Original research |
Peer reviewed |
Effects of Mycoplasma hyopneumoniae vaccination in pigs co-infected with M hyopneumoniae and porcine circovirus type 2
Effectos de la vacunación de Mycoplasma hyopneumoniae en cerdos coinfectados con M hyopneumoniae y circovirus porcino tipo 2
Effets d’une vaccination contre Mycoplasma hyopneumoniae chez des porcs co-infectés avec M hyopneumoniae et le circovirus porcin de type 2
Vicki J. Rapp-Gabrielson, MS, PhD; Thayer Hoover, DVM; Steve Sornsen, DVM, MS; Lyle Kesl, DVM, PhD; Lucas Taylor, MS; Rika Jolie, DVM, PhD, MBA; Paul Runnels, DVM, PhD; Daniel Weigel, MS, PhD; Shan Yu, DVM, PhD; Tanja Opriessnig, DVM, PhD; Kristin Ruebling-Jass, MS; Erin Strait, DVM; Patrick G. Halbur, DVM, PhD
VJRG, LT, RJ, PR, DW: Veterinary Medicine Research and Development, Pfizer Animal Health, Division of Pfizer, Inc, Kalamazoo, Michigan. TH, SS: Swine Veterinary Operations, Pfizer Animal Health, Division of Pfizer, Inc, New York, New York. LK: Veterinary Resources, Inc, Ames, Iowa. SY, KRJ, ES: Department of Veterinary Microbiology and Preventive Medicine, College of Veterinary Medicine, Iowa State University, Ames, Iowa. TO, PGH: Department of Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, Ames, Iowa. Dr Thayer Hoover, Dr Rika Jolie, Dr Vicki Rapp-Gabrielson, Dr Paul Runnels, Dr Steve Sornsen, Lucas Taylor, and Dr Daniel Weigel were employed by Pfizer Animal Health while this study was being conducted. Corresponding author: Dr Vicki J. Rapp-Gabrielson, Pfizer Animal Health, RIC-190–39, 7000 Portage Road, Kalamazoo, MI 49001; Tel: 269-833-2634; Fax: 269-833-3305; E-mail: vicki.j.rapp-gabrielson@pfizer.com.
Cite as: Rapp-Gabrielson VJ, Hoover T, Sornsen S, et al. Effects of Mycoplasma hyopneumoniae vaccination in pigs co-infected with M hyopneumoniae and porcine circovirus type 2. J Swine Health Prod. 2008;16(1):16–26.
Also available as a PDF.
SummaryObjectives: To evaluate clinical and production parameters in pigs vaccinated with commercially available Mycoplasma hyopneumoniae (M hyo) bacterins, then co-infected with M hyo and porcine circovirus type 2 (PCV2). Materials and methods: Weaned pigs (n = 296) were each vaccinated with one of three M hyo bacterins or a placebo and challenged 3 and 5 weeks after the second vaccination with M hyo and PCV2, respectively. A subset of pigs, necropsied 2 or 4 weeks after PCV2 challenge, were evaluated for clinical signs, lesions, PCV2 serum levels, and M hyo in bronchial alveolar lavage fluids. Antibody titers to M hyo and PCV2 and average daily gain (ADG) were determined periodically during the trial. Estimated carcass value was determined at market weight. Results: In the placebo group, the challenge caused severe respiratory disease associated with M hyo and PCV2. There was no evidence of vaccine-enhanced PCV2 associated disease, in that the vaccine and placebo groups did not differ in PCV2 serum levels, severity of PCV2 lesions, or incidence of PCV2-associated disease. Vaccinated groups had lower lung lesion scores at necropsy 2 weeks post challenge and higher body weights and ADG at the study conclusion. Implications: When short-term clinical and long-term production parameters are evaluated, control of M hyo infection by M hyo vaccination is an important tool in reducing respiratory disease and production losses in herds infected with both PCV2 and M hyo. Under the conditions of this study, M hyo vaccination does not enhance disease associated with PCV2 infection. | ResumenObjetivos: Evaluar los parámetros clínicos y de producción en cerdos vacunados con bacterinas de Mycoplasma hyopneumoniae (M hyo por sus siglas en inglés) disponible comercialmente, y luego coinfectados con M hyo y circovirus porcino tipo 2 (PCV2 por sus siglas en inglés). Materiales y métodos: Cerdos destetados (n = 296) fueron vacunados con una de tres bacterinas de M hyo o un placebo y retados 3 y 5 semanas después de la segunda vacunación con M hyo y PCV2, respectivamente. Un subconjunto de cerdos fueron sacrificados 2 o 4 semanas después del reto con PCV2 y se evaluaron en busca de signos clínicos, lesiones, niveles en suero de PCV2, y M hyo en fluídos de lavado bronquioalveolar. Los títulos de anticuerpos contra M hyo y PCV2 y la ganancia diaria promedio (ADG por sus siglas en inglés) fueron determinados periódicamente durante la prueba. El valor estimado de la canal se determinó a peso de mercado. Resultados: En el grupo de placebo, el reto causó enfermedad respiratoria severa asociada con M hyo y PCV2. No hubo evidencia de aumento de la enfermedad asociada a PCV2 debido al efecto de la vacunación contra PCV2, ya que los grupos de vacuna y placebo no difirieron en los niveles de suero de PCV2, severidad de lesiones de PCV2, o incidencia de enfermedad asociada con PCV2. Los grupos vacunados tuvieron calificaciones menores de lesión pulmonar a la necropsia 2 semanas después del reto y mayor peso corporal y ADG al concluir el estudio. Implicaciónes: Cuando se evalúan los parámetros de producción a largo plazo y clínicos a corto plazo, el control de la infección de M hyo con vacunación de M hyo es una herramienta importante en la reducción de las enfermedades respiratorias y las pérdidas de producción en hatos infectados con ambos PCV2 y M hyo. Bajo las condiciones de este estudio, la vacunación de M hyo no aumentó la enfermedad asociada con la infección de PCV2. | ResuméObjectifs: Évaluer des paramètres cliniques et zootechniques chez des porcs vaccinés contre Mycoplasma hyopneumoniae avec des bactérines commerciales, puis co-infectés avec M hyopneumoniae et du circovirus porcin de type 2 (PCV2). Matériels et méthodes: Des porcs sevrés (n = 296) ont chacun été vaccinés avec une des trois bactérines de M hyopneumoniae ou un placebo et inoculés 3 et 5 semaines après la seconde vaccination avec, respectivement, M hyopneumoniae et PCV2. Chez un sous-groupe de porc, soumis à une nécropsie 2 ou 4 semaines après l’inoculation avec PCV2, les signes cliniques, les lésions, les niveaux sériques de PCV2, et de M hyopneumoniae dans les lavages broncho-alvéolaires ont été évalués. Les titres d’anticorps dirigés contre M hyopneumoniae et PCV2 et le gain quotidien moyen (ADG) ont été déterminés périodiquement durant la durée de l’expérience. La valeur estimée de la carcasse a été déterminée au poids du marché. Résultats: Dans le groupe placebo, l’inoculation a causé une maladie respiratoire sévère associée à M hyopneumoniae et PCV2. Il n’y avait aucune évidence d’augmentation de la sévérité de la maladie associée à la vaccination, tel que démontré par le fait qu’il n’y avait pas de différence dans les niveaux sériques de PCV2, dans la sévérité des lésions associées à PCV2, ou l’incidence de maladie associée à PCV2 entre les groupes vaccinés et placebo. Les groupes vaccinés avaient un pointage de lésions pulmonaires plus faibles 2 semaines post-inoculation et des poids corporels et un ADG plus élevés à la fin de l’étude. Implications: Lorsque des paramètres de production à long terme et des paramètres cliniques à court terme sont évalués, la maîtrise de l’infection par M hyopneumoniae au moyen d’une vaccination envers M hyopneumoniae est un outil important pour réduire les maladies respiratoires et les pertes de production dans les troupeaux infectés conjointement par PCV2 et M hyopneumoniae. Dans les conditions expérimentales de la présente étude, la vaccination contre M hyopneumoniae n’a pas exacerbé la maladie associée à une infection par PCV2. |
Keywords: swine, Mycoplasma
hyopneumoniae, porcine circovirus type 2, co-infection, postweaning
multisystemic wasting syndrome, PCV, PMWS
Search the AASV web site
for pages with similar keywords.
Received: July
20, 2007
Accepted: September
14, 2007
Association of postweaning multisystemic wasting syndrome (PMWS) with a porcine circovirus was first reported in 1997 and 1998 in swine from Western Canada, the United States, and Europe.1-4 Since those early reports, PMWS associated with porcine circovirus type 2 (PCV2) has become established as a disease of major economic importance in the key swine production areas of the world. Porcine circovirus type 2 is ubiquitous, and although essential for the development of PMWS, PCV2 infection alone is usually not sufficient for the development of PMWS or the other disease syndromes associated with PCV2.5,6 The terminology “porcine circovirus diseases” (PCVD)6 and “porcine circovirus associated disease” (PCVAD)7 have been adopted to describe the variety of clinical presentations coincident with PCV2 infection.
The pathogenesis of PCVAD is complex and multifactorial. It has been postulated that immune stimulation is a key event in the clinical expression of PCV2 disease.8,9 However, inoculation of a macrophage activator with tandem-cloned PCV2 DNA had no effect on viral replication or the appearance of disease.10 Experimental studies5,11-17 and epidemiological case-controlled studies18-20 indicate that clinical expression of PCVAD is also associated with co-infection with other swine pathogens, including Mycoplasma hyopneumoniae (M hyo), porcine reproductive and respiratory syndrome virus (PRRSV), and porcine parvovirus (PPV). Virulence and genetic differences among PCV2 isolates have been reported,21-27 and introduction of a new PCV2 strain into a population may be associated with a sudden increase in incidence and severity of clinical disease. Breed-associated differences in genetic susceptibility to the virus have also been reported.28 Although epidemiological spread of the disease suggests involvement of a new unidentified agent in PCVAD,29,30 no such agent has yet been identified.
Concurrent M hyo infection is associated with greater replication of PCV2, more severe PCV2-induced lesions, and a higher incidence of PMWS in co-infected pigs.16 Thus, vaccination to minimize the impact of M hyo infection in herds where both M hyo and PCV2 are co-circulating should be beneficial. However, in some experimental models and field studies, oil-based adjuvants or adjuvanted vaccines enhanced PCV2 replication and the severity of lymphoid depletion, compared to placebos or aluminum-based adjuvants.31-34 This association has not been demonstrated in other studies,35-37 and field experience does not indicate temporal or epidemiological relationships between the use of vaccines in piglets and the onset of PCVAD.38 A recent study demonstrated that the interval between administration of an M hyo bacterin and experimental challenge with PCV2 affects the clinical outcome.39
The present study was conducted to evaluate clinical signs, lesions, and production losses or gains following M hyo and PCV2 co-infection in groups of pigs each treated with a placebo or vaccinated with one of three commercially available M hyo bacterins, which each contained either an oil- or aqueous-based adjuvant. The bacterins were administered to pigs 3 and 5 weeks of age. The challenge model was a dual M hyo-PCV2 challenge that had previously been demonstrated to produce severe lesions.16 The pigs were serologically negative to PCV2 virus at the time of PCV2 challenge, in order to circumvent the protective effect of even low levels of maternal PCV2 antibodies.39,40 Following challenge, pigs were evaluated for both short-term and long-term outcomes to evaluate the effects that M hyo vaccination had not only on PCV2 replication and lesions, but also on market-weight production parameters.
Materials and methods
Study animals
Two hundred ninety-six weaned cross-bred barrows ([Landrace × York dams] × Large White boars) were obtained from a herd that had no history of vaccination or disease due to M hyo or PCV2. Animals were excluded from the study under any of the following conditions: injury, illness, or other adverse event unrelated to the study procedures that might compromise evaluation of the bacterins or challenge model; serologically positive to M hyo on study Day 0; or serologically positive to PCV2 at the time of PCV2 challenge (Day 49).
Study design
The study was a blinded generalized block design with the blocking factor based on body weight and pen location. Study design is summarized in Table 1. A block contained two animals from each of the four treatment groups, with pig the experimental unit. At the time of allotment, seven blocks (14 pigs) per group and one block of eight nonvaccinated, nonchallenged (NTX) pigs were randomly selected for necropsy on Day 63 (2 weeks after PCV2 inoculation) or on Day 77 (4 weeks after PCV2 inoculation). Individuals performing clinical observations, necropsy scoring, and laboratory analyses were blinded to the allocation of animals to treatment group. The study was completed on Day 131, when the average weight of the pigs in the pen estimated to be the heaviest was approximately 118.2 to 120.5 kg.
Table 1: Summary of study design to evaluate the effects of Mycoplasma hyopneumoniae (M hyo) vaccination in pigs co-infected with M hyo and porcine circovirus type 2 (PCV2)
* Seven blocks (14 pigs each for Placebo and Vacc1, Vacc2, and Vacc3) and one block (eight pigs) of NTX pigs randomly selected for necropsy each day. NTX pigs: nonvaccinated, nonchallenged procedural controls housed at a different facility, not included in the analysis; NA = not applicable. † Pfizer Animal Health, New York, New York. ‡ Schering-Plough Animal Health, Summit, New Jersey. § Fort Dodge Animal Health, Fort Dodge, Iowa. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Housing and animal management
The vaccine and placebo groups were housed in a single commercial, fan-ventilated swine building with double-sided curtains and slatted floors. Each pen contained a stainless steel feeder and a nipple cup waterer with approximately 6.3 m2 of floor space. Pigs were housed in 34 pens with eight pigs per pen (two pigs per treatment). To prevent exposure of the nonvaccinated, nonchallenged (NTX) pigs to the challenges, they were housed in three pens, eight pigs per pen, at a different facility with a similar configuration. After arrival at the study sites, pigs were treated according to label directions with ceftiofur sodium (Pfizer Animal Health, New York, New York) for 3 consecutive days to help prevent stress-related infections associated with weaning and shipping. Pigs were acclimated for 7 days prior to the first vaccination. Animal feed contained 55 g per tonne carbadox (Mecadox; Phibro Animal Health, Fairfield, New Jersey) until Day 20, 15 days prior to M hyo challenge, at which time animals were introduced to an age-appropriate nonmedicated diet. Throughout the study, pigs were fed two stages of nursery diets and five stages of grow-finish diets (1.20%, 1.05%, 1.0%, 0.9%, and 0.75 % lysine content, respectively). Each ration was a pelleted corn-soy diet with 3% fat added, formulated for maximum lean-gain accretion to exceed NRC requirements.41 All diets and water were provided ad libitum.
Pigs were observed daily for general heath and, in the event of clinical illness prior to the completion of the study, treatments that were considered necessary for the animal’s welfare were administered at the discretion of the site veterinarian. Moribund or injured animals were euthanized and necropsied. This study was approved by the Institutional Animal Care and Use Committee of Veterinary Resources, Inc, Ames, Iowa.
Vaccination and challenge procedures
Pigs in the three vaccine groups were vaccinated intramuscularly at approximately 3 and 5 weeks of age (Days 0 and 14) with commercially available bacterins at the doses recommended on the labels (Table 1). The placebo was a sterile 0.9% saline solution (Phoenix Pharmaceuticals, St Joseph, Missouri).
The M hyo lung homogenate, administered on Day 35, contained isolate 232, a derivative of M hyopneumoniae strain 11.42 Each pig was manually restrained and the inoculum was administered endotracheally (10 mL containing 105 color changing units per mL)
The PCV2 challenge inoculum, administered on Day 49, was PK-15 cell-culture passaged live virus prepared from PCV2 40985 molecular clone.43 Each pig was manually restrained and inoculated with 3 mL of virus: 1 mL given in the right neck muscle and 2 mL given intranasally (approximately 1 mL per nostril). The titer of the PCV2 challenge was 104.54 median tissue culture infectious doses (TCID50) per mL, as determined by back-titration using an indirect fluorescent antibody assay on PK-15 cells.43
Serological testing
Blood samples were collected on Days -2, 34, 48, 62, 76, 100, and 131, and the sera were tested for PCV2 and M hyo antibodies by ELISA.44,45 For the Tween 20 M hyo ELISA, a normalized optical density (OD) of < 0.20 is negative and a value of > 0.24 is positive.46 For the PCV2 ELISA, a sample-to-positive (S:P) ratio of < 0.12 is negative and an S:P ratio of > 0.20 is positive. For analysis of the frequency of positive or negative sera at any time point, ELISA values that fell between the established negative and positive values were treated as negative. Sera from selected time points and pigs were submitted to the Iowa State University Veterinary Diagnostic Laboratory (ISU VDL) for testing for antibodies to PRRSV, swine influenza virus (SIV H1N1 and H3N2) and PPV to ascertain the status of the study animals relative to these pathogens.
Clinical signs and necropsy
Pigs in the blocks randomly selected at the time of allotment for necropsy on Day 63 or Day 77 were observed for clinical signs, and body temperatures were recorded three times weekly after PCV2 challenge. The following clinical signs were scored as absent or present (score of 0 or 1, respectively): respiratory distress, sneezing, coughing, lethargy, wasting, and icterus.
At necropsy, macroscopic lesions of all organs and the percent of lung involvement were scored visually.47 Percent lung involvement was recorded for dorsal and ventral sides of the left and right cranial, middle, and caudal lobes, and for the accessory lobe. Pooled lymph nodes from each pig were scored for hyperplasia (score of 0 to 3). Other organs were scored for macroscopic lesions as absent (0) or present (1). Fixed tissue samples from the liver, heart, lung, tonsils, thymus, spleen, kidney, and the lymph-node pool were examined histologically. Tissue samples were scored for microscopic lesions and immunohistochemical staining for the presence of PCV2 antigen as previously described.16,32,48 Briefly, the scores ranged from 0 to 3 (normal, mild, moderate, severe) for the spleen, tonsil, and lymph-node pool. The lymph-node pool was scored for lymphoid depletion, histiocytic replacement, and the presence of PCV2 by immunohistochemistry (IHC); the spleen and tonsils were scored for lymphoid depletion and for the presence of PCV2 by IHC. Lung tissue sections were scored from 0 to 3 for the presence of PCV2 by IHC and also for the presence and severity of pneumonia as follows: 0 = normal; 1 = mild multifocal; 2 = mild diffuse; 3 = moderate multifocal; 4 = moderate diffuse; 5 = severe multifocal; and 6 = severe diffuse. The lesion scoring system was 0 (absent) or 1 (present) for the other tissues.
Polymerase chain reaction
Serum samples collected on Days 48, 62, and 76 were tested for PCV2 DNA copy number by real-time quantitative polymerase chain reaction (qPCR).32 Titers are presented as genome copy number per mL.
At necropsy, bronchial swabs were collected for routine bacterial culture, and bronchial alveolar lavage (BAL) fluids were collected for M hyo quantification by qPCR.42 One-mL aliquots of BAL from each pig were centrifuged at 16,000g for 10 minutes, and the resulting pellet was resuspended in 200 μL of phosphate buffered saline. Isolation of DNA was performed using the “blood and body fluid protocol” of the QIAamp DNA mini kit (Qiagen, Valencia, California). Primers were designed using Invitrogen’s Lux designer Web-based software (www.invitrogen.com/dlux). The target was based on a previously published PCR protocol specific to M hyo.49 The forward primer was FAM-labeled 5’ctacctTTGAACCTCGGGCTTAGGtAG 3’. The reverse primer was 5’CAACCCGTACCCGCATAAGT 3’. The qPCR reactions were set up using Platinum Quantitative PCR SuperMix-UDG (Invitrogen, Carlsbad, California). The master mix consisted of the following: 1.5 U Platinum Taq DNA Polymerase, 20 mM Tris-HCL (pH 8.4), 50 mM KCL, 3mM MgCl2, 200 μM dGTP, 200 μM dATP, 200 μM dCTP, 400 μM dUTP, 1 U UDG, and 0.05 μM of the forward and reverse primer. Next, 5 uL of the DNA sample or standard DNA was added to 20 μL of master mix for a 25-μL reaction. All samples were performed in triplicate. The qPCR reactions were performed on a Rotorgene 3000 (Corbett Research, San Francisco, California) using the following program: 50°C for 2 minutes, 95°C for 2 minutes, then 38 cycles of denaturation at 95°C for 15 seconds, primer annealing at 55°C for 15 seconds, and elongation at 72°C for 15 seconds. A melt curve was performed to verify target amplification in the range of 70°C to 89°C. Samples were quantified by comparison to a standard curve generated with DNA isolated from the M hyo challenge strain, and results are presented as ng per μL M hyo DNA.
Market weight outcomes
Pigs were observed daily and those that presented as wasting or moribund were euthanized and necropsied. Tissues were submitted to the ISU VDL to assist in determination of the cause of death and evidence of PMWS. Pigs were weighed on Days -2, 34, 62, 76, 100, and 131 for analysis of body weight and average daily gain (ADG). “Sort loss” was defined as a pig with a body weight of < 104.3 kg at study conclusion on Day 131. The case definition of PMWS was met if a pig demonstrated the following: clinical signs of wasting (weight loss or ill thrift); microscopic lesions of lymphoid depletion or lymphohistiocytic inflammation in lymphoid tissues; and PCV2 antigen associated with lymphoid lesions confirmed by IHC.50 Pigs remaining at the study site at the conclusion of the study were not necropsied, so the PMWS case definition could be confirmed only for pigs that died or were euthanized.
Data analysis
The primary outcomes were body weight and ADG for all pigs and the percent-lung-involvement score for the pigs necropsied on Days 63 and 77. Serum antibody ELISA S:P ratios, PCV2 and M hyo quantification by qPCR, post-challenge clinical signs, macroscopic and microscopic lesions at necropsy, sort loss, carcass value at market, and occurrence of PMWS were analyzed as secondary outcomes.
Percentage of total lung with lesions was calculated using the following formula: total percentage = 100 × {(0.05 × left cranial-dorsal side) + (0.05 × left cranial-ventral side) + (0.05 × left middle-dorsal side) + (0.05 × left middle-ventral side) + (0.15 × left caudal-dorsal side) + (0.125 × left caudal-ventral side) + (0.05 × right cranial-dorsal side)+ (0.05 × right cranial-ventral side) + (0.05 × right middle-dorsal side) + (0.05 × right middle-ventral side) + (0.15 × right caudal-dorsal side) + (0.125 × right caudal-ventral side) + (0.05 × accessory)}. The arcsine square root transformation was applied prior to analysis using a general linear mixed model with fixed effects treatment, time point, and treatment × time point, and random effect block. For analysis of macroscopic and microscopic lesions that were scored from 0 to 3, scores of 0 or 1 were considered within the normal range and were compared to scores of 2 or 3. For analysis of the microscopic lung lesions (scored as 0 to 6), scores of 0 to 2 (normal to mild) were compared to scores of 3 to 6 (moderate to severe). Sort loss, presence-absence of microscopic or macroscopic lesions, occurrence of PMWS, and positive-negative antibody status were analyzed using a generalized linear mixed model with fixed effects treatment, time point, and treatment × time point, and random effect block. The percent of days that a pig showed at least one clinical sign was analyzed using a general linear mixed model, with fixed effects treatment, time point, and treatment × time point, and random effect block. Body weight, PCV2 and M hyo antibody ELISA S:P ratios, PCR titers, and rectal temperatures were analyzed using a general linear mixed model with repeated measures, with fixed effects treatment, time point, and treatment × time point, with random effects block and animal within block × treatment, which is the animal term. Where appropriate, ELISA or PCR values were transformed for analysis. Frequency distributions of the Day 131 body weights were summarized by treatment group and weight category for pigs ≤ 90.7 kg and in increments of 4.5 kg for pigs weighing between 90.7 and 149.7 kg. Market value in $US was based on an industry grid for market weight value (Table 2) and was analyzed using a general linear mixed model with fixed effect treatment, and random effect block. Pigs that died or were euthanized at the farm were treated as missing data points for analysis of body weights, ADG, and estimate of market value.
Table 2: Industry grid for estimating market value from live weight at slaughter.
|
The NTX pigs, housed in separate facilities, were procedural controls and were not included in the statistical analysis. Statistical analysis was performed with SAS-STAT Guide Version 8 (SAS Institute, Inc, Cary, North Carolina). The 5% level of significance (P < .05) was used to assess all statistical differences.
Results
Animal exclusion
One pig (Vacc1) died prior to revaccination due to a trauma-induced injury unrelated to the study procedures. Sixteen pigs were excluded from all analyses: 14 pigs (four each in Vacc1, Vacc2, and Vacc3, and two NTX) were positive for PCV2 antibodies on Day 48; one pig (Vacc3) was incorrectly vaccinated; and one pig (Vacc3) was excluded because of lesions at necropsy that were considered by the pathologist to be unrelated to the test procedures but that may have compromised evaluation of the bacterins. One pig in the Vacc2 group with a chronic tail-bite injury was not included in the analysis of sort loss at the end of the study.
Serological results
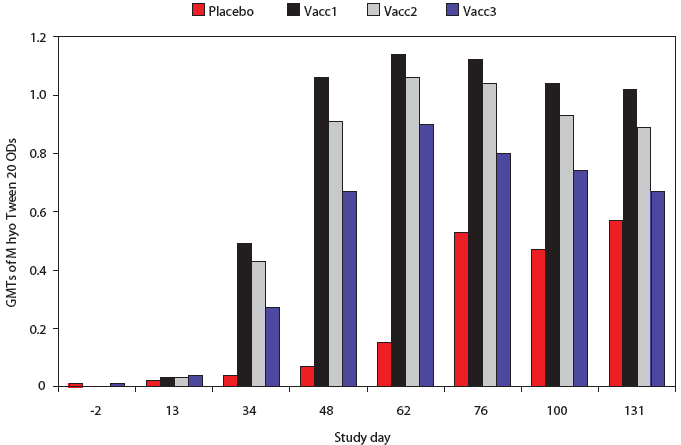
All pigs were negative by M hyo Tween 20 ELISA on Days -2 and 13 (Figure 1). At all subsequent time points, M hyo Tween 20 OD values were significantly higher in all vaccine groups than in the Placebo group. The percent of pigs serologically positive for M hyo was significantly higher in all vaccine groups than in the Placebo group on Days 34, 48, and 62 (P < .001, data not shown).
| Figure 1: Mycoplasma hyopneumoniae (M hyo)
Tween 20 ELISA results for pigs vaccinated with M hyo bacterins (described
in Table 1) or placebo on Days 0 and 14 and challenged with M hyo on Day
35 and with porcine circovirus type 2 (PCV2) on Day 49. Data are presented
as back-transformed geometric mean titers (GMTs). Normalized OD values
were log transformed and analyzed using a general linear mixed model with
repeated measures with fixed effects treatment, time point, and treatment × time
point, with random effects block and animal within block ×
treatment. Treatments did not differ on Days -2 and 13 (P > .05).
For all subsequent time points, GMTs of all vaccine treatment groups were
significantly higher than those of the Placebo group (P < .001
each day except Placebo to Vacc3 contrast, Day 131; P < .05).
The GMT of the Vacc1 group was significantly higher than that of the Vacc2
group on Day 48 (P < .001), Day 100 (P < .05) and Day
131 (P < .01) and was significantly higher than the Vacc3 group
on Days 34, 48, 62, 76, 100, and 131 (P <
.001).
|
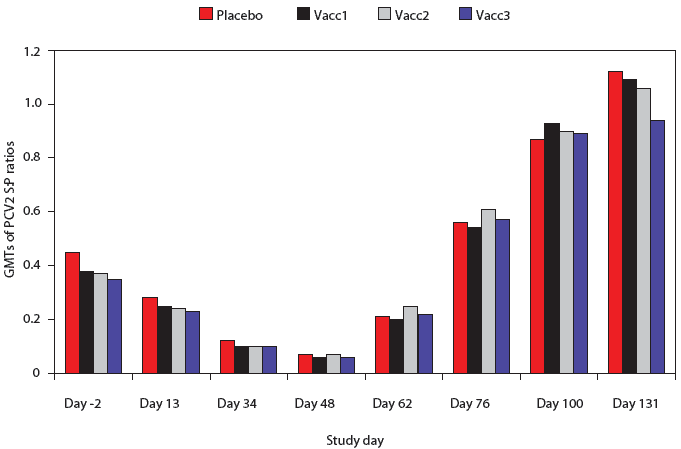
The PCV2 ELISA antibody S:P ratios were not significantly different among any of the groups at any time point (Figure 2). Antibodies to PCV2 were evident at Day -2, and both the S:P ratios and numbers of positive pigs declined prior to PCV2 challenge on Day 48. There were no significant differences among groups in the percent of pigs positive for PCV2 antibodies at any time point (data not shown). Approximately 50% of pigs were PCV2-positive by 2 weeks post challenge, approximately 90% were positive by 4 weeks post challenge, and nearly 100% were positive by Day 100.
| Figure 2: Porcine circovirus type 2 (PCV2) ELISA
antibody sample:positive (S:P) ratios in pigs vaccinated with Mycoplasma
hyopneumoniae bacterins (described in Table 1) or placebo on Days 0
and 14 and challenged with M hyopneumoniae on Day 35 and with PCV2
on Day 49. Data are presented as back-transformed geometric mean titers
(GMTs). The S:P ratios were log transformed and analyzed using a general
linear mixed model with repeated measures with fixed effects treatment,
time point, and treatment ×
time point, and random effects block and animal within block. There were
no differences among treatments at any time point (P < .05).
|
Sera from selected pigs tested at all time points showed no serological evidence of exposure to PRRSV, SIV H1N1, or PPV. Pigs at the farm site seroconverted to SIV H3N2 between Day 100 and 131; however, no clinical signs were apparent in the pigs during this time.
Clinical signs post challenge
The percentage of days in which any clinical sign was observed in a pig was significantly lower in Vacc1 (P < .001) and Vacc3 (P < .05) than in Placebo (Table 3). The percent of days in which a clinical sign was observed in a pig was also significantly lower in the Vacc1 group than the Vacc2 group (P < .05). The most frequent observation was coughing; lethargy, respiratory distress, and wasting were also observed in low frequencies in all groups (data not shown). The mean percent of days in which individual clinical signs were observed in pigs in a group did not differ among groups, and at no time points were mean body temperatures ≥ 40°C in any group (data not shown). Clinical signs were not seen in nonchallenged NTX pigs observed at the same time points as challenged pigs.
Table 3: Percentage of days (back-transformed least squares means ± SE) in which at least one clinical sign was observed* after challenge with porcine circovirus type 2 (PCV2) in pigs vaccinated with Mycoplasma hyopneumoniae bacterins (described in Table 1) or placebo on Days 0 and 14 and challenged with M hyopneumoniae on Day 35 and PCV2 on Day 49
* Pigs were observed three times weekly after PCV2 challenge until necropsy on Days 63 and 77 for respiratory distress, sneezing, coughing, lethargy, wasting, and icterus. The percentage of days when at least one clinical sign was observed was transformed using the arcsine of the square root of the percentage ÷100, and the transformed percentage was analyzed using a general linear mixed model with fixed effect treatment and random effect block. † Compared to Placebo. abc Values with no common superscript differ significantly (P < .05). NA = not applicable. |
||||||||||||||||||||||||||||||||
Necropsies
At the Day 63 necropsy (2 weeks after PCV2 challenge), mean percentage lung involvement was significantly lower in all vaccine groups than in the Placebo group (Table 4). The percentage lung involvement in Vacc1 (P < .001) and Vacc3 (P < .01) were also significantly lower than in Vacc2. At Day 77 necropsy (4 weeks after PCV2 challenge), mean percentage lung involvement was lower than in the Placebo only in Vacc1 (P < .05).
Table 4: Percentage of macroscopic lung-lesion involvement (back-transformed least squares means ± SE) scored visually in pigs vaccinated with Mycoplasma hyopneumoniae bacterins or placebo on Days 0 and 14, challenged with M hyopneumoniae on Day 35 and with porcine circovirus type 2 on Day 49, and necropsied on Day 63 or 77
* The arcsine square root transformation was applied prior to analysis using a general linear mixed model with fixed effects treatment, time point, and treatment × time point, and random effect block. † Compared to Placebo. ‡ Nonvaccinated, nonchallenged procedural controls not included in analysis. Values are mean ± SD. abc Values within a column with no common superscript differ significantly (P < .05). NA = not applicable. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Macroscopic and microscopic lymph-node lesions associated with PCV2 were present in a high percentage of pigs in all groups (Table 5). Similar results were observed for the spleen and tonsils (data not shown). The percentages of pigs with positive scores for pneumonia (range 75% to 100% on Day 63 and 35.7% to 71.4% on Day 77) and lung specimens with positive scores for PCV2 by IHC (range 41.7% to 78.6% on Day 63 and 7.1% to 35.7% on Day 77) were also high in all groups. There were no significant differences (treatment or treatment × time-point effects) in the macroscopic or microscopic lesion scores for the lymph-node pool, lungs, or any of the other tissues examined (data not shown).
Table 5: Incidence of microscopic lesions in lymph-node pools* at necropsy in pigs vaccinated with Mycoplasma hyopneumoniae bacterins or placebo on Days 0 and 14, challenged with M hyopneumoniae on Day 35 and with porcine circovirus type 2 on Day 49, and necropsied on Day 63 or 77
* Lymph nodes from each pig were pooled. † Positive = lymph-node pool scored 2 or 3 in a range of 0 to 3. Presence or absence of microscopic lesions was analyzed with a generalized linear mixed model (GLIMMIX macro) with fixed effects treatment, time point, and treatment × time point, and random effect block, or with Fisher’s exact test. Treatment or treatment × time point interactions were not significant for any lymph-node score. ‡ Nonvaccinated, nonchallenged procedural controls not included in analysis. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PCR and bacterial isolation
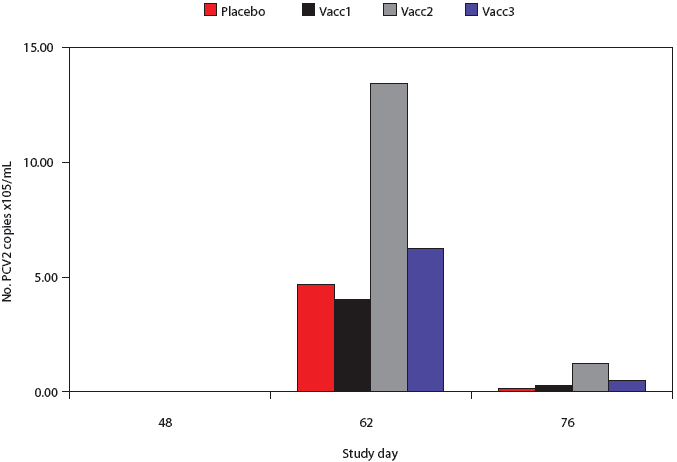
All pigs were negative by PCR for PCV2 nucleic acid in serum at Day 48 (pre-challenge) (Figure 3). High levels of PCV2 DNA were detected in the sera of pigs post challenge, but there was considerable variability in the individual pig values and there were no significant differences among groups at either the Day 62 or 76 post-challenge time points.
| Figure 3: Porcine circovirus type 2 (PCV2) real-time
quantitative polymerase chain reaction (qPCR) genome copy numbers in serum
from pigs vaccinated with Mycoplasma hyopneumoniae bacterins (described
in Table 1) or placebo on Days 0 and 14 and challenged with M hyopneumoniae on
Day 35 and with PCV2 on Day 49. Data are presented as back-transformed
geometric mean titers (GMTs). Titers were log transformed and analyzed
using a general linear mixed model with repeated measures with fixed effects
treatment, time point, and treatment × time point, and random effects
block and animal within block. There were no differences among treatments
at any time point.
|
On Day 63, M hyo DNA titers in BAL samples were significantly lower in Vacc1 than in Placebo (P < .001), Vacc2 (P < .01), or Vacc3 (P < .01) (Table 6). On Day 77, M hyo DNA titers in BAL samples were significantly lower in all vaccinated groups than Placebo (P < .05), but did not differ among vaccinated treatment groups.
Table 6: Mycoplasma hyopneumoniae real-time polymerase chain reaction (qPCR) DNA titers (ng/μL) in bronchial alveolar lavage fluids in pigs at necropsy on Days 63 and 77 after vaccination with M hyopneumoniae bacterins or placebo on Days 0 and 14 and challenge with M hyopneumoniae on Day 35 and PCV2 on Day 49
* Back-transformed geometric mean titer (GMT) ± SE. A log transformation was applied prior to analysis with a general linear mixed model with fixed effects treatment, time point, and treatment × time point, with random effect block. † Compared to Placebo. ‡ Nonvaccinated, nonchallenged procedural controls not included in analysis. Values are mean ± SD. abc Values within a column with no common superscript differ significantly (P < .05). NA = not applicable. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The following bacterial pathogens were identified in the 128 bronchial swabs collected: Streptococcus suis (14 swabs); Pasteurella multocida (three swabs); Actinobacillus suis, Haemophilus parasuis, and Bordetella bronchiseptica (two swabs each); Actinobacillus equuli and Arcanobacterium pyogenes (one swab each). The number of colony forming units detected per culture ranged from a few to approximately 200.
Occurrence of PMWS
The percentage of pigs meeting the case definition of PMWS was not significantly different among groups at either necropsy time point. The case definition of PMWS was met for two of 14 Placebo pigs (14.3%) at both the Day 63 and Day 77 necropsies. On Day 63, one of 12 pigs in Vacc1 and in Vacc2 (8.3%) met the case definition of PMWS. On Day 77, one of 14 pigs in Vacc1 (7.1%) and four of 14 pigs in Vacc2 (28.6%) met the case definition of PMWS. None of the NTX sentinel controls (zero of seven) met the case definition of PMWS at either necropsy (not included in statistical analysis). Eight pigs (three in Placebo, one in Vacc1, and two each in Vacc2 and Vacc3) died or became moribund, and were euthanized and necropsied at the study site during the postchallenge phase of the study. One necropsied pig in each group that died at the farm met the case definition of PMWS.
Market-weight outcomes
The production parameters of body weight, ADG, sort loss, and estimated carcass market value at study conclusion on Day 131 are summarized in Table 7. There were no differences in body weight among groups on Day -2, or at any time point prior to Day 76 (data not shown). On Day 76, the weights were significantly higher in Vacc1 than in the Placebo (P < .05), and on Day 100, the weights of all vaccinated groups were higher than that of the Placebo group (data not shown). The body weights of all vaccinated groups were significantly higher (P < .01) than that of the Placebo group on Day 131. There were no differences among the vaccinated groups at any time point. The ADG between Days -2 and 131 was significantly higher for all vaccinated groups (P < .01) than for the Placebo group, and there were no differences among vaccinated groups (Table 7). Average daily gain was also calculated at interim time points and the results were similar to the overall ADG for the study period (data not shown).
Table 7: Live body weight at Day 131, average daily gain (ADG) Days -2 through 131, sort loss, and estimated market value ($US) of pigs vaccinated with Mycoplasma hyopneumoniae bacterins (described in Table 1) or placebo on Days 0 and 14 and challenged with M hyopneumoniae on Day 35 and porcine circovirus type 2 (PCV2) on Day 49
* Least-squares means ± SE. Body weights and average daily gain were analyzed using a general linear mixed model with repeated measures with fixed effects treatment, time point, and treatment × time point, with random effects block and animal within block × treatment. Pigs that died or were euthanized prior to day 131 were treated as missing data. † Compared to Placebo. ‡ Sort loss (body weight < 104.3 kg) was analyzed using a generalized linear mixed model (GLIMMIX macro) with fixed effects treatment, time point, and treatment × time point, and random effect block. Overall treatment effect was not significant (P > .05). § Carcass value was estimated from market grid in place at the time the study (Table 2) using a general linear mixed model with fixed effect treatment and random effect block. There was a tendency for an overall treatment effect (P < .10). abc Values within a column with different superscripts differ significantly (P < .05). NA = not applicable. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The percentage of pigs meeting the definition of sort loss (body weight < 104.3 kg) at the study conclusion was not different among groups (Table 7).
Using the market grid estimate (Table 2), there was a tendency for an overall treatment effect on estimated carcass value (P < .10). All vaccine groups had an estimated higher market value than Placebo, with a carcass value that exceeded the Placebo value by $9.63 for Vacc1, $5.83 for Vacc2, and $7.13 for Vacc3.
Discussion
This study was conducted to evaluate the effect of vaccination with commercially available M hyo bacterins, formulated with either oil- or aqueous-based adjuvants, on the development of PCVAD in a co-infection model where pigs were challenged first with M hyo and 2 weeks later with PCV2. Pigs were vaccinated at 3 to 4 weeks of age, reflecting field practices where pigs are vaccinated around the time of weaning. Pigs were challenged with PCV2 at 10 weeks of age, when antibodies, presumed to be maternally derived, had declined to negative S:P ratios, to reduce the confounding effect that PCV2 antibodies would have on protection from PCV2 disease and to mimic the time when exposure typically occurs in the field in the United States. The study was concluded when pigs reached typical market weight, in order to assess not only the short-term effect that vaccination might have on the development of clinical signs, pneumonia, and other PCV2-associated lesions, but also the long-term impact on production parameters of body weight, ADG, and carcass value.
Consistent with the previous report,16 co-infection of pigs with M hyo and PCV2 resulted in severe respiratory disease in control pigs (Placebo), as measured by clinical signs, pneumonia associated with both M hyo and PCV2, and severe microscopic lesions associated with PCV2, including lymphoid depletion and histiocytic replacement. The short-term parameters evaluated in this study indicate that M hyo vaccination was effective in reducing respiratory disease associated with M hyo and PCV co-infection. There was no evidence of enhancement of PCVAD in groups vaccinated with M hyo bacterins containing either an oil- or aqueous-based adjuvant, as measured by microscopic and macroscopic lesion scores, quantitation of PCV2 in serum, development of clinical signs, or incidence of low body weights or PMWS. Vaccination with M hyo had a positive impact on the production parameters of body weight, ADG, and estimated market value. Although significant differences among vaccinated groups for some of the short-term parameters were evident, the significant advantage in ADG and numerical advantage in market value was apparent in all three M hyo vaccine groups.
The co-factors or triggers involved in the development of lesions and clinical expression of disease in PCV2-infected pigs are not fully understood; however, PCVAD is apparently a consequence of the inability of some pigs to develop an effective immune response to a virus that is ubiquitous in the pig population. Experimental challenge studies and case-controlled epidemiological studies, as well as anecdotal information from field observations, yield conflicting results as to whether routine swine vaccinations influence the clinical outcome of PCV2 infection.31-39 However, there is no compelling evidence of a cause-effect association of the introduction of M hyo vaccination into a herd and the onset of severe PCVAD.38 Differences in the results and conclusions of studies evaluating the effect of immune stimulation in general, and administration of vaccines in particular, may reflect differences in the disease models, interval between vaccination and PCV2 exposure, virulence of the virus strains, differences in susceptibility and immune maturity of the animals, and the presence of other swine pathogens in the study population.
Under the conditions of this study, where PCV2 and M hyo co-infected pigs were housed in a production setting, the data are consistent with other reports that do not demonstrate an association of M hyo vaccination or adjuvant type with the development of PCVAD.35-38 In contrast, although severe lesions attributable to infection with both PCV2 and M hyo were evident in all groups at necropsy, vaccination with the commercial bacterins had a positive effect in reducing M hyo infection, and all vaccine groups out-performed the controls in market-weight outcomes.
Implications
- When both short-term effects and long-term impact on production parameters are assessed, control of M hyo infection by the appropriate timing of M hyo vaccination is an important tool in reducing respiratory disease and production losses in herds where both PCV2 and M hyo are present.
- Under the conditions of this study, M hyo vaccination does not enhance disease associated with PCV2 infection.
Acknowledgements
Funding support for this study was provided by Pfizer Animal Health. Preparation of the challenge inocula, the antibody assays, and M hyo qPCR were performed in the laboratory of Dr Eileen Thacker, College of Veterinary Medicine, Iowa State University, Ames, Iowa. The authors would also like to thank Dr Kent Schwartz, Iowa State University, and the students and staff in the laboratories of Dr Halbur, Dr Thacker, the Iowa State University Veterinary Diagnostic Laboratory, and Veterinary Resources, Inc, who assisted with this study.
References
*1. Harding JCS, Clark EG. Recognising and diagnosing postweaning multisystemic wasting syndrome (PMWS). Swine Health Prod. 1997;5:201–203.
2. Allan GM, McNeilly F, Kennedy S, Daft B, Clarke EG, Ellis JA, Haines DM, Meehan BM, Adair BM. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J Vet Diagn Invest. 1998;10:3–10.
3. Morozov I, Sirinarumitr T, Sorden SD, Halbur PG, Morgan MK, Yoon K-J, Paul PS. Detection of a novel strain of porcine circovirus in pigs with postweaning multisystemic wasting syndrome. J Clin Microbiol. 1998;36:2535–2541.
4. Kiupel M, Stevenson GW, Mittal SK, Clark EG, Haines DM. Circovirus-like viral associated disease in weaned pigs in Indiana. Vet Pathol. 1998;35:303–307.
5. Allan GM, Kennedy S, McNeilly F, Foster JC, Ellis JA, Krakowka S, Meehan BM, Adair BM. Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J Comp Pathol. 1999;121:1–11.
6. Allan GM, McNeilly F, Meehan B, Kennedy S, Johnston D, Ellis J, Krakowka S, Fossum C, Wattrang E, Wallgren P. Reproduction of PMWS with a 1993 Swedish isolate of PCV-2. Vet Rec. 2002;150:255–256.
*7. AASV Porcine Circovirus Ad Hoc Committee. Porcine circovirus associated disease (PCVAD) case definition. J Swine Health Prod. 2007;15:45.
8. Krakowka S, Ellis JA, McNeilly F, Ringler S, Rings DM, Allan G. Activation of the immune system is the pivotal event in the production of wasting disease in pigs infected with porcine circovirus (PCV2). Vet Pathol. 2001;38:31–42.
9. Grasland B, Loizel C, Blanchard P, Oger A, Nignol A-C, Bigarré L, Morvan H, Cariolet R, Jestin A. Reproduction of PMWS in immuno-stimulated SPF piglets transfected with infectious cloned genomic DNA of type 2 porcine circovirus. Vet Res. 2005;36:685–697.
10. Loizel C, Blanchard P, Grasland B, Dory D, Oger A, Nignol A-C, Cariolet R, Jestin A. Effect of granulocyte-macrophage colony-stimulating factor on post-weaning multisystemic wasting syndrome in porcine circovirus type-2-transfected piglets. Int J Exp Path. 2005;86:33–43.
11. Allan GM, McNeilly F, Ellis JA, Krakowka S, Meehan B, McNair I. Experimental infection of colostrum deprived piglets with porcine circovirus type 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch Virol. 2000;145:2421–2429.
12. Kennedy S, Moffett D, McNeilly F, Meehan B, Ellis J, Krakowka S, Allan GM. Reproduction of lesions of postweaning multisystemic wasting syndrome by infection of conventional pigs with porcine circovirus type 2 alone and in combination with porcine parvovirus. J Comp Pathol. 2000;122:9–24.
13. Krakowka S, Ellis JA, Meehan BM, Kennedy S, McNeilly F, Allan G. Viral wasting syndrome of swine: experimental reproduction of postweaning multisystemic wasting syndrome in gnotobiotic swine by coinfection with porcine circovirus 2 and porcine parvovirus. Vet Pathol. 2000;37:254–263.
14. Harms PA, Sorden SD, Halbur PG, Bolin SR, Lager KM, Morozov I, Paul PS. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet Pathol. 2001;38:528–539.
15. Rovira A, Balasch M, Segalés J, García L, Plana-Durán J, Rosell C, Ellerbrok H, Mankertz A, Domingo M. Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2. J Virol. 2002;76:3232–3239.
16. Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng XJ, Halbur PG. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol. 2004;41:624–640.
17. Ostanello F, Caprioli A, Di Francesco A, Battilani M, Sala G, Sarli G, Mandrioli L, McNeilly F, Allan GM, Prosperi S. Experimental infection of 3-week-old conventional colostrum-fed pigs with porcine circovirus type 2 and porcine parvovirus. Vet Microbiol. 2005;108:179–186.
18. Wellenberg GJ, Stockhofe-Zurwieden N, Boersma WJA, de Jong MF, Elbers ARW. The presence of co-infections in pigs with clinical signs of PMWS in The Netherlands: a case control study. Res Vet Sci. 2004;77:177–184.
19. Dewey CE, Johnston T, Gould L, Whiting TL. Postweaning mortality in Manitoba swine. Can J Vet Res. 2006;70:161–167.
20. Dorr PM, Baker RB, Almond GW, Wayne SR, Gebreyes A. Epidemiologic assessment of porcine circovirus type 2 coinfection with other pathogens in swine. JAVMA. 2007;230:244–250.
21. Fenaux M, Opriessnig T, Halbur PG, Elvinger F, Meng XJ. Two amino acid mutations in the capsid protein of type 2 porcine circovirus (PCV2) enhanced PCV2 replication in vitro and attenuated the virus in vivo. J Virol. 2004;78:13440–13446.
22. Wen L, Guo X, Yang H. Genotyping of porcine circovirus type 2 from a variety of clinical conditions in China. Vet Microbiol. 2005;110:141–146.
23. Opriessnig T, McKeown NE, Zhou E-M, Meng X-J, Halbur PG. Genetic and experimental comparison of porcine circovirus type 2 (PCV2) isolates from cases with and without PCV2-associated lesions provides evidence for differences in virulence. J Gen Virol. 2006;87:2923–2932.
24. Olvera A, Cortey M, Segalés J. Molecular evolution of porcine circovirus type 2 genomes: Phylogeny and clonality. Virology. 2007;357:175–185.
25. Cheung AK, Lager KM, Kohutyuk OI, Vincent AL, Henry SC, Baker RB, Rowland RR, Dunham AG. Detection of two porcine circovirus type 2 genotypic groups in United States swine herds. Arch Virol. 2007;152:1035–1044.
*26. Gagnon CA, Tremblay D, Tijssen P, Venne M-H, Houde A, Elahi SM. PCV2 strain variation: what does it mean? Proc AASV. Orlando, Florida. 2007;535–540.
27. Horlen KP, Schneider PS, Anderson J, Nieterfled JC, Henry SC, Tokach LM. A cluster of farms experiencing severe porcine circovirus associated disease: Clinical features and association with the PCV2b genotype. J Swine Health Prod. 2007;15:270–278.
28. Opriessnig T, Fenaux M, Thomas P, Hoogland MJ, Rothschild MF, Meng XJ, Halbur PG. Evidence of breed-dependent differences in susceptibility to porcine circovirus type-2-associated disease and lesions. Vet Pathol. 2006;43:281–293.
29. Vigre H, Bækbo P, Jorsal SE, Bille-Hansen V, Hassing
A-G, Enře C, Břtner A. Spatial and temporal patterns of pig herds
diagnosed with Postweaning Multisystemic Wasting Syndrome (PMWS)
during the first two years of its occurrence in Denmark. Vet
Microbiol. 2005:110:17–26.
*30. Jaros P, McIntyre LH, Morris RS, Johnstone AC, Garkavenko O, Neumann E. Experimental evidence that an agent other than PCV2 is a necessary cause of PMWS. Proc IPVS. Copenhagen, Denmark. 2006;1:168.
31. Kyriakis SC, Saoulidis K, Lekkas S, Miliotis ChC, Papoutsis PA, Kennedy S. The effects of immuno-modulation of the clinical and pathological expression of postweaning multisystemic wasting syndrome. J Comp Path. 2002;126:38–46.
32. Opriessnig T, Yu S, Gallup JM, Evans RB, Fenaux M, Pallares F, Thacker EL, Brockus CW, Ackermann MR, Thomas P, Meng XJ, Halbur PG. Effect of vaccination with selective bacterins on conventional pigs infected with type 2 porcine circovirus. Vet Pathol. 2003;40:521–529.
33. Krakowka S, Ellis J, McNeilly F, Waldner C, Rings DM, Allan G. Mycoplasma hyopneumoniae bacterins and porcine circovirus type 2 (PCV2) infection: Induction of postweaning multisystemic wasting syndrome (PMWS) in the gnotobiotic swine model of PCV2-associated disease. Can Vet J. 2007;48:716–724.
34. Hoogland MJ, Opriessnig T, Halbur PG. Effects of adjuvants on porcine circovirus type 2-associated lesions. J Swine Health Prod. 2006;14:133–139.
*35. Harvey RE. The effect of vaccination against Mycoplasma hyopneumoniae on the mortality rate of growing pigs affected with post-weaning multisystemic wasting syndrome. Pig J. 2003;52:201–205.
36. Resendes A, Segalés J, Balasch M, Calsamiglia M, Sibilia M, Ellerbrok H, Mateu E, Plana-Durán J, Mankertz A, Domingo M. Lack of an effect of a commercial vaccine adjuvant on the development of postweaning multisystemic wasting syndrome (PMWS) in porcine circovirus type 2 (PCV2) experimentally infected conventional pigs. Vet Res. 2004;35:83–90.
37. Haruna J, Hanna P, Hurnik D, Ikede B, Miller L, Yason C. The role of immunostimulation in the development of postweaning multisystemic wasting syndrome in pigs under field conditions. Can J Vet Res. 2006;70:269–276.
38. MacKinnon JD. Vaccination ramification? An objective look at how vaccination might affect post-weaning multisystemic wasting syndrome (PMWS) and porcine dermatitis and nephropathy syndrome (PNDS). Pig J. 2003;51:36–63.
39. Opriessnig T, Halbur PG, Yu S, Thacker EL, Fenaux M, Meng XJ. Effects of the timing of the administration of Mycoplasma hyopneumoniae bacterin on the development of lesions associated with porcine circovirus type 2. Vet Rec. 2006;158:149–154.
40. McKeown NE, Opriessnig T, Thomas P, Guenette DK, Elvinger F, Fenaux M, Halbur PG, Meng XJ. Effects of porcine circovirus type 2 (PCV2) maternal antibodies on experimental infection of piglets with PCV2. Clin Diag Lab Immunol. 2005;12:1347–1351.
41. Nutrient Requirements of Swine. 10th rev ed. National Research Council, National Academy of Sciences, National Academy Press; 1998.
42. Thacker EL, Thacker BJ, Kuhn M, Hawkins PA, Waters WR. Evaluation of local and systemic immune responses induced by intramuscular injection of Mycoplasma hyopneumoniae bacterin in pigs. Am J Vet Res. 2000;61:1384–1389.
43. Fenaux M, Halbur PG, Haqshenas G, Royer R, Thomas P, Nawagitgul P, Gill M, Toth TE, Meng XJ. Cloned genomic DNA of type 2 porcine circovirus is infectious when injected directly into the liver and lymph nodes of pigs: characterization of clinical disease, virus distribution, and pathologic lesions. J Virol. 2002;76:541–551.
44. Bereiter M, Young T, Joo HS, Ross RF. Evaluation of the ELISA and comparison to the complement fixation test and radial immunodiffusion enzyme assay for detection of antibodies against Mycoplasma hyopneumoniae. Vet Microbiol. 1990;25:177–192.
45. Nawagitgul P, Harms PA, Morozov I, Thacker BJ, Sorden SD, Lekcharoensuk C, Paul PS. Modified indirect porcine circovirus (PCV) type 2-based and recombinant capsid protein (ORF2)-based enzyme-linked immunosorbent assays for detection of antibodies to PCV. Clin Diagn Lab Immunol. 2002;9:33–40.
46. Erlandson KR, Evans R, Thacker B, Wegner M, Thacker E. Evaluation of three serum antibody ELISA tests for Mycoplasma hyopneumoniae. J Swine Health Prod. 2005;13:198–203.
47. Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, Lum MA, Andrews JJ, Rathje JA. Comparison of the pathogenicity of two U.S. porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad Virus. Vet Pathol. 1995;32:648–660.
48. Sorden SD, Harms PA, Nawagitgul P, Cavanaugh D, Paul PS. Development of a polyclonal-antibody-based immunohistochemical method for the detection of type 2 porcine circovirus in formalin-fixed, paraffin-embedded tissue. J Vet Diagn Invest. 1999;11:528–530.
49. Kurth KT, Hsu T, Snook ER, Thacker EL, Thacker BJ, Minion C. Use of a Mycoplasma hyopneumoniae nested polymerase chain reaction test to determine the optimal sampling sites in swine. J Vet Diagn Invest 2002;14:463–469.
*50. Sorden SD. Update on porcine circovirus and postweaning multisystemic wasting syndrome. J Swine Health Prod. 2000;8:133–136.
*Non-refereed references.