des étiquettes, le coût, la commodité, l’importance de l’antibiotique pour la santé humaine et les directives d’utilisation prudente des antibiotiques. |
Keywords: swine, antibiotics, meta-analysis, respiratory disease, systematic review
Search the AASV web site
for pages with similar keywords.
Received: May 9, 2018
Accepted: December 3, 2018
Respiratory disease represents a major health issue in swine production. Al – though prevention of respiratory disease is the preferred management approach, antibiotic treatment is required to ensure the best possible outcome regarding animal health and well-being when cases of swine respiratory disease (SRD) do occur. Many products are registered around the world for the treatment of SRD. Ideally, veterinarians would read the available literature about the efficacy of SRD treatments and combine the information. However, there are numerous barriers to such synthesis. First, veterinarians often lack both the access to and time for review of the literature. Further, many studies conducted and published for registration purposes often compare response to treatment in untreated animals. Such comparisons are often of little interest to producers or veterinarians who might be interested in comparisons between two or more active products. It is also extremely difficult, without statistical methods, to appropriately combine and compare studies from different trials and sample sizes. Because of these factors, the comparative efficacy of many antibiotic treatments for SRD are rarely known, despite this being critical information for producers and veterinarians. Knowledge of comparative efficacy is critical because it establishes a baseline for antibiotic selection.
Although comparative efficacy is important it is clearly not the only metric of importance in antibiotic selection. Veterinarians should also consider this alongside other relevant factors for antibiotic treatment selection, which may include sensitivity testing results for target animals, pathogen susceptibility monitoring data for the farm, local antibiotic prescribing policies, the recommendations for use and warnings on the product labels and leaflets, cost, convenience, importance of the antibiotic to human health, and guidelines for prudent antibiotic use.
Ideally, comparative efficacy would be assessed in large multi-arm randomized controlled clinical trials; however, such trials are rarely conducted or publicly available. An alternative approach to assessing comparative efficacy in large trials is a network meta-analysis, also known as a mixed treatment comparison meta-analysis. This approach has been widely used in human health, and evidence from bovine respiratory disease suggests that estimates of comparative efficacy obtained from network meta-analysis are very reasonable approximations of those observed in controlled trials.1,2
The objective of this study was to evaluate the comparative efficacy of injectable antibiotic treatments for SRD and assess the risk-of-bias potential associated with the body of work. The project sought to provide estimates of comparative efficacy and ranking of efficacy based on the first treatment failure between 5 and 14 days post-treatment for antibiotics used to treat swine. The review question was framed using a format that explicitly defined the population, the intervention, the comparator, and the outcome of interest (sometimes known as the PICO format): In weaned swine with naturally occurring undifferentiated or differentiated SRD in modern production systems (population), what is the comparative efficacy of injectable antibiotic treatments (interventions, comparator) for the first treatment failure occurring between 5 and 14 days post-treatment (outcome)?
Materials and methods
Protocol and registration
The review protocol was developed before the start of the review. Development of a protocol prior to conduct of the review is standard practice for systematic reviews, and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement provides the following rationale:
A protocol is important because it pre-specifies the objectives and methods of the systematic review. For instance, a protocol specifies outcomes of primary interest, how reviewers will extract information about those outcomes, and methods that reviewers might use to quantitatively summarize the outcome data (see Item 13). Having a protocol can help restrict the likelihood of biased post hoc decisions in review methods, such as selective outcome reporting.
As a pharmaceutical company funded this review, concerns about selective inclusion of literature or selective reporting of outcomes and the influence of the company on the report might be relevant to readers, therefore a protocol is particularly important. The final protocol was approved and time-stamped on September 30, 2017. There is no mechanism to register protocols for systematic reviews in livestock at present, therefore, the time-stamped protocol was made and is included in the supplementary materials (SM1: Protocol). This report is prepared based on the PRISMA extension for network meta-analyses published in 2015.3
Eligibility criteria
The eligibility criteria described herein do not differ from those proposed in the protocol.
Population. The population of interest was weaned swine, which might variably be described as nursery pigs, grower pigs, finishers, or based on weight and age. The swine also had to be diagnosed with naturally occurring, undifferentiated or differentiated SRD in modern swine production systems. Studies based only on sows, gilts, or boars were not considered relevant. No restrictions were placed on the country of conduct.
Interventions. Individual animal interventions of interest included injectable antibiotics listed in Table 1. The list of known SRD treatment regimens was provided by the sponsor designate (Dr Shane), who consulted work colleagues about treatment regimens of interest. These regimens were the registered label dose of the antibiotic in either Europe or the United States, and thus multiple antibiotic treatments and regimens would be considered extra-label use in the United States. Treatment regimens of parenteral products for SRD control, SRD control interventions added to food or water, antibiotics combined with non-steroidal anti-inflammatory drugs, and off-label use regimens were not considered relevant to the conclusions of the review. When the label included multiple dose regimens, these were combined into a single treatment. For example, if a three-arm trial had one placebo group, a second group that assessed a single intramuscular dose of 3.0 mg/kg of ceftiofur sodium, and a third group that assessed a single intramuscular dose of 5.0 mg/kg of ceftiofur sodium, the second and third groups would be combined and compared to the placebo because these two doses are listed as equivalent on the product label and, therefore, these data were considered to represent one treatment. The rationale for this approach was that if labeled as such, the regimens were assumed to be therapeutically non-inferior. All non-active controls including placebo, saline, non-drug sterile diluent, or no treatment were combined into one group defined as non-active controls. A single comparator of interest was not identified, as the purpose of the review was to compare the efficacy across all the available interventions.
Table 1: Injectable antibiotic regimens reported in studies and the final regimes used in a mixed-treatment comparisons meta-analysis
| Antibiotic regimen | Short name | Prespecified regimen | Abbreviation |
|---|---|---|---|
| Amoxicillin: 15 mg/kg 2 doses 48 hours apart | Amoxicillin | Yes | AMX |
| Amoxicillin and clavulanic acid: 7.0 and 1.75 mg/kg, respectively, once daily for 3 days | Amoxicillin/clavulanic acid (7.0/1.75 mg/kg 3 days) | No | AMXOL |
| Ampicillin: 6 mg/kg once | Ampicillin | Yes | . |
| Ceftiofur (HCl or NA not reported); 3 mg/kg once daily for 3 days | Ceftiofur (HCl or NA) | No | CEFOL1 |
| Ceftiofur crystalline free acid: 5.0 mg CE/kg once | Ceftiofur CFA | Yes (FDA) | CCFA |
| Ceftiofur hydrochloride: 3-5 mg/kg once daily for 3 days | Ceftiofur HCL (MD) | Yes (FDA) | . |
| Ceftiofur hydrochloride: 5 mg/kg once | Ceftiofur HCl (5 mg/kg once) | No | CEFOL3 |
| Ceftiofur sodium: 1-2 mg/kg once daily for 3 days | Ceftiofur NA (1-2 mg/kg 3 days) | No | CEFOL4 |
| Ceftiofur sodium: 3-5 mg/kg once daily for 3 days | Ceftiofur NA (MD) | Yes (FDA) | CEF |
| Danofloxacin: 1.25 mg/kg or 2.5 mg/kg once | Danofloxacin (1.25 or 2.5 mg/kg once) | No | DANOF |
| Danofloxacin: 1.25 mg/kg once daily for 3 days | Danofloxacin | Yes | . |
| Enrofloxacin: 2.5 mg/kg once daily for 3 days | Enrofloxacin (2.5 mg/kg 3 days) | No | ENFOL1 |
| Enrofloxacin: 7.5 mg/kg once or 2.5-5 mg/kg once daily for 3-5 days | Enrofloxacin | Yes | ENF |
| Enrofloxacin: 7.5 mg/kg once or once daily for 2 days | Enrofloxacin (7.5 mg/kg once or twice) | No | ENFOL2 |
| Florfenicol: 15 mg/kg twice 48 hours apart | Florfenicol | Yes | FLO |
| Gamithromycin: 6 mg/kg once | Gamithromycin | Yes | GAM |
| Gentamicin sulfate: 2-5 mg/kg twice daily for 3 days | Gentamicin | Yes | . |
| Lincomycin hydrochloride: 5 mg/lb (2.27 mg/kg) once | Lincomycin hydrochloride | Yes | . |
| Marbofloxacin: 8 mg/kg once or 2 mg/kg once daily for 3 days | Marbofloxacin | Yes | MAR |
| No treatment: saline, non-drug, sterile diluent, placebo | Non-active control | Yes (FDA) | NAC |
| Oxytetracycline: 9 mg/lb (4.1 mg/kg) once or 5-10 mg/kg once | Oxytetracycline | Yes (FDA) | OXY |
| Penicillin: 3000 units/lb once daily for 4 days or 15 IU/kg once daily for 4 days | Penicillin | Yes (FDA) | . |
| Tiamulin: 15 mg/kg once daily for 3 days | Tiamulin | No | TIAOL |
| Tildipirosin: 4 mg/kg once | Tildipirosin | Yes | TIL |
| Tulathromycin: 2.5 mg/kg once | Tulathromycin | Yes (FDA) | TUL |
| Tylosin Injectable: 4 mg/lb (1.8 mg/kg) once | Tylosin | Yes (FDA) | . |
Outcome. The outcome of interest was first-treatment failure risk measured in the 5 to 14 days post-treatment. When the day of treatment was defined as day 0, then outcomes measured on days 4 and 13 were within the relevant follow-up period. When the day of treatment was defined as day 1, then outcomes measured on days 5 and 14 were within the relevant follow-up period. When the outcome was measured on multiple days in the 5 to 14 day period, the results closest to 7 days post-treatment were used. The rationale was that this period is commonly used by the US Food and Drug Administration (FDA) for registration purposes. The definition of treatment failure, or the inverse of treatment success, was described by the investigators of the original research report. For the meta-analysis, when the success risk was defined, this was converted to failure risk.
Study design. Studies relevant to the review had to contain a concurrent control group (active comparator or placebo) and at least one of the registered antibiotic regimens listed in the protocol (Table 1). Experimental challenge trials, cluster-randomized trials, and observational studies were not considered relevant. Experimental challenge studies were not considered relevant, as the external validity of the disease model to practice can be unclear. Cluster-randomized trials were not considered because the treatments are administered to an individual pig at diagnosis with SRD and cluster-randomized studies are a design associated more commonly with prophylactic or metaphylactic antibiotic uses. Observational studies were excluded because the potential for bias due to indication is very high for such studies. Random allocation to treatment group was not used as an exclusion criterion due to evidence that this may be rare in SRD trials.
Report characteristics. Eligible studies had to be written in English and publicly available, although not necessarily open access, in conference proceedings or peer-reviewed journals.
Information sources
The information sources used were Cambridge Agricultural and Biological Index (CABI), MEDLINE, the Swine Information Library (SIL), and FDA Freedom of Information (FOI) New Animal Drug Approval (NADA) summaries for registered regimens, and the bibliographies of relevant studies and potentially relevant reviews identified during screening. The European Medicines Authority data was not searched because neither the European Public Assessment Report nor the product information provides data similar to the FDA FOI NADA summaries. The Iowa State University Web of Science interface was used to search CABI and MEDLINE for literature from 1970-2017. The rationale for this limit was that few studies of antibiotics of greatest interest would be published before 1970 and the authors’ experience suggests that such studies are often very poorly reported and of little value for meta-analyses. One impact of this approach is that pre-1970 literature may include placebo versus penicillin studies and these studies have no opportunity to be considered for the review. However, the decision was made that the benefit of finding such studies for inclusion was not considered sufficient relative to the cost needed to screen, retrieve, and extract data from them. The SIL enables access to the American Association of Swine Veterinarians Annual Meeting Proceedings (1999-2017), the International Pig Veterinary Society Congress proceedings (2000-2016), the Iowa State University Swine Diseases Conference proceedings (1996-2016), and the Allen D. Leman Swine Conference proceedings (2007-2016). These dates were dictated by the availability of electronic versions. The FDA FOI NADA summaries were available online (https://animaldrugsatfda.fda.gov/adafda/views/#/foiDrugSummaries).
Search
The citation searches began on October 5, 2017 and were completed on November 30, 2017 after all relevant studies had been identified and their bibliographies assessed. The CABI search results are reported in the supplementary materials (SM2: Table S1). Details about the conduct of the search such as how the SIL was searched as it doesn’t have indexation, handling of duplicates, and linked references are available in the supplementary materials (SM2: Tables and Figures).
Study selection
The screening was conducted using systematic review management software (Distiller SR; Evidence Partners, Ontario, Canada). Forms for study selection and data extraction were pre-tested during the protocol drafting phase to ensure consistent interpretation of relevant studies and data by the two independent reviewers. The two reviewers (Drs O’Connor and Totton), both experienced systematic reviewers and veterinary epidemiologists, independently assessed the abstracts and titles for relevance based on the eligibility criteria. The entire article was acquired if one reviewer indicated the record might meet the inclusion criteria. The full text was then assessed for relevance by both reviewers. Four sequential questions based on the PICO elements of eligibility criteria were used to evaluate relevant studies. If a study failed a question, no further evaluation was conducted. All relevant studies were included in the systematic review. However, studies were only eligible for the meta-analysis if the numerical outcome data could be extracted and at least one treatment arm was connected to the rest of the evidence network.
Duplication refers to multiple citations of the same publication. Duplicates were removed initially in the reference management software, then again in the systematic review management software. Linked publications, ie, the same studies reported in part or in full in different sources, were sometimes identified during the relevance screening but more commonly during data extraction.4 For linked publications, the more complete record was used as the citation. Reference lists from relevant reports and reviews were hand searched for additional relevant manuscripts. If these studies were published in years outside the original search range, they were still included. When disagreements arose about the relevance of the study between the two reviewers, these were resolved by discussion. It was not found necessary to consult the sponsor designate during the eligibility assessment.
Data collection process
The systematic review management software was used to extract data into pre-tested forms by two reviewers (Drs O’Connor and Totton) working independently. The unit of concern for dataset extraction was the study level if available. As investigators can vary in reporting the outcome, the order of preference for extracting the outcome dataset was as follows: an adjusted estimate of the summary effect size, an unadjusted estimate of the effect size, and the group-level frequency data. The rationale for this preference was that swine populations are clustered in pens, rooms, and barns and often across multiple sites, therefore adjusted estimates that correctly account for non-independence of observations provide the least biased estimate of the variance. Interestingly all studies reported group-level data rather than summary-level data. Investigators were not contacted when data were missing. If studies were linked, all the available information was used but the version that was the most complete was cited, which was usually the one with site-specific results.
Data items. Data items extracted related to the conduct of the study, the definition of SRD, the trial interventions, and the outcome. The detailed list of items extracted from each paper is provided in the protocol (SM1: Protocol).
Geometry of the network. Network geometry was assessed using an approach previously proposed.5 The probability of an inter-species encounter (PIE) index was calculated using custom-written R script and the C-score test was performed via R package EcoSimR (version 0.1.0, ).6 The PIE index is a continuous variable that decreases in value as unevenness increases. Values < 0.75 can be considered to reflect the limited diversity of interventions. Co-occurrence was also assessed using the C-score, which describes, based on a checkerboard analysis, if pairwise comparisons of specific treatments are preferred or avoided.5
Risk of bias within individual studies. The risk-of-bias form was based on the Cochrane Risk of Bias (ROB) 2.0 tool for randomized trials. However, this form was modified as follows to ensure relevance to the topic area.7
To assess bias due to the randomization process (ROB1), the ROB 2.0 tool provides the following signaling questions (SQ) to guide the reviewer:
SQ 1.1 – Was the allocation sequence random?
SQ 1.2 – Was the allocation sequence concealed until participants were recruited and assigned to interventions?
SQ 1.3 – Were there baseline imbalances that suggest a problem with the randomization process?
In addition to the Cochrane guidance for SQ 1.1, yes was indicated if the study was conducted for regulatory purposes, ie, an FDA study or if the study was conducted using Good Clinical Practice Guidelines.
Also, the response to SQ 1.2 about allocation concealment was ignored. In ROB 2.0, any study that did not report allocation concealment was automatically at high risk of bias. The response to SQ 1.2 was not considered in the overall assessment of bias due to randomization. The schema used was as follows: If the response to SQ 1.1 was yes or probably yes and the response to SQ 1.3 was no or probably no, the study was considered low risk of bias for that domain. If the response to SQ 1.1 and SQ 1.3 was no information, the study was considered high risk of bias for that domain. If the response to SQ 1.1 was no or probably no, the answer to SQ 1.3 was not influential and the study was considered high risk of bias. If the response to SQ 1.1 was yes or probably yes and the response to SQ 1.3 was no information, the study was considered to be of some concern of bias for that domain. If the response to SQ 1.1 was no information and the response to SQ 1.3 was no or probably no, the study was considered to be of some concern of bias for that domain.
The rationale for this modification was that it was considered unlikely in swine production settings that caregivers would have differential preferences for groups of animals to receive a particular intervention. This modification was planned in the protocol.
Bias due to deviations from intended interventions (ROB2) refers to deviations due to care-giving or failure to complete an allocated treatment. The potential for this bias is very low in commercial settings using short-duration antibiotic treatments, so few or no deviations were assumed even in the absence of reporting on blinding of outcome assessors. No changes to the Cochrane ROB 2.0 SQs or ROB algorithm were made.
Bias due to missing outcome data (ROB3) refers to loss to follow-up, and neither the SQs nor the risk algorithm proposed by Cochrane ROB 2.0 tool were modified.
Bias in the measurement of the outcome (ROB4) refers to bias introduced due to knowledge of the intervention by outcome assessors. Even if outcome assessors were aware of the intervention or if this was unclear, the risk of bias was considered low if the definition of treatment success included an objective measure such as temperature and that a threshold for considering an animal to be pyrexic was reported.
Bias in selection of the reported results (ROB5) was also assessed. For this review, only studies that reported the results at 5 to 14 days post-treatment were included, and other studies that were potentially relevant but reported a different outcome were not included. Bias was considered possible when multiple poorly defined or undefined metrics of the outcome were used.
The risk-of-bias information was not included in the meta-analysis nor used as exclusion criteria. Instead the risk of bias was included mainly to convey to end users that substantial information about the conduct of the studies is missing, and the impact of this information on the certainty of the conclusions that can be reached.
Summary measures. The primary approach to summarizing the data was the comparative efficacy rankings. The rationale for using these as the primary outcome is that they are a relative measure of efficacy. Given the potential for publication bias in the topic area, it is theoretically possible that all companies owning products relevant to the review are publishing the most promising studies. Therefore, the actual magnitude of effect size observed in the studies might be biased upwards. For example, companies owning products relevant to the review might have conducted several placebo-vs-active trials but presented only the one with the largest effect size. If this occurs, the effect sizes might be distorted. However, if all companies owning products relevant to the review engage in this practice, the relative comparisons should still be reasonable. Interestingly, it was previously speculated that this bias might occur; however, previous research in bovine respiratory disease did not find empirical evidence of this bias.1,2 For each simulation based on the probability of treatment failure, each treatment received a ranking. Lower rankings indicated a lower probability of treatment failure. All treatment regimens included in the meta-analysis received a ranking including off-label regimens, therefore, the range of rankings was 1 to 19 for each simulation. The reported data are the mean rankings and related 95% CI. Despite some reservations, the risk ratio (RR) and related 95% CI for all possible comparisons was also reported. This outcome was chosen because ease of interpretation is greater for the RR than for the odds ratio. The extracted data were organized such that an event (treatment failure) was an adverse outcome. Drugs with greater efficacy had lower event percentages. This approach was used because some studies reported success percentages (ie, failure to retreat), while others reported failure percentages (ie, retreatments). The data items, randomization to treatment arm (reported/not reported), outcome assessor blinding (reported/not reported), and pharmaceutical company sponsorship of treatment were also extracted and used for the assessment of methodological heterogeneity. When the RR is < 1, this implies that the drug in the numerator has a lower treatment failure risk than the drug in the denominator and is, therefore, more effective at treating SRD. When the RR is > 1, this implies that the drug in the numerator has a higher treatment failure risk than the drug in the denominator and is less effective at treating SRD. The baseline risk used to convert the odds ratios to the RR was obtained by using the distribution of the placebo group. Using these data, the prior distribution of the log odds ratio ( N [mean (SD)]) was reported as N (−0.9633 [0.7344]).
Planned method of statistical analysis
The proposed method has been previously described in detail.8 Briefly:
rjk ~ Bin(pjk, njk), θjk = logit(pjk)
and
µ jb, if k = b;b = A,B,C, …
θjk = {µ jb +δjbk , if k > b;b = A,B,C, …
where pjk is the probability of the event in trial j under treatment k and δjbk is the trial-specific log odds ratio of treatment k relative to the corresponding baseline treatment b in trial j. The trial-specific treatment effects are distributed as:
δjbk ~ N (dbk, σ2bk ),
with priors
dbk ~ N (0 [10000]),
and under the homogeneous variance assumption that σ2bk = σ2, where σ ~ U (0, 5).
Handling of multi-arm trials. The co-variance between δjAB and δjAC was assumed to be σ2/2 for multi-arm trials.9,10
Selection of prior distributions in Bayesian analysis. The prior distributions were originally based on the previously reported approach.10,11 In prior similar models, σ ~ U (0, 2) and σ ~ U (0, 5) were assessed, and σ ~ U (0, 5) was preferred. That assessment was repeated and the same prior used in a previous model was retained.1,2
Implementation and output. All posterior samples were generated using Markov Chain Monte Carlo (MCMC) simulation implemented using Just Another Gibbs Sampler (JAGS) software (version 3.4.0). All statistical analyses were performed using R software (version 3.2.1).12 The model was fitted using JAGS, an MCMC sampler, by calling JAGS from R through the rjags package.13 Three chains were simulated, and the convergence was assessed using Gelman-Rubin diagnostics. Five thousand “burn-in” iterations were discarded and inferences were based on an additional 10,000 iterations. The model output included all possible pairwise comparisons using log odds ratios for inconsistency assessment, RRs for comparative efficacy reporting, and the treatment failure rankings for comparative efficacy reporting.
Assessment of model fit. The fit of the model was assessed based on the log odds ratio by examining the residual deviance between the predicted values from the network meta-analysis model and the observed value for each study.8 The deviance to the number of data points were compared and a ratio of one was vaguely equated for these two numbers as a good fit. When this ratio seemed subjectively large, the output was searched for signs of potential issues, including unrealistic outcomes such as rankings with no variation or very large credible intervals. If these were noted, treatment groups were combined or studies that appeared to be associated with the poor fit were removed and the reduced model was re-evaluated. Trace plots for the treatment effects were monitored to identify major issues with convergence.
Assessment of inconsistency. The back-calculation method was used to assess the consistency assumption.8 The inconsistency evaluation did not rely only on the P values. The estimates from the direct and indirect models were also compared and the standard deviation of each estimate was considered. Comparisons for which the direct and indirect estimates had different signs were further evaluated and discussed.
Risk-of-bias assessment. The potential systematic biases resulting from the methodological variables, blinding, randomization, and sponsorship were described using indicator variables. The effect size and related 95% CI were reported. The impact of small-study effects was not assessed, as the potential to detect asymmetry was limited by the number of valid pairs available and any funnel plots would be too sparse to be meaningfully interpreted.
Additional analyses
No additional analyses were conducted.
Results and discussion
Study selection
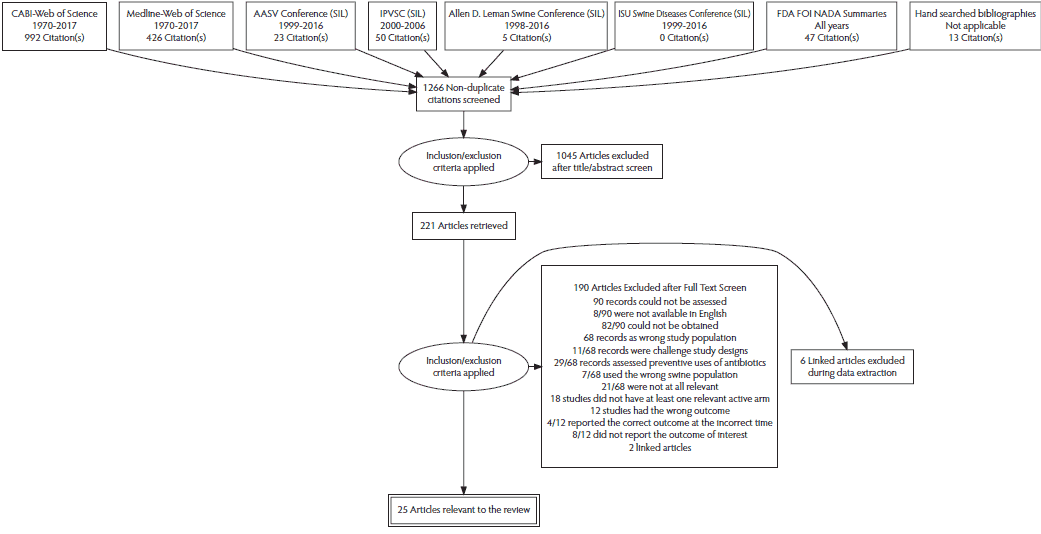
The flow chart for records retrieved for the review is reported in Figure 1. There were 1266 records screened, and 25 relevant records describing 41 relevant studies were identified. Thirty-four of the 41 relevant studies could be included in the meta-analysis. Of 1266 records screened, 221 were retrieved for full-text evaluation. One hundred ninety of the 221 full texts were excluded (see SM2: Table S2). This included two sets of linked publications, so exclusion reasons are available for 188 records. Thirty-one records were determined to contain studies relevant to the review. These are listed as 25 relevant articles in Figure 1 due to 6 linked publications.14-38 Those 25 records contained 41 unique studies considered relevant to the review. Four unique studies from 3 records were excluded from the meta-analysis because, although meeting all the relevance criteria, they did not report the outcome data.16,29,31 One unique study compared danofloxacin (1.25 mg/kg once daily for 3 days) to benzyl penicillin with dihydrostreptomycin. This was the sole study that evaluated these treatments, and therefore there was no link to the remaining evidence network. Consequently, this study was also excluded from the meta-analysis.36
Figure 1: The PRISMA flowchart describing the flow of literature through the review. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses; CABI = Cambridge Agricultural and Biological Index; AASV = American Association of Swine Veterinarians; SIL = Swine Information Library; IPVSC = International Pig Veterinary Society Congress; ISU = Iowa State University; FDA FOI NADA = Food and Drug Administration’s Freedom of Information New Animal Drug Application.
During the model assessment, two unique studies in the same manuscript were removed from the network meta-analysis because the results were inconsistent with the network.35 These 2 studies reported results for treatment failure where arm 1 was a non-active control (Farm A: 29 of 29; Farm B: 30 of 30), arm 2 was ceftiofur hydrochloride (3 mg/kg once daily for 3 days; Farm A: 8 of 30; Farm B: 2 of 30), and arm 3 was ceftiofur hydrochloride (5 mg/kg once daily for 3 days; Farm A: 7 of 30; Farm B: 0 of 30). As these doses were both on the same label, this represented two arms of multi-dose ceftiofur hydrochloride. This extremely high level of efficacy was unusual for ceftiofur regimens in the dataset. When these data were included in the model, the model was unstable. For example, multi-dose ceftiofur hydrochloride was ranked the highest with zero rank variation, yet the next nearest ceftiofur regimen was nine regimens lower. To explore the issue, the impact of creating a single category of multi-dose ceftiofur (3-5 mg/kg once daily for 3 days), which ignored the sodium or hydrochloride, was evaluated. However, this approach did not solve the issue. For example, several RR estimates were greater than 1000 indicating a major issue with model fit. Finally, the impact of excluding the 2 studies was assessed, which resolved the issue and the resulting model is reported here. Exclusion of this manuscript does not represent a deviation from the protocol, as consistency assessment is a required aspect of the meta-analysis.8 Therefore, a total of 7 of the 41 relevant studies were excluded and the resulting 34 studies were used in the final reported meta-analysis.
Presentation of network structure
The final evidence network used in the meta-analysis represented 34 studies and 73 arms. Some arms used treatment regimens that were off-label. These off-label arms were included in the network meta-analysis because they contributed data for estimation of regimens that were of interest. These non-protocol regimens are listed in Table 1. Information about the number of arms and the reporting of blinding and randomization is presented in Table 2.
Table 2: Characteristics of relevant studies included in the meta-analyses
| Reference number | Year | Country | Arm 1 Regimen | Arm 1 Events* | Arm 1 Total | Arm 2 Regimen | Arm 2 Events* | Arm 2 Total | Arm 3 Regimen | Arm 3 Events* | Arm 3 Total | Random | Blinded |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 | 1996 | United States | Enrofloxacin (7.5 mg/kg once) | 33 | 49 | Non-active control | 49 | 49 | NA | NA | NA | No | Yes |
| 14 | 1996 | United States | Enrofloxacin (7.5 mg/kg once) | 4 | 39 | Non-active control | 33 | 36 | NA | NA | NA | No | Yes |
| 15 | 2010 | United States | Enrofloxacin (7.5 mg/kg once) | 29 | 75 | Non-active control | 55 | 75 | NA | NA | NA | Yes | Yes |
| 15 | 2010 | United States | Enrofloxacin (7.5 mg/kg once) | 6 | 75 | Non-active control | 50 | 75 | NA | NA | NA | Yes | Yes |
| 17 | NR | United States | Ceftiofur CFA | 175 | 233 | Non-active control | 195 | 237 | NA | NA | NA | Yes | Yes |
| 18 | NR | France, Germany | Florfenicol | 14 | 109 | Oxytetracycline (20 mg/kg once) | 31 | 110 | NA | NA | NA | No | No |
| 19 | NR | Denmark, France, Germany | Amoxicillin | 4 | 77 | Ceftiofur CFA | 3 | 77 | NA | NA | NA | Yes | Yes |
| 20 | NR | Italy | Enrofloxacin (2.5 mg/kg for 3 days) | 30 | 67 | Enrofloxacin (5 mg/kg for 3 days) | 10 | 48 | NA | NA | NA | No | Yes |
| 21 | 2007 | Spain | Florfenicol | 8 | 31 | Amoxicillin | 12 | 29 | NA | NA | NA | No | Yes |
| 22 | NR | Slovenia | Amoxicillin and clavulanic acid (7.0 and 1.75 mg/kg, respectively, on days 0, 1, and 2) | 21 | 34 | Tulathromycin | 22 | 35 | NA | NA | NA | Yes | Yes |
| 22 | NR | Germany | Amoxicillin and clavulanic acid (7.0 and 1.75 mg/kg, respectively, on days 0, 1, and 2) | 5 | 26 | Tulathromycin | 2 | 19 | NA | NA | NA | Yes | Yes |
| 23 | NR | Spain | Florfenicol | 1 | 25 | Ceftiofur (unclear if HCl or Sodium) | 4 | 25 | NA | NA | NA | Yes | Yes |
| 24 | NR | Germany | Amoxicillin | 23 | 102 | Enrofloxacin (7.5 mg/kg once or twice) | 14 | 96 | NA | NA | NA | Yes | Yes |
| 24 | NR | Denmark, Germany | Enrofloxacin (7.5 mg/kg once or twice) | 2 | 85 | Florfenicol | 6 | 84 | NA | NA | NA | Yes | Yes |
| 25 | NR | Germany | Tildipirosin | 17 | 254 | Tulathromycin | 20 | 254 | NA | NA | NA | Yes | Yes |
| 26 | NR | Germany | Tildipirosin | 6 | 96 | Tulathromycin | 6 | 96 | NA | NA | NA | Yes | Yes |
| 27 | NR | Japan | Gamithromycin | 7 | 42 | Danofloxacin (1.25 mg/kg or 2.5 mg/kg) | 6 | 21 | NA | NA | NA | No | Yes |
| 27 | NR | Germany | Gamithromycin | 5 | 15 | Tildipirosin | 11 | 152 | NA | NA | NA | No | Yes |
| 30 | NR | France, United States | Ceftiofur HCl (5 mg/kg once) | 140 | 152 | Non-active control | 139 | 152 | NA | NA | NA | No | Yes |
| 32 | NR | United States | Tulathromycin | 10 | 48 | Non-active control | 14 | 48 | NA | NA | NA | Yes | Yes |
| 32 | NR | United States | Tulathromycin | 14 | 44 | Non-active control | 23 | 44 | Ceftiofur Sodium (3 mg/kg for 3 days) | 9 | 44 | Yes | Yes |
| 32 | NR | United States | Tulathromycin | 17 | 48 | Non-active control | 35 | 49 | Ceftiofur Sodium (3 mg/kg for 3 days) | 29 | 47 | Yes | Yes |
| 32 | NR | United States | Tulathromycin | 9 | 48 | Non-active control | 29 | 48 | Ceftiofur Sodium (3 mg/kg for 3 days) | 11 | 48 | Yes | Yes |
| 32 | NR | United States | Tulathromycin | 9 | 48 | Non-active control | 25 | 48 | Ceftiofur Sodium (3 mg/kg for 3 days) | 20 | 48 | Yes | Yes |
| 32 | NR | Canada | Tulathromycin | 18 | 30 | Non-active control | 17 | 30 | NA | NA | NA | Yes | Yes |
| 32 | NR | France | Tulathromycin | 4 | 40 | Florfenicol | 1 | 20 | NA | NA | NA | Yes | Yes |
| 32 | NR | Germany | Tulathromycin | 8 | 78 | Tiamulin | 7 | 39 | NA | NA | NA | Yes | Yes |
| 32 | NR | The Netherlands | Tulathromycin | 13 | 44 | Tiamulin | 13 | 22 | NA | NA | NA | Yes | Yes |
| 32 | NR | United Kingdom | Tulathromycin | 17 | 41 | Tiamulin | 13 | 20 | NA | NA | NA | Yes | Yes |
| 32 | NR | United Kingdom | Tulathromycin | 1 | 37 | Tiamulin | 3 | 16 | NA | NA | NA | Yes | Yes |
| 33 | NR | Canada | Florfenicol | 19 | 71 | Non-active control | 25 | 42 | NA | NA | NA | Yes | Yes |
| 34 | 2009 | United States | Tildipirosin | 155 | 434 | Non-active control | 261 | 434 | Tulathromycin | 155 | 535 | No | Yes |
| 37 | 1992 | Korea | Ceftiofur Sodium (3 mg/kg for 3 days) | 6 | 30 | Ceftiofur Sodium (2 mg/kg for 3 days) | 35 | 60 | NA | NA | NA | No | No |
| 38 | 2013-2014 | Germany, Hungary | Marbofloxacin (8 mg/kg once) | 22 | 121 | Enrofloxacin (7.5 mg/kg once or twice) | 22 | 118 | NA | NA | NA | Yes | Yes |
*The event is first-treatment failure . NA = not applicable; NR = not reported; CFA = crystalline free acid; HCl = hydrochloride.
Summary of network geometry
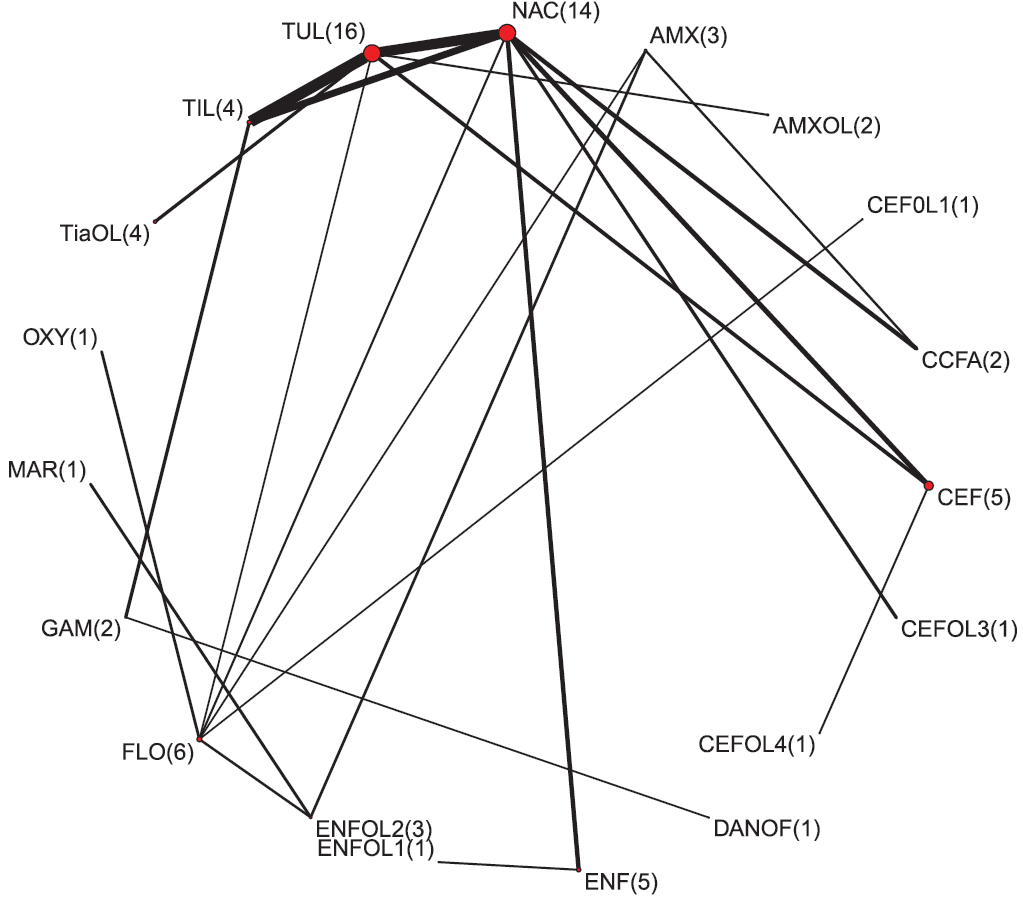
The geometry of the network was sparse, with most regimens being assessed only once. The network would be considered quite diverse as measured by the PIE index (0.79). A PIE index > 0.75 often indicates the network was quite diverse.5 This result is consistent with the visual examination of the network which includes a large number of treatments (Figure 2). However, this analysis can only consider the treatments included in the analysis, the diversity of which is bolstered by treatments not relevant to the review. Further, no studies were found for 5 of the 17 antibiotic regimens identified as relevant to the review in the protocol (Table 1). Therefore, the real diversity was considered to be lower than the PIE suggested, as it includes non-relevant regimens. However, the regimens for which data were available were likely of greatest interest to producers and those regimens for which no reports were found are likely of less interest. The C-score was 10.11 and the C-score test had a large P value (P = .55). These metrics seek to evaluate how random encounters occur in ecological populations and, when used in a network meta-analysis, they assess if there are particular pairwise comparisons that occur more or less often than expected by random encounter. Although the results of hypothesis testing suggest little evidence of non-random pairs, visual examination of the network does suggest pairwise comparisons used in the network are not random, with a strong preference for comparisons with placebo-controlled trial arms.
Figure 2: The network of treatment arms used in the mixed-treatment comparison meta-analysis. The size of the dot is a relative indicator of the number of arms and the width of the lines is a relative indicator of the number of indirect comparisons. The number of study arms reporting the injectable antibiotic regimen is presented in parentheses. Antibiotic regimen abbreviation definitions are listed in Table 1.
Study characteristics and study results
The descriptive information for the studies included in the meta-analysis is provided in Table 2. As the population definition was quite narrow, that information is not presented due to space limitations. The definitions of SRD (SM2: Table S3) and treatment success (SM2: Table S4) are presented in the supplementary materials. Studies varied in how success or failure was defined. Interestingly most studies tended to report metrics of success, and this differs from a review of bovine respiratory disease where most studies tended to define the outcome based on failure, ie, first-treatment failure risk.
Individual risk of bias
For each study eligible for the review, the risk-of-bias judgment for each bias domain is presented in Table 3. The impact of modification on the risk of bias due to allocation can be seen. As no studies reported using allocation concealment, the original schema would have resulted in all studies being classified as high risk of bias for this domain. As the Cochrane ROB tool assigns the highest risk of bias across the domains to the report, then all reports would have been given an overall high risk of bias. Based on the change, some studies, generally those conducted for regulatory purposes and those reporting using Good Clinical Practices, are at low risk of bias. However, because the Cochrane ROB tool was modified, an overall ROB was not explicitly provided.
Table 3: Risk of Bias for all 25 relevant studies identified in the systematic review7
| Reference number | SQ 1.1* | SQ 1.2† | SQ 1.3‡ | Original ROB1§ | Modified ROB1¶ | ROB2** | ROB3†† | ROB4‡‡ | ROB5§§ |
|---|---|---|---|---|---|---|---|---|---|
| 14 | Probably yes | Probably no | Probably no | High | Low | Low | Low | Low | Low |
| 15 | Probably yes | Probably no | Probably no | High | Low | Low | Low | Low | Low |
| 16 | Probably yes | Probably no | Probably no | High | Low | Low | Concerns | Low | Low |
| 17 | Probably yes | Probably no | Probably no | High | Low | Low | Low | Low | Low |
| 18 | No information | Probably no | No information | High | High | Low | Concerns | Low | Concerns |
| 19 | Probably yes | Probably no | No information | High | Concerns | Low | Low | Low | Low |
| 20 | No information | Probably no | No information | High | High | Low | Low | High | High |
| 21 | No information | Probably no | No information | High | High | Low | Concerns | Low | Concerns |
| 22 | No information | Probably no | No information | High | High | Low | High | Low | Concerns |
| 23 | No information | Probably no | Probably no | High | Concerns | Low | Concerns | Low | Concerns |
| 24 | Probably yes | Probably no | No information | High | Concerns | Low | Low | Low | Low |
| 25 | Probably yes | Probably no | Probably no | High | Low | Low | Concerns | Low | Low |
| 26 | No information | Probably o | No | High | Concerns | Low | Concerns | Low | Concerns |
| 27 | No information | Probably no | No information | High | High | Low | Concerns | Low | Concerns |
| 28 | No information | Probably no | No information | High | High | Low | Low | Concerns | Concerns |
| 29 | No information | Probably no | No information | High | High | Low | Concerns | Low | Concerns |
| 30 | No information | Probably no | No information | High | High | Low | Low | Low | Concerns |
| 31 | No information | Probably no | No information | High | High | Low | Concerns | Low | Concerns |
| 32 | No information | Probably no | No information | High | High | Low | Low | Low | Concerns |
| 33 | Probably no | Probably no | No information | High | High | Concerns | Concerns | Low | Concerns |
| 34 | No information | Probably no | No information | High | High | Low | Concerns | Low | Concerns |
| 35 | No information | Probably no | No information | High | High | Low | Low | Low | Concerns |
| 36 | No information | Probably no | No | High | Concerns | Low | Concerns | Low | Concerns |
| 37 | No information | Probably no | No information | High | High | Low | High | Concerns | Concerns |
| 38 | Probably yes | Probably yes | No | Low | Low | Low | Low | Low | Low |
* Was the allocation sequence random?
† Was the allocation sequence concealed until participants were recruited and assigned to interventions?
‡ Were there baseline imbalances that suggest a problem with the randomization process?
§ Risk of bias due to randomization process.
¶ In ROB 2.0, any study that did not report allocation concealment was automatically at high risk of bias, however this item was not considered in the overall assessment of bias due to randomization.
** Risk of bias due to deviations from intended interventions.
†† Risk of bias due to missing outcome data.
‡‡ Risk of bias in the measurement of the outcome.
§§ Risk of bias in selection of the reported results.
SQ = signaling question; ROB = risk of bias.
Individual study results
The individual results for studies included in the final meta-analysis are reported in Table 2.
Synthesis of results
The final meta-analysis included results from 34 of the 41 relevant studies. For the final model, the deviance was 80, while the number of data points was 73, suggesting reasonable fit of the model as the deviance should be close to the number of data points. Convergence of the Bayesian model was within normal limits based on visual inspection of trace plots. The results of the model are presented several ways. The estimates of mean rank are provided in Figure 3. This plot only includes label-dose regimens, ie, those identified in the protocol a priori. The rankings for all regimens used in the meta-analysis, including off-label regimens, are provided in Table 4. Off-label regimens were excluded from Figure 3 to avoid the perception of promoting the use of off-label regimens. However, for transparency of the results, the ranks for all regimens in the meta-analysis are presented in the tables, knowing that most people will rely upon the figures for the results. Lower rankings are associated with fewer treatment failures. Not surprisingly, there is considerable overlap of confidence intervals of the rankings. This reflects the small number of studies informing some ranking estimates and the variation in observed results reported in the primary research. For example, marbofloxacin had a high level of efficacy. However, without more publicly available studies, the result remains a single, potentially random observation, and therefore the point estimate is tempered by the measures of uncertainty. Table 4 also shows that the other ceftiofur regimens were clustered together with mid-level rankings at best, which supports the decision to remove the inconsistent study.35 The distribution of probability of treatment response for the label-dose protocols are presented in the supplementary materials (SM2: Table S5 and Figure S1). The top 4 model-estimated SRD treatments based on the mean rank were the enrofloxacin (7.5 mg/kg once or 2.5-5 mg/kg once daily for 3-5 days; n = 5; rank = 2; 95% CI, 1-4), gamithromycin (6 mg/kg once; n = 2; rank = 5; 95% CI, 1-14), marbofloxacin (8 mg/kg once; n = 1; rank = 6; 95% CI, 1-16) and florfenicol (15 mg/kg twice 48 hours apart; n = 6; rank = 7; 95% CI, 3-13).
Figure 3: The ranking plot of relevant treatments. A ranking of 1 has the lowest treatment failure risk and 19 has the highest treatment failure risk. Ranking means (2.5 % lower limit of CI, 97.5% upper limit of CI) are reported for registered antibiotic regimens only. The number of study arms are presented in parentheses for each injectable antibiotic regimen reported. Antibiotic regimen abbreviation definitions are listed in Table 1.
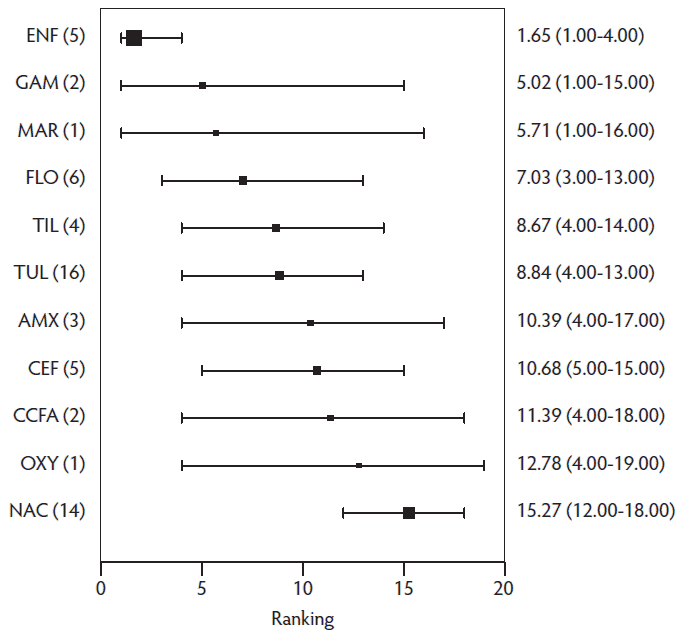
Table 4: Mean ranking for treatment efficacy for antibiotic regimens for SRD based on mixed-treatment comparison meta-analysis.
| Treatment arm | Ranking,* mean (SD) | 95% Credible Interval and median rank | ||
|---|---|---|---|---|
| 2.50% | 50% | 97.5% | ||
| Enrofloxacin | 1.65 (1.01) | 1 | 1 | 4 |
| Gamithromycin | 4.82 (3.53) | 1 | 4 | 14 |
| Enrofloxacin (7.5 mg/kg once or twice) | 5.34 (3.15) | 1 | 5 | 13 |
| Enrofloxacin (2.5 mg/kg 3 days) | 5.45 (3.73) | 1 | 4 | 15 |
| Marbofloxacin | 5.76 (4.27) | 1 | 4 | 16 |
| Florfenicol | 7.06 (2.76) | 3 | 7 | 13 |
| Danofloxacin (1.25 or 2.5 mg/kg once) | 8.42 (5.45) | 1 | 7 | 19 |
| Tildipirosin | 8.68 (2.92) | 4 | 9 | 14 |
| Tulathromycin | 8.83 (2.32) | 4 | 9 | 13 |
| Amoxicillin | 10.44 (3.69) | 4 | 11 | 17 |
| Amoxicillin/clavulanic acid (7.0/1.75 mg/kg 3 days) | 10.45 (4.09) | 3 | 11 | 18 |
| Ceftiofur (MD) | 10.69 (2.77) | 5 | 11 | 15 |
| Ceftiofur CFA | 11.41 (3.53) | 4 | 12 | 17 |
| Oxytetracycline | 12.80 (4.26) | 4 | 14 | 19 |
| Ceftiofur HCl (5 mg/kg once) | 14.84 (3.55) | 5 | 16 | 19 |
| Ceftiofur (HCl or NA) | 15.07 (4.75) | 3 | 17 | 19 |
| Non-active control | 15.27 (1.59) | 12 | 15 | 18 |
| Tiamulin | 15.44 (2.43) | 10 | 16 | 19 |
| Ceftiofur NA (1-2 mg/kg 3 days) | 17.57 (2.31) | 11 | 18 | 19 |
* A ranking of 1 has the lowest treatment failure risk and 19 has the highest treatment failure risk. Rankings are reported for all regimens included in the meta-analysis.
MD = Multidose; CFA = crystalline free acid; HCl = hydrochloride; NA = sodium.
Table 5 provides the comparative RRs for only the label-dose regimens, ie, those identified in the protocol a priori. The data are organized such that the event is the risk of treatment failure for the treatment in the row divided by the risk of treatment failure in the column. For example, in the first row of the table, all the RR estimates are greater than one, meaning that the risk of treatment failure was higher in the non-active control groups when compared to all other antibiotics. The upper right-hand quadrant reports the estimated RR and the lower quadrant reports the 95% CI. The risk of treatment failure was 16-fold higher for untreated animals compared to enrofloxacin (RR = 16; 95% CI, 4-48). Only 3 antibiotics did not have a credible interval that excluded one when compared to non-active control: oxytetracycline, amoxicillin, and marbofloxacin. Given the point estimate and mean rank for marbofloxacin, this finding is likely a function of identification of only one publicly available study reporting the efficacy of marbofloxacin.
Table 5: Risk ratio of all possible comparisons within the evidence network. The upper right-hand quadrant represents the estimated risk ratio and the lower quadrant represents the 95% CI. Risk ratios are reported for registered antibiotic regimens only.
| NAC | 2.46 | 1.92 | 1.88 | 16.46 | 3.53 | 8.86 | 9.42 | 1.95 | 2.54 | 2.30 |
| (0.65-8.01) | AMX | 1.04 | 1.16 | 10.15 | 1.76 | 5.32 | 3.89 | 0.99 | 1.55 | 1.40 |
| (0.69-5.32) | (0.22-3) | CCFA | 1.28 | 11.07 | 2.24 | 5.92 | 5.37 | 1.24 | 1.71 | 1.55 |
| (1-3.65) | (0.21-3.45) | (0.31-3.4) | CEF | 9.45 | 2.03 | 4.94 | 5.40 | 1.13 | 1.44 | 1.30 |
| (4.17-43.18) | (1.54-37.44) | (2.1-36.28) | (2.59-26.95) | ENF | 0.27 | 0.67 | 0.67 | 0.15 | 0.20 | 0.18 |
| (1.09-9.96) | (0.52-4.45) | (0.49-6.94) | (0.52-5.92) | (0.05-0.82) | FLO | 3.26 | 2.55 | 0.57 | 0.96 | 0.87 |
| (1.02-40.76) | (0.34-27.1) | (0.46-28.95) | (0.54-22.05) | (0.05-3.1) | (0.25-15.78) | GAM | 3.67 | 0.56 | 0.59 | 0.60 |
| (0.77-46.01) | (0.49-15.93) | (0.41-26.91) | (0.37-26.55) | (0.04-3.29) | (0.28-11.47) | (0.06-13.97) | MAR | 0.56 | 0.87 | 0.79 |
| (0.39-7.36) | (0.15-3.44) | (0.16-4.7) | (0.17-4.3) | (0.02-0.58) | (0.14-1.56) | (0.02-2.53) | (0.03-2.35) | OXY | 2.35 | 2.11 |
| (1.07-5.75) | (0.26-4.97) | (0.36-4.99) | (0.52-3.38) | (0.04-0.53) | (0.2-2.74) | (0.08-1.87) | (0.05-3.64) | (0.29-8.57) | TIL | 1.02 |
| (1.24-4) | (0.28-4.06) | (0.4-3.92) | (0.65-2.33) | (0.05-0.44) | (0.22-2.13) | (0.06-2.06) | (0.05-3.17) | (0.3-6.98) | (0.46-1.92) | TUL |
Antibiotic regimen abbreviation definitions are listed in Table 1.
Exploration of inconsistency
The consistency between the direct and indirect sources of evidence of the final model using 34 trials and 73 arms is reported in Table 6. In this model, no evidence of inconsistency was found between the direct and indirect estimates. However, this should not be interpreted as proof that inconsistency does not exist. The small number of studies available means that the precision of direct estimates is low (ie, wide credible intervals) making it difficult to detect differences in direct and indirect estimates.
Table 6: Results of the indirect comparison for the consistency assumption.
| Comparison* | Dir, d (SD)† | MTC, d (SD)‡ | Rest, d (SD)§ | w (SD) | P value¶ |
|---|---|---|---|---|---|
| Enrofloxacin vs Enrofloxacin (2.5 mg/kg 3 days) | 1.16 (2.91) | 1.17 (0.80) | 1.17 (0.84) | -0.01 (3.03) | 1.00 |
| Enrofloxacin (7.5 mg/kg once or twice) vs Marbofloxacin | -0.04 (2.90) | -0.03 (1.06) | -0.02 (1.14) | -0.01 (3.11) | 1.00 |
| Enrofloxacin (7.5 mg/kg once or twice) vs Amoxicillin | -0.55 (2.94) | 0.96 (0.84) | 1.09 (0.88) | -1.64 (3.07) | 0.59 |
| Florfenicol vs Enrofloxacin (7.5 mg/kg once or twice) | 1.37 (3.03) | -0.43 (0.79) | -0.57 (0.82) | 1.93 (3.14) | 0.54 |
| Florfenicol vs Tulathromycin | 1.18 (3.17) | 0.33 (0.53) | 0.31 (0.54) | 0.87 (3.21) | 0.79 |
| Oxytetracycline vs Florfenicol | 1.00 (2.90) | -1.00 (0.86) | -1.19 (0.9) | 2.19 (3.04) | 0.47 |
| Tiamulin vs Tulathromycin | -1.16 (0.66) | -1.13 (0.48) | -1.1 (0.69) | -0.06 (0.95) | 0.95 |
| Tildipirosin vs Gamithromycin | 0.87 (2.93) | -0.92 (0.87) | -1.1 (0.91) | 1.97 (3.07) | 0.52 |
| Tulathromycin vs Tildipirosin | 0.04 (0.65) | -0.05 (0.41) | -0.11 (0.53) | 0.15 (0.84) | 0.86 |
| Tulathromycin vs Amoxicillin/clavulanic acid (7.0/1.75 mg/kg 3 days) | -0.31 (1.69) | 0.24 (0.64) | 0.33 (0.69) | -0.64 (1.83) | 0.73 |
| Non-active control vs Enrofloxacin | -3.73 (1.50) | -3.04 (0.48) | -2.96 (0.5) | -0.76 (1.58) | 0.63 |
| Non-active control vs Florfenicol | -1.39 (2.95) | -1.35 (0.62) | -1.35 (0.63) | -0.04 (3.02) | 0.99 |
| Non-active control vs Tildipirosin | -0.99 (2.88) | -1.07 (0.47) | -1.07 (0.48) | 0.08 (2.92) | 0.98 |
| Non-active control vs Tulathromycin | -1.05 (0.31) | -1.02 (0.29) | -0.82 (0.78) | -0.24 (0.84) | 0.78 |
| Non-active control vs Ceftiofur CFA | -0.44 (2.90) | -0.65 (0.64) | -0.66 (0.65) | 0.22 (2.97) | 0.94 |
| Non-active control vs Ceftiofur (MD) | -1.00 (0.65) | -0.76 (0.38) | -0.64 (0.47) | -0.36 (0.8) | 0.65 |
| Non-active control vs Ceftiofur HCl (5 mg/kg once) | 0.11 (2.95) | 0.08 (0.79) | 0.08 (0.82) | 0.03 (3.06) | 0.99 |
| Amoxicillin vs Florfenicol | -0.75 (2.96) | -0.52 (0.71) | -0.51 (0.73) | -0.25 (3.05) | 0.94 |
| Ceftiofur (HCl or NA) vs Florfenicol | -1.93 (3.22) | -1.92 (1.47) | -1.92 (1.66) | -0.01 (3.62) | 1.00 |
| Ceftiofur CFA vs Amoxicillin | -0.35 (3.01) | -0.19 (0.72) | -0.18 (0.74) | -0.17 (3.1) | 0.96 |
| Ceftiofur (MD) vs Tulathromycin | -0.49 (0.79) | -0.26 (0.34) | -0.21 (0.38) | -0.28 (0.87) | 0.75 |
| Ceftiofur NA (1-2 mg/kg 3 days) vs Ceftiofur NA (1-2 mg/kg 3 days) | 1.07 (2.93) | 0.00 (0.94) | -0.12 (1) | 1.19 (3.09) | 0.70 |
| Danofloxacin (1.25 or 2.5 mg/kg once) vs Gamithromycin | -0.68 (2.90) | -0.71 (1.23) | -0.72 (1.35) | 0.04 (3.2) | 0.99 |
* The first treatment listed is the reference (denominator) and the second treatment listed is the comparator (numerator).
† Posterior mean (d) and SD of log-odds ratio of treatment effects calculated using direct evidence only.
‡ Posterior mean (d) and SD of log-odds ratio of treatment effects calculated using all the evidence.
§ Posterior mean (d) and SD of log-odds ratio of treatment effects calculated using indirect evidence only.
¶ The Z distribution test was used.
Dir = direct evidence; d = posterior mean; MTC = all evidence; rest = indirect evidence; w = inconsistency estimate; CFA = crystalline free acid;
MD = multidose; HCl = hydrochloride; NA = sodium.
Assessing sources of systematic bias
The beta for the sponsorship indicator variable was -0.08 (95% CI, -1.39 to 1.32), while βrandomization = -4.27 (95% CI, -18.59 to 10), and βblinding = -1.13 (95% CI, -4.47 to 1.14). These results do not suggest systematic bias in either direction thus they were not included in the final network meta-analysis model.
Risk of bias across studies
Risk of bias across studies, such as looking for evidence of small-studies effect, was not assessed because the number of individual studies available for assessment within each treatment and pairwise comparison was low.
Limitations
The major limitation of this review is the paucity of data available for inclusion in the review. Although SRD is an important disease, it is surprising that only 41 publicly available studies could be identified for inclusion in the review and data from only 34 studies could be included in the meta-analysis. If company websites had been included as a source of evidence, more studies might have been identified. Such sites were not included because they are not a time-stamped source and, therefore, not a reproducible source of data. After a review is published, relevant studies can be added to or removed from company websites without traceable documentation. This is not possible with conference proceedings and journals indexed in the SIL or CABI. Another aspect of the scientific literature in this body of work that should be addressed is the poor reporting associated with conference proceedings. As reported previously, many studies in swine production are not published in peer-reviewed journals.39 Therefore, the studies in conference proceedings are a vital resource for practitioners and research synthesis. Further, conference proceedings are not subjected to peer review and authors are not required to indicate if the findings presented are the final results, which has the potential to increase favorable findings.
Another possible concern is the potential omission of antibiotic regimens of interest. A post hoc evaluation by the sponsor designate of possible SRD antibiotics did identify several registered antibiotic regimens in Europe that were not included in the protocol. For completeness, we re-assessed if studies excluded at level 2, because they were considered to have not used a relevant regimen, used these European-registered regimens. One study featured a treatment arm with oxytetracycline given at a dose of 20 mg/kg.40 If the pigs were still sick 48 h after the first injection, they were given a second injection at the same dose. Injecting twice at this dose is not a registered use in the United States. The results for this arm were presented without distinguishing which pigs received 1 vs 2 injections and, therefore, this study would not have been eligible for the review. A second study included one treatment arm with amoxicillin at 7 mg/kg for 3 or 5 days (treatment was only given for 5 days if pigs were still sick at that point).41 The outcome reported was cure risk by day 5. The other treatment arm received marbofloxacin at 2mg/kg once daily for 3 to 5 days rather than 3 days, which was the regimen of interest in the protocol. The combined registered (2mg/kg once daily for 3 days) and unregistered (2mg/kg once daily for 5 days) marbofloxacin dose regimen was the rationale for exclusion. The amoxicillin regimen was not identified a priori as a regimen of interest, although it is registered in Europe. If either regimen had been of interest, the results of the study could not have been included in the meta-analysis because neither treatment arm linked to the rest of the evidence network, ie, both arms were unique treatment regimens. As these are post-hoc regimens introduced for discussion and transparency, these studies are not included in the PRISMA diagram (Figure 1).
Another possible concern is the impact of the funding source on the meta-analysis. The highest-ranked product found by the review is owned by the sponsoring company. However, the data informing the review are publicly available data and are verifiable even though the company likely has additional data that could further narrow the 95% CI. Therefore, the authors propose that others using the same criteria would reach the same conclusion. To further address this concern several steps were taken: 1) a time-stamped a priori protocol was created and followed with no deviations from the protocol, 2) the role of the sponsor designate was transparently reported and documented, and 3) once the protocol was time-stamped the sponsor designate was not responsible for the steps of the review from the search to the first draft of the full manuscript. Once the first draft was written, no further analyses were conducted, and the sponsor designate was only able to contribute to the interpretation and discussion.
It is important to recognize that a systematic review is neither a formal guideline for clinical use nor a recommendation for use. Inference is limited to the review question, which was comparative efficacy, whereas guidelines for clinical use should consider multiple factors. Comparative efficacy is only one dimension that should be considered when selecting an antibiotic. Other dimensions should include the spectrum (broad or narrow) of antibiotic, the sensitivity and specificity of the diagnosis of SRD, the organism likely to be involved based on the veterinarian’s knowledge of the farm where the animals are raised, and guidelines from leading agencies about antibiotic stewardship in swine production.
Implications
- The results of network meta-analysis can provide information about the comparative efficacy of antibiotics when primary studies of active-to-active trials are missing. This gives producers and veterinarians information that might overwise not be available.
- The network used was reasonably small due to an absence of publicly indexed data; however, the estimates suggest that the top 4 model-estimated SRD treatments based on the mean rank were enrofloxacin (7.5 mg/kg once or 2.5-5 mg/kg once daily for 3-5 days; n = 5; rank = 2; 95% CI, 1-4), gamithromycin (6 mg/kg once, n = 2; rank = 5; 95% CI, 1-14), marbofloxacin (8 mg/kg once, n = 1; rank = 6; 95% CI, 1-16), and florfenicol (15 mg/kg twice 48 hours apart, n = 6; rank = 7; 95% CI, 3-13).
- Producers would have greater confidence in the comparable efficacy of products available if more, better-reported trial results were available in publicly indexed locations.
- With respect to antibiotic choices, comparative efficacy is only one metric that should be considered when selecting an antibiotic. Other metrics should include the antibiotic spectrum (broad or narrow), the organism likely to be involved based on the veterinarian’s knowledge of the system the animals are raised in, and guidelines from leading agencies about appropriate antibiotic stewardship in swine production.
Acknowledgments
Authorship roles
Dr O’Connor developed the review protocol, coordinated the project team, conducted relevance screening, extracted data, conducted the data analysis, interpreted the results, and wrote the first manuscript draft. Dr Totton assisted with development of the protocol, conducted relevance screening, extracted data, provided guidance for the interpretation of results, commented on manuscript drafts, and approved the final manuscript version. Dr Shane conceived of the project question and the eligibility criteria, assisted with identifying relevant information sources, assisted with designing the terms used in the search strategy, approved the review protocol, and contributed to the interpretation of the results and conclusions. Dr Shane did not participate in relevance screening of retrieved records, extraction of data, or conduct of the data analysis.
Publication declaration
The authors declare that this is a full and accurate description of the project and no important information or analyses are omitted.
Sponsorship
This study was funded by Bayer Animal Health. The sponsor and sponsor designate had a role in developing the protocol to ensure that the review studied the target swine populations, interventions, outcomes, and study designs of interest. It was determined a priori (SM1: Protocol) that during the review process, if needed, the sponsor designate would provide feedback about potentially relevant studies only when the 2 main reviewers were in conflict about eligibility. The sponsor designate was otherwise not involved in eligibility assessment of citations retrieved by the search, data extraction, or conduct of the analysis. The first draft of the results was provided for the sponsor designate’s interpretation of the results and the discussion. It was decided a priori (SM1: Protocol) that the sponsor had a role in developing the protocol, providing feedback on the draft of the discussion and conclusions, and, consistent with standards for authorship, should be listed as a co-author on any publications and conflicts of interest noted.
Conflict of interest
Drs O’Connor and Totton were funded by Bayer Animal Health for the conduct of the review. Dr Shane is the sponsor designate and employed by Bayer Animal Health.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. O’Connor AM, Coetzee JF, da Silva N, Wang C. A mixed treatment comparison meta-analysis of antibiotic treatments for bovine respiratory disease. Prev Vet Med. 2013;110(2):77-87.
2. O’Connor AM, Yuan C, Cullen JN, Coetzee JF, da Silva N, Wang C. A mixed treatment meta-analysis of antibiotic treatment options for bovine respiratory disease – An update. Prev Vet Med. 2016;132:130-139.
3. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784.
4. von Elm E, Poglia G, Walder B, Tramer MR. Different patterns of duplicate publication: an analysis of articles used in systematic reviews. JAMA. 2004;291(8):974-980.
5. Salanti G, Kavvoura FK, Ioannidis JP. Exploring the geometry of treatment networks. Ann Intern Med. 2008;148(7):544-553.
*6. EcoSim: Null models software for ecology [computer program]. Version 0.1.0. Gotelli NJ, Ellison AM; 2015. http://www.uvm.edu/~ngotelli/EcoSim/EcoSim.html.
*7. Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, Reeves B, Eldridge S. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016;10(Suppl 1):29-31.
8. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7-8):932-944.
9. Higgins JP, Whitehead A. Borrowing strength from external trials in a meta-analysis. Stat Med. 1996;15(24):2733-2749.
10. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105-3124.
*11. Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 2: A generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. London, UK: National Institute for Health and Clinical Excellence; 2011. https://www.ncbi.nlm.nih.gov/books/NBK310366/. Updated April 2014. Accessed January 2018.
*12. R: A Language and Environment for Statistical Computing [computer program]. Version 3.2.1. Vienna, Austria: R Foundation for Statistical Computing; 2015. https://www.R-project.org/.
*13. Package ‘rjags’ [computer program]. Version 4.3. Plummer, M: The comprehensive R archive network; 2017. http://CRAN.R-project.org/package=rjags.
*14. Bayer HealthCare LLC Animal Health Division. Baytril 100 Enrofloxacin injectable solution swine for the treatment and control of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, and Streptococcus suis. https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/605. NADA 141-068 FOI Summary Supplemental New Animal Drug Application. Approved March 14, 2008. Accessed October 2017.
*15. Bayer HealthCare LLC Animal Health Division. Baytril 100 injectable solution, Enrofloxacin swine. For the treatment and control of swine respiratory disease (SRD) associated with Bordetella bronchiseptica and Mycoplasma hyopneumoniae. https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/608. NADA 141-068 FOI Summary Supplemental New Animal Drug Application. Approved November 21, 2012. Accessed October 2017.
*16. The Upjohn Company. NAXCEL® Sterile Powder. https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/1716. NADA 140-388 FOI Summary Supplemental New Animal Drug Application. Approved August 4, 1992. Accessed October 2017.
*17. Zoetis Inc (Original sponsor: Pharmacia & Upjohn Company A Division of Pfizer Inc). EXCEDE for swine (ceftiofur crystalline free acid) sterile suspension for the treatment of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, and Streptococcus suis. https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/776. NADA 141-235 FOI Summary Supplemental New Animal Drug Application. Approved June 18, 2004. Accessed October 2017.
*18. Glattleider D, Bossow H, Sommer J, Laval A, Abric JL, Goossens L, Lockwood PW. Comparative efficacy of florfenicol (FFC) and oxytetracycline LA (OTC LA) in the treatment of naturally occurring swine respiratory diseases in Europe. Proc IPVS. Melbourne, Australia. 2000:107.
*19. Schmitt EJ, Bryson WL, Van Oye SN, Lens ST, Crane JP. Efficacy of ceftiofur crystalline free acid for the treatment of swine respiratory disease under European field conditions. Proc IPVS. Ames, IA 2002. Abstract P484.
*20. Terreni M, Guazzetti S, Nisoli L, Martelli P. Efficacy of enrofloxacin (BAYTRIL 5%®) at two different dosages schemes in the treatment of swine acute respiratory disease. Proc IPVS. Hamburg, Germany. 2004:181.
*21. Collell M, Menjón R, Bollo J, Calvo H, Tejedor M. Comparison of the clinical efficacy of florfenicol vs amoxicillin for the treatment of an acute outbreak of Actinobacillus pleuropneumoniae in swine. Proc IPVS. Durban, South Africa. 2008. Abstract P03.008.
*22. Keane CJ, Braun G, Siciliano S, Hellmann K, Godinho KS. Use of tulathromycin (Draxxin® injectable solution) in the treatment, and prevention in swine at risk of developing respiratory disease, associated with Haemophilus parasuis and Bordetella bronchiseptica. Proc IPVS. Durban, South Africa. 2008. Abstract P05.018.
*23. Bringas J, Bollo JM, Menjón R, Calvo E. Clinical efficacy of two injectable treatments for an acute respiratory outbreak in swine: Florfenicol (Nuflor swine injectable®) and ceftiofur. Proc IPVS. Durban, South Africa. 2008. Abstract P05.029.
*24. Daube GW, Radeloff I, Braun G, Hellmann K, Stephan B. Efficacy and safety of Baytril® Max 10% injectable in the treatment of naturally occuring porcine respiratory disease. Proc IPVS. Jeju, Korea. 2012:789.
*25. Petersen I, Zschiesche E, Wolf O, Rose M. Multi-center field study on the therapeutic efficacy of Zuprevo® (tildipirosin), a novel macrolide, for the management of natural outbreaks of swine respiratory disease in Europe. Proc IPVS. Jeju, Korea. 2012:790.
*26. Moyaert H, Palzer A, Noé L, Liu B, Stegemann M. Efficacy of tulathromycin (DRAXXIN 25 mg/mL) for the treatment of swine respiratory disease associated with B. bronchiseptica. Proc IPVS. Cancun, Mexico. 2014:10.
*27. Kondo Y, Nakanishi N, Wakui Y, Richard-Mazet A, Kinoshita G, Jeannin P. Therapeutic efficacy of ZACTRAN® (gamithromycin injectable solution) against swine respiratory disease in a field trial in Japan. Proc IPVS. Dublin, Ireland. 2016:822.
*28. Richard-Mazet A, Dietmar H, Voisin F, Bohne I, Fraisse F, Winter R, Dumont P, Jeannin P. Therapeutic efficacy of ZACTRAN® (gamithromycin) against swine respiratory disease in a multi-center field trial in Europe. Proc IPVS. Dublin, Ireland. 2016:833.
*29. Jackson J, Rodibaugh M, Harker J, Bales S, Katz T, Lockwood P. Comparative efficacies of florfenicol and ceftiofur in the treatment of naturally occurring swine respiratory disease. Proc AASV. Orlando, FL. 1999:213.
*30. Meeuwse D, Kausche F, Bryson L, Dame K. Efficacy of a single intramuscular dose of ceftiofur hydrochloride (Excenel® RTU) at 5mg ceftiofur equivalents/kg body weight for the treatment of naturally occurring bacterial swine respiratory disease. Proc AASV. Kansas City, MO. 2002:203.
*31. Zolynas R, Cao J, Simmons R. Evaluation of the efficacy and safety of Nuflor® injectable solution (15 mg/kg twice 48 hours apart) in the treatment of swine respiratory disease (SRD). Proc AASV. Orlando, FL. 2003:211-214.
32. Nutsch RG, Hart FJ, Rooney KA, Weigel DJ, Kilgore WR, Skogerboe TL. Efficacy of tulathromycin injectable solution for the treatment of naturally occurring swine respiratory disease. Vet Ther. 2005;6(2):214-224.
*33. Jackson JA, Moore C, Kirkwood R, Cegielski A, Berman R, Machell N, Katz TL, Lockwood PW. Efficacy of florfenicol administered intramuscularly in treating naturally occurring swine respiratory disease in Canada. Proc AASV. Indianapolis, IN. 2000:209.
*34. Kniffen TS, Wray MI. Scientific and practical considerations regarding the use of a novel, injectable anti-infective in swine: Zuprevo® from Merck Animal Health. Proc AASV. Denver, CO. 2012:131-134.
*35. Kubo K, Nakanishi N, Ibayashi T, Sasaki A, Ando N, Gillette GL. A study on the effects of ceftiofur on respiratory diseases in swine. Proc IPVS. Bangkok, Thailand. 1994:179.
*36. Uchida K, Fujii T, Tomiyasu T, Nakamura Y, Yamamoto K. Assessment of the efficacy of danofloxacin, compared to penicillin/streptomycin, for the treatment of acute bacterial pneumonia outbreaks in five Japanese swine herds. Proc IPVS. Bangkok, Thailand. 1994:335.
*37. Kim BH, Chung B. Field efficacy studies of ceftiofur against acute swine bacterial respiratory disease. Proc IPVS. Bangkok, Thailand. 1994:341.
38. Grandemange E, Perrin P-A, Cvejic D, Haas M, Rowan T, Hellmann K. Randomised controlled field study to evaluate the efficacy and clinical safety of a single 8mg/kg injectable dose of marbofloxacin compared with one or two doses of 7.5mg/kg injectable enrofloxacin for the treatment of Actinobacillus pleuropneumoniae infections in growing-fattening pigs in Europe. Porcine Health Manag. 2017;3:10. doi:10.1186/s40813-017-0057-2.
39. Brace S, Taylor D, O’Connor AM. The quality of reporting and publication status of vaccines trials presented at veterinary conferences from 1988 to 2003. Vaccine. 2010;28(32):5306-5314.
40. Hoflack G, Maes D, Mateusen B, Verdonck M, de Kruif A. Efficacy of tilmicosin phosphate (Pulmotil premix) in feed for the treatment of a clinical outbreak of Actinobacillus pleuropneumoniae infection in growing-finishing pigs. J Vet Med B Infect Dis Vet Public Health. 2001;48(9):655-664.
41. Thomas E, Grandemange E, Pommier P, Wessel-Robert S, Davot JL. Field evaluation of efficacy and tolerance of a 2% marbofloxacin injectable solution for the treatment of respiratory disease in fattening pigs. Vet Q. 2000;22(3):131-135.
* Non-refereed references.