| Diagnostic notes | Peer reviewed |
Cite as: Jin LY, Hyoung-Joon M, Bo-Kyu K, et al. In vitro antimicrobial susceptibility of Mycoplasma hyorhinis field isolates collected from swine lung specimens in Korea. J Swine Health Prod. 2014;22(4):193–196.
Also available as a PDF.
SummaryMycoplasma hyorhinis is a very common inhabitant of the respiratory tract of pigs with or without pneumonia. Because there is no vaccine available to control M hyorhinis, chemotherapy is the most practical way to treat disease associated with M hyorhinis infection. Therefore, we tested the antimicrobial susceptibility of M hyorhinis isolates recovered from lung specimens of pigs using the liquid minimum inhibitory concentration (MIC) method in tests with 12 antimicrobial agents. The MIC50, MIC90, and range of MICs against 10 field isolates from Korea and the reference strain (ATCC 17981) were investigated. Mycoplasma hyorhinis field isolates were sensitive to lincomycin and tylosin but resistant to erythromycin, spectinomycin, and streptomycin. The MIC90s for lincomycin and tylosin were 0.5 µg per mL and 1.0 µg per mL, respectively. The MIC90s for amoxicillin, erythromycin, penicillin, spectinomycin, and streptomycin were ≥ 64 µg per mL. The MIC90s for gentamicin, kanamycin, and neomycin were 4.0 µg per mL, 8.0 µg per mL and 16 µg per mL, respectively. For oxytetracycline and tetracycline, the MIC50 was 4.0 µg per mL and the MIC90 was 16 µg per mL. These results provide practical information for treatment of M hyorhinis infection in pigs. | ResumenEl Mycoplasma hyorhinis es un habitante muy común del tracto respiratorio de cerdos con o sin neumonía. Debido a que no hay vacuna disponible para controlar el M hyorhinis, la quimioterapia es la manera más práctica para tratar la enfermedad asociada con la infección de M hyorhinis. Por tanto, pusimos a prueba la susceptibilidad antimicrobiana de los aislados del M hyorhinis recuperados de las muestras de pulmón de cerdos utilizando el método de concentración mínima inhibitoria (MIC por sus siglas en inglés) de líquido en pruebas con 12 agentes antimicrobianos. Se investigaron el MIC50, MIC90, y el rango de los MICs contra 10 aislados de campo de Corea y la cepa de referencia (ATCC 17981). Los aislados de campo del M hyorhinis fueron positivos a la lincomicina y a la tilosina pero resistentes a la eritromicina, espectinomicina, y estreptomicina. El MIC90s para la lincomicina y la tilosina fueron de 0.5 µg por mL y 1.0 µg por mL, respectivamente. El MIC90s para la eritromicina, espectinomicina, amoxicilina, penicilina, y estreptomicina fueron ≥ 64 µg por mL. El MIC90s para la gentamicina, kanamicina, y neomicina fueron de 4.0 µg por mL, 8.0 µg por mL, y 16 µg por mL, respectivamente. Para oxitetraciclina y tetraciclina, el MIC50 fue 4.0 µg por mL y el MIC90 fue 16 µg por mL. Estos resultados proveen información práctica para el tratamiento de la infección de M hyorhinis en cerdos. | ResuméMycoplasma hyorhinis est un habitant très fréquent du tractus respiratoire des porcs avec et sans pneumonie. Étant donné qu’il n’y a aucun vaccin disponible pour limiter M hyorhinis, l’utilisation d’antimicrobien est le moyen le plus pratique pour traiter la maladie associée à l’infection par M hyo-rhinis. Ainsi, nous avons testé la sensibilité antimicrobienne d’isolats de M hyorhinis obtenus de spécimens de poumons de porcs en utilisant la méthode de concentration minimale inhibitrice (CMI) en milieu liquide avec 12 agents antimicrobiens. Les CMI50 et CMI90, de même que l’étendue des CMI de 10 isolats de champs provenant de la Corée et de la souche de référence (ATCC 17981) ont été étudiées. Les isolats de champs de M hyorhinis étaient sensibles à la lincomycine et le tylosin mais résistants à l’érythromycine, la spectinomycine, et la streptomycine. Les CMI90 pour la lincomycine et le tylosin étaient respectivement de 0,5 µg par mL et 1,0 µg par mL. Les CMI90 pour l’érythromycine, la spectinomycine, l’amoxicilline, la pénicilline, et la streptomycine étaient ≥ 64 µg par mL. Les CMI90 pour la gentamicine, la kanamycine, et la néomycine étaient respectivement de 4 µg par mL, 8 µg par mL, et 16 µg par mL. Pour la tétracycline et l’oxytétracycline, la CMI50 était de 4 µg par mL et la CMI90 de 16 µg par mL. Ces résultats fournissent des informations pratiques pour le traitement des infections à M hyorhinis chez les porcs. |
Keywords: swine, Mycoplasma hyorhinis, antimicrobial susceptibility, minimum inhibitory concentrations
Search the AASV web site
for pages with similar keywords.
Received: May 16, 2013
Accepted: October 23, 2013
Mycoplasma hyorhinis is a common isolate from the upper respiratory tract and tonsils of pigs exhibiting pleuritis, peritonitis, pericarditis, polyserositis, or polyarthritis.1 However, it may be isolated from swine lungs with or without pneumonia.2,3 Mycoplasma hyorhinis is responsible for considerable economic losses through growth retardation, poor feed conversion, inflammation, immunosuppression, and increased susceptibility to other infectious swine diseases.1 It may occur as a secondary agent associated with both catarrhal bronchopneumonia and interstitial pneumonia.4
Chemotherapy is the most practical way to treat disease associated with M hyorhinis infection, because no vaccine is available. Several studies have been conducted using the broth dilution method to examine the antimicrobial susceptibility of M hyorhinis.3-12 In Korea, M hyorhinis infection is gradually increasing as a secondary infection with porcine reproductive and respiratory syndrome virus, but the antimicrobial susceptibility of M hyorhinis has not been thoroughly investigated. Therefore, we tested the antimicrobial susceptibility of M hyorhinis isolated from pig lung specimens using the liquid minimum inhibitory concentration (MIC) method described by Hannan.11
Isolation and identification of M hyorhinis
A total of 10 M hyorhinis field isolates were tested in this study. Isolates were collected directly from diagnostic swine lung specimens submitted to the Research Unit at Green Cross Veterinary Products Co, Ltd, Yongin, Korea, during 2011 and 2012. No animal-use protocol was necessary because only laboratory specimens were used.
The lung specimens had been collected from 23 weaned pigs, 30 to 70 days old, from nine swine farms. Isolates were cultured in Friis broth and on agar plates,1 then tested for purity as single colonies on agar as follows. The lung specimen was cultured in Friis broth. The multiplex polymerase chain reaction (PCR) method for Mycoplasma hyopneumoniae and M hyorhinis was performed and samples in which M hyorhinis was identified as a single band were inoculated on Friis agar. Single colonies were re-inoculated into Friis broth and PCR was again performed to identify M hyorhinis. Mycoplasma hyorhinis colonies were then passaged 10 times in Friis broth. It was possible to repeatedly passage only 10 isolates in Friis broth and on agar. These 10 isolates were identified using the multiplex PCR method for M hyopneumoniae and M hyorhinis.13 Mycoplasma hyorhinis ATCC 17981, isolated from the nasal cavity of a pig, was used as the reference strain for comparison with field isolates.
Antimicrobial susceptibility testing
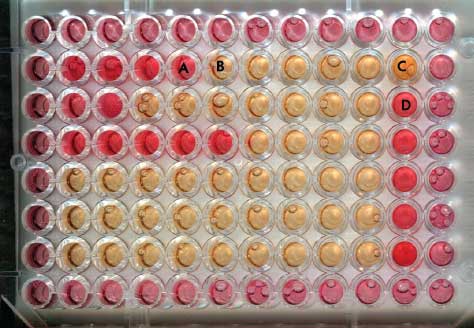
The antimicrobial susceptibility of M hyorhinis was tested by the liquid MIC method,10-12 which is simple to perform and convenient for testing small numbers of isolates.11 The following 12 antimicrobial agents were examined: amoxicillin, erythromycin, gentamicin, kanamycin, lincomycin, neomycin, oxytetracycline, penicillin, spectinomycin, streptomycin, tetracycline, and tylosin (Sigma-Aldrich Co, St Louis, Missouri; Table 1). The inoculum concentration of M hyorhinis field isolates used in the MICs was determined by serial tenfold dilutions in Friis broth. Specifically, the lowest dilution to show a color change (red to yellow) denoted the reciprocal number of color changing units (CCU), and the inoculum standard number of organisms was 103 CCU per mL. A final volume of 100 µL of each antimicrobial agent was prepared by serial twofold dilutions (64 to 0.25 µg per mL) in sterile distilled water in a 96-well microplate. The same volume of isolate culture (103 CCU per mL) was inoculated into each well of plates containing diluted antimicrobials. Each plate contained uninoculated Friis broth as a sterility control and drug-free inoculum as a growth control. Plates were sealed, incubated at 37°C for 5 to 7 days, and observed daily until color changes in the wells were complete. The value of the MIC was defined as the lowest antimicrobial concentration to inhibit color change when the growth control changed from red to yellow (Figure 1).
Table 1: Description of the minimum inhibitory concentrations (MICs) for antimicrobial agents tested against Mycoplasma hyorhinis field isolates and a reference strain*
| Antimicrobial | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| Field strains (n = 10) | Reference strain | ||||
| Class | Agent | MIC50† | MIC90‡ | Range | |
| Aminoglycoside | Gentamicin | 2.0 | 4.0 | 0.5-8.0 | 16 |
| Kanamycin | 2.0 | 8.0 | 1.0-8.0 | 32 | |
| Neomycin | 8.0 | 16 | 2.0-32 | ≥ 64 | |
| Spectinomycin | ≥ 64 | ≥ 64 | 4.0 to ≥ 64 | 4.0 | |
| Streptomycin | ≥ 64 | ≥ 64 | ≥ 64 | 4.0 | |
| β–lactam | Amoxicillin | ≥ 64 | ≥ 64 | ≥ 64 | ≥ 64 |
| Penicillin | ≥ 64 | ≥ 64 | ≥ 64 | ≥ 64 | |
| Lincosamide | Lincomycin | ≤ 0.25 | 0.5 | ≤ 0.25-1.0 | 2.0 |
| Macrolide | Erythromycin | 16 | ≥ 64 | 8.0 to ≥ 64 | ≤ 0.25 |
| Tylosin | 0.5 | 1.0 | ≤ 0.25-2.0 | ≤ 0.25 | |
| Tetracycline | Oxytetracycline | 4.0 | 16 | ≤ 0.25-32 | ≤ 0.25 |
| Tetracycline | 4.0 | 16 | ≤ 0.25-32 | ≤ 0.25 | |
* Mycoplasma hyorhinis reference strain ATCC 17981. Field strains were isolated from lung specimens submitted to the Research Unit at Green Cross Veterinary Products Co, Ltd, Yongin, Korea, during 2011 and 2012. Specimens were from 23 weaned pigs (30-70 days old) from nine swine farms.
† MIC required to inhibit growth of 50% of M hyorhinis isolates.
‡ MIC required to inhibit growth of 90% of M hyorhinis isolates.
Figure 1: A 96-well microplate showing the result of minimum inhibitory concentration (MIC) testing of antimicrobials against Mycoplasma hyorhinis cultured in Friis broth (isolates described in Table 1). Outside rows and columns were inoculated with 100 mL of Friis broth to prevent dehydration during the culture period of 5 to 7 days. The antimicrobial dilutions were performed from left to right using only the inner 60 wells. MIC = lowest antimicrobial concentration to inhibit color change (red to yellow). A: Inhibited growth of M hyorhinis; B: Uninhibited growth of M hyorhinis; C: Growth control (drug-free inoculum); D: Sterility control (uninoculated Friis broth).
Results
Table 1 shows the MIC50, MIC90, and range of MICs against the 10 M hyorhinis field isolates and the reference strain. Against the field isolates, the MIC90 was ≥ 64 µg per mL for amoxicillin, erythromycin, penicillin, spectinomycin, and streptomycin; 1.0 µg per mL for lincomycin; and 0.5 µg per mL for tylosin. Against the reference strain, the MIC was ≥ 64 µg per mL for amoxicillin, neomycin, and penicillin; ≤ 0.25 µg per mL for erythromycin, oxytetracycline, tetracycline, and tylosin; and 2.0 µg per mL for lincomycin.
Discussion
In this test, 10 M hyorhinis field isolates were resistant to spectinomycin and streptomycin (MIC ≥ 64 µg per mL). However, Ter Laak et al6 and Wu et al9 reported low MIC90s for these antimicrobials against M hyorhinus (4 µg per mL and 2 µg per mL, respectively). In this study, the MIC of spectinomycin against the reference strain was low (4 µg per mL).
Susceptibility of the field isolates to oxytetracycline and tetracycline in this study was poor, with an MIC50 of 4.0 µg per mL and an MIC90 of 16 µg per mL for both oxytetracycline and tetracycline. Aarestrup et al,8 Hannan et al,7 Ter Laak et al,6 and Wu et al9 found MIC90s of 0.25, 1.0, 2.0, and 2.5 µg per mL, respectively, for tetracycline.
In this study, the MIC90 for erythromycin was high (≥ 64 µg per mL), in agreement with the results reported by Ter Laak et al6 and Wu et al,9 who found MIC90 values ≥ 16 µg per mL, and Kobayashi et al, 5 who reported MIC90 values ≥ 100 µg per mL. The MIC90 was 1.0 µg per mL for tylosin and 0.5 µg per mL for lincomycin against the field isolates, in agreement with the results of other studies.5,6,8,9
In general, Mycoplasma species are difficult to isolate and culture because of fastidious growth requirements and slow growth.14 For this reason, the MIC test for Mycoplasma species is complex and difficult to study. The results of this study provide new data regarding the susceptibility of Korean M hyorhinis field isolates to 12 antimicrobial agents. To the authors’ knowledge, these are the first published data concerning the antimicrobial susceptibility of Korean M hyorhinis isolates. Further investigations should be conducted at regular intervals to determine the MICs of antimicrobials against additional field strains. Appropriate use of antimicrobial agents after a susceptibility test is the most practical way to treat M hyorhinis infection in pigs.
Implication
The Korean field strains of M hyorhinis tested in this study are sensitive to lincomycin and tylosin but resistant to erythromycin, spectinomycin, and streptomycin.
Acknowledgements
This research was part of a project titled “The production of amphidinol from marine microalgae culture and development of mycoplasma elimination kit using amphidinol,” funded by the Ministry of Land, Transport and Maritime Affairs, Korea.
Conflict of interest
None reported.
References
1. Friis NF, Feenstra AA. Mycoplasma hyorhinis in the etiology of serositis among piglets. Acta Vet Scand. 1994;35:93–98.
2. Kobisch M, Friis NF. Swine mycoplasmosis. Rev Sci Tech Off Int Epiz. 1996;15:1569–1605.
3. Lin JH, Chen SP, Yeh KS, Weng CN. Mycoplasma hyorhinis in Taiwan: Diagnosis and isolation of swine pneumonia pathogen. Vet Microbiol. 2006;115:111–116.
4. Schilman A, Estola T, Garry-Anderson AS. On the occurrence of Mycoplasma hyorhinis in the respiratory organs of pigs, with special reference to enzootic pneumonia. Zentralblat Veterinarmed. 1970;17:549–553.
5. Kobayashi H, Morozumi T, Munthall G, Mitani K, Ito N, Yamamoto K. Macrolide susceptibility of Mycoplasma hyorhinis isolated from piglets. Antimicrob Agents Chemother. 1996;40:1030–1032.
6. Ter Laak EA, Pijpers A, Noordergraaf JH, Schoevers EC, Verheijden JH. Comparison of methods for in vitro testing of susceptibility of porcine Mycoplasma species to antimicrobial agents. Antimicrob Agents Chemother. 1991;35:228–233.
7. Hannan PCT, Windsor GD, DeJong A, Schmeer N, Stegemann M. Comparative susceptibilities of various animal pathogenic Mycoplasmas to fluoroquinolines. Antimicrob Agents Chemother. 1997;41:2037–2040.
8. Aarestrup FM, Friis NF, Szancer J. Antimicrobial susceptibility of Mycoplasma hyorhinis in a liquid medium compared to a disc assay. Acta Vet Scand. 1998;39:145–147.
9. Wu CC, Shryock TR, Lin TL, Faderan M, Veenhuizen MF. Antimicrobial susceptibility of Mycoplasma hyorhinis. Vet Microbiol. 2000;76:25–30.
10. Tanner AC, Wu CC. Adaptation of the Sensititre broth microdilution technique to antimicrobial susceptibility testing of Mycoplasma gallisepticum. Avian Dis. 1992;36:714–717.
11. Hannan PCT. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary Mycoplasma species. Vet Res. 2000;31:373–395.
12. Bébéar C, Robertson JA. Determination of minimal inhibitory concentration. In: Tully JG, Razin S, eds. Molecular and Diagnostic Procedures in Mycoplasmology. Volume II: Diagnostic Procedures. New York, New York: Academic Press; 1996;189–197.
13. Caron J, Ouardani M, Dea S. Diagnosis and differentiation of Mycoplasma hyopneumoniae and Mycoplasma hyorhinis infections in pigs by PCR amplification of the p36 and p46 genes. J Clin Microbiol. 2000;38:1390–1396.
14. Wu CC, Shryock TR, Lin TL, Veenhuizen MF. Testing antimicrobial susceptibility against Mycoplasma hyopneumoniae in vitro. Swine Health Prod. 1997;5:227–230.